Abstract
Cancer vaccines can induce robust activation of tumor-specific CD8+ T cells that can destroy tumors. Understanding the mechanism by which cancer vaccines work is essential in designing next-generation vaccines with more potent therapeutic activity. We recently reported that short peptides emulsified in poorly biodegradable, Incomplete Freund’s Adjuvant (IFA) primed CD8+ T cells that did not localize to the tumor site but accumulated at the persisting, antigen-rich vaccination site. The vaccination site eventually became a T cell graveyard where T cells responded to chronically released gp100 peptide by releasing cytokines, including interferon - γ (IFN-γ), which in turn upregulated Fas ligand (FasL) on host cells, causing apoptosis of Fas+ T cells. T cells that escaped apoptosis rapidly became exhausted, memory formation was poor, and therapeutic impact was minimal. Replacing the non-biodegradable IFA-based formulation with water-based, short-lived formulation in the presence of immunostimulatory molecules allowed T cells to traffic to tumors, causing their regression. In this review, we discuss recent advances in immunotherapeutic approaches that could enhance vaccine-primed immune cells fitness and render the tumor microenvironment more accessible for immune cell infiltration.
1. Introduction
Cancer vaccines given to treat established tumors have shown some therapeutic efficacy, yet challenges remain. Tumor regression has been rare1,2 despite the presence of vaccination-induced circulating tumor-specific CD8+ cytotoxic T cells (CTLs) in the peripheral blood of patients with cancer3. While peptide vaccines can induce successful T-cell priming, therapeutic success may require other essential features of vaccine-primed T cells, including attaining expansion to sufficient numbers, function and memory formation and traffic to - and long-term survival in the hostile tumor microenvironment.
CD8+ CTLs recognize their target antigens as small protein fragments presented by Major Histocompatibility Complex I (MHC–I) molecules on the surface of antigen presenting cells (APCs). The principle behind peptide-based vaccination is that the peptide epitope, the exact MHC-I binding antigenic fragment, in the vaccine will be taken up by APCs such as dendritic cells (DCs) that then travel to the vaccine draining lymph node (VdLN) and present the antigen to circulating antigen-specific CD8+ T cells. In this approach optimal DC activation and migration to the VdLN is crucial and can be supported by co-administration of immunostimulatory agents such as Toll-like receptor ligands and CD40 agonist antibodies4. Thus activated, DCs can present otherwise nonimmunogenic peptides in an immunogenic fashion to T cells, promoting their activation in turn.
Peptide vaccines currently used to treat patients of cancer are formulated as water-in-oil emulsions of antigen in mineral oil, IFA, with mannide monooleate as a surfactant5. It is widely believed that IFA causes local inflammation and forms a poorly biodegradable depot that protects the antigen from degradation as it is slowly released6,7. As such, IFA has been in the forefront as an adjuvant of choice in many clinical trials. In United States alone, 86 federally registered IFA-based cancer vaccines trials have been completed and currently 39 trials are active (www.ClinicalTrials.gov).
2. Understanding the mechanism of adjuvanticity of IFA
2.1. Background
Despite the widespread use of IFA in several vaccines to treat various maladies such as colorectal cancer, prostate cancer, pancreatic cancer, glioblastoma, leukemia, anemia, renal cell carcinoma, liver cancer, esophageal cancer, breast cancer, lung cancer, ovarian cancer, gastric cancer, melanoma, HIV and malaria, its mechanism of action remains poorly understood. While the explanation for the unexpectedly low therapeutic outcome1 of peptide/IFA-based cancer vaccines likely lies in part with tumor-induced immunoregulatory cells and factors8-10, we recently addressed the possibility that IFA-based vaccines may have intrinsic properties that limit their efficacy11, resulting in only rare therapeutic benefit and instead causing inflammatory reactions at vaccine injection sites.
2. 2. Peptide/IFA vaccination site as a T cell sink and graveyard
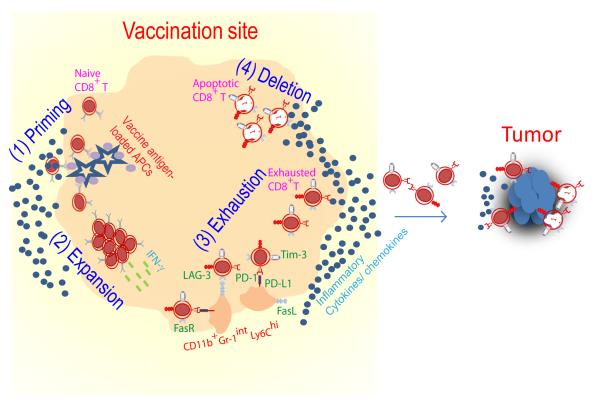
Whereas vaccination with the minimal gp100 peptide epitope emulsified in IFA is capable of priming tumor-specific T cells, primed T cells become sequestered at the vaccination site rather than tumor site. In addition, the injection site turns into a “graveyard” for terminally differentiated apoptotic T cells (Fig. 1). We confirmed that sequestration of tumor-specific CD8+ T cells at the vaccine injection site requires persistence of antigen in IFA, as vaccines consisting of antigen and water failed to trap T cells at the vaccination sites11. Tumor-specific CD8+ T cells retained at the vaccination sites were strikingly dysfunctional as evidenced by reduced secretion of proinflammatory cytokines (IFN-γ) and expression of inhibitory surface markers, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte activation gene 3 (LAG-3), programmed death 1 (PD-1) and hepatitis A virus cellular receptor 2 (HAVCR-2 also referred as Tim-3) (Fig. 1). Furthermore, we observed IFN-γ-driven accumulation of host CD11bhiGr-1intLy6Chi inflammatory myeloid cells that upregulated FasL and PDL-1 at the vaccination site (Fig. 1). A similar subset of myeloid cells has recently been shown to cause T cell tolerization after closely associating with tumor specific CD8+ T cells in splenic marginal zone of tumor-bearing mice12. These findings help to explain why several IFA-based vaccines that successfully prime blood tumor–specific responses in mice and humans often fail to promote robust tumor regression12.
Fig. 1. Fate of CD8+ T cells primed by IFA-based vaccine.
(1) Naive CD8+ T cells travel to antigen-rich and highly inflamed vaccination site and become primed. (2) Effector CD8+ T cells respond to persistent antigen presentation by continued expansion and secretion of inflamma tory cytokines (IFN-γ). (3) Activated CD8+ T cells up regulate Fas and other inhibitory surface markers including PD-1, LAG-3, Tim-3 and CTLA-4 in response to antigen, IFN-γ and other inflammatory cytokines; these conditions also promote accumulation of host cells expressing PD-L1 and FasL. PD-1/PD-L1 engagement leads to T cell exhaustion; Fas/FasL engagement results in T cell apoptosis. (4) As a result, very few primed T cells reach the tumor.
2.3. Clinical observations
Accumulating data from clinical trials point to IFA-based peptide vaccines causing reactogenicity (tissue inflammation), sequestration and deletion of primed T cells at vaccination sites. Patients with melanoma vaccinated with Melan A/MART-1 peptide emulsified in IFA in combination with Toll-like-receptor 9 agonist, CpG developed inflammatory reaction at the old vaccination site after a recall vaccination11,12, suggesting that the old vaccination site is still potent enough to attract activated T cells, which in turn produce more chemokines thereby causing more effectors T cells to join them and creating a highly inflammatory environment. Carl June and colleagues reported that >90% of patients of myeloma developed skin rejections following infusion of co-stimulated autologous T cells in combination with peptide emulsified in Montanide, TLR-3 agonist Poly-ICLC and GM-CSF13. Similarly, repeated vaccination of patients with Acute Myeloid Leukemia (AML) with leukemia-associated antigens (PR1 and WT1) peptides in IFA resulted in short-lived expansion of high-avidity tumor-specific CD8+ T cells followed by their deletion. Interestingly, low-avidity tumor-specific CD8+ T cells continued to expand, resulting in poor clinical outcome in all patients14. These observations lead us to speculate that the low-avidity tumor-specific CD8+ T cells surviving from earlier vaccination site and VdLN preferentially expanded and localized to and inflamed, persisting, peptide-rich vaccination sites rather than tumor sites as shown in (Fig. 1).
2.4. Short vs long peptide: all IFA-based vaccines are not equal
Not all peptide epitopes behave the same when formulated in IFA. Recent studies show that vaccination with synthetic long peptides (~20-mer) may be a promising solution for the many shortcomings of (9–12-mer) minimal epitope peptide vaccines15. Long gp100 peptide is presented by rare DCs in the VdLN only, while short gp100 peptide is presented by multiple VdLN cell types including ubiquitous B cells11,16. The presence of MHC Class II-restricted helper epitopes in the long peptides can result in the stimulation of CD4+ T helper cells that express CD40L, which interacts with CD40 or the immature DC, resulting in maturation of the DC and upregulation of costimulatory molecules, such as CD8615. Indeed, we found that long peptides induced minimal T cell sequestration at the vaccination site, no rapid contraction of the T cell response and superior antitumor activity. Together this data suggests the need for efficient dendritic-cell targeting to induce potent T-cell response. A clinical study of peptides (~20-mer) derived from the HPV-16 E6 and E7 viral oncoproteins administered in IFA showed clinical responses in 74% and complete response in 47% women with vulvar intraepithelial neoplasia17. Tumor regressions were associated with the generation of HPV-specific, IFN-γ-producing CD4+ and CD8+ T cells. Vaccination with long peptides induced strong antigen-specific T-cell response with long lasting memory and tumor regression in mouse models18. Long gp100 peptide is presented by rare DCs in the VdLN only, while short gp100 peptide is presented by multiple VdLN cell types including ubiquitous B cells11,16. The presence of MHC Class II-restricted helper epitopes in the long peptides can result in the stimulation of CD4+ T helper cells that express CD40L, which interacts with CD40 or the immature DC, resulting in maturation of the DC and upregulation of costimulatory molecules, such as CD8615. Indeed, we found that long peptides induced minimal T cell sequestration at the vaccination site, no rapid contraction of the T cell response and superior antitumor activity. Together this data suggests the need for efficient dendritic-cell targeting to induce potent T-cell response.
3. Towards enhanced tumor-trafficking of vaccine-primed T cells
The use of peptide vaccines to activate the immune system to cause the rejection of established tumor remains an intensely studied approach. Recently, there has been unprecedented evidence that T cells can efficiently attack and kill large tumors and that their antitumor activity can be enhanced19-21. Here, we focus on how the current and next-generation peptide-based cancer vaccine approaches could benefit from increased understanding of T cell biology and the tumor microenvironment - and highlight clinically available approaches that improve immune cell tumor-trafficking leading to enhanced tumor rejection.
3.1. Replacing IFA and addition of immunostimulatory agents
The first major issue we, therefore, addressed was replacing the persistent IFA with a less persistent formulation, saline. However, when we simply replaced IFA with saline we observed no T cell priming, likely due to a lack of any adjuvant activity by saline. We then added a costimulatory vaccine cocktail, called covax, consisting of toll-like receptor 7 (TLR7) agonist (imiquimod cream), agonistic anti-CD40 antibody, and IL-2. Concurrent vaccinations with gp100 peptide emulsion in IFA and covax induced strong T cell priming; however, the majority of vaccine-primed T cells still remained sequestered at the vaccination site and with negligible anti-tumor activity. In contrast, vaccination with gp100 peptide in saline and covax induced slightly weaker T cell expansion; however, these T cells did not become sequestered at the vaccination site and reached the tumor, thereby suppressing tumor growth.
The triple combination of a TLR7 agonist, CD40 agonist and IL-2 was critical for the induction of effective anti-tumor immunity as we recently reported11. However, most clinical trials of peptide or protein vaccines thus far have used IFA and TLR agonist22 or TLR agonist and anti-CD4023 or IL-21, but never this powerful triple combination (covax). If the murine system is at all predictive of human T cell responses, the full potential of anti-tumor T cell responses will not be unlocked until multiple synergistic immunostimulators, which by themselves may prove quite weak, are combined24. While adding immunostimulatory agents can boost the activity of cancer vaccines22,23,25, IFA-based vaccines may be a special case26. The addition of covax to IFA-based vaccine increased numbers and survival of circulating T cells, but it did not overcome their entrapment at the vaccination site. As a result, we observed increased reactogenicity and tissue damage at the vaccination site, while tumor growth remained unimpeded consistent with clinical data.
Conversely, adding covax to water-based vaccine caused no reactogenicity but strongly boosted the priming of T cells, which trafficked to the tumor and suppressed its growth. We also confirmed that T cell entrapment at the vaccination site correlated with local antigen peptide presentation, which lasted a few days after vaccination with gp100 in saline vs. more than 3 months after vaccination with gp100 in IFA. This suggests that the duration of antigen presentation could be manipulated through the choice of an adjuvant, and it should neither be too-short (causing poor priming) nor too-long (causing T cell sequestration, dysfunction and tissue damage).
3.2. Enlisting chemokine and adhesion molecules
Chemokine receptors are expressed on a variety of immune cells in the tumor microenvironment, including tumor cells. Therefore, chemokines and adhesion molecules may serve as a suitable target for manipulating the tumor microenvironment to facilitate immune cell recruitment and tumor homing27-29. Data from multiple clinical studies suggest that inadequate recruitment of tumor-specific CD8+ T cells may be a rate limiting factor in tumor regression8,30 Recent clinical studies show T-cell infiltrate in patients of melanoma express higher levels of the chemokines CXCL9 and CXCL10 in primary lesions31 and the CXCR3/CCR5 chemokine ligands is found to be critical for immune mediated tumor-rejection in patients with metastatic melanoma32. In line with this, observations in mouse xenograft models suggested that the tumor could be manipulated to support recruitment of tumor-specific T cells through endogenously produced CXCR3/CCR5 ligands33. Similarly, pmel-1 transfectant T cells overexpressing CXCR2 preferentially localized to tumor sites and showed enhanced anti-tumor activity against MC38 tumor model which naturally expressed CXC- chemokine receptor 2 Ligand (CXCL2)34.
Cancer inflammatory reaction caused by tumor-infiltrating cytotoxic T cells (CTL) results in the expression of high levels of pro-angiogenic factors by tumors thereby the creation of immunosppressive environment that limit T cell trafficking and homing of the tumor microenvironment35. Data from recent gene expression profiling of vascular endothelial cells in patients with ovarian cancer showed over expression of endothelin B was associated with the absence of a T cell infiltrate and short patient survival, suggesting that endothelin B receptor could be manipulated towards improved T cell trafficking36. In another study it was shown that selective targeted delivery of TNF-α fused with NGR-TNF, a tumor homing peptide, to tumor vessels induced up-regulation of VCAM-1 and ICAM-2 on the endothelial lining of tumor vessels37,38, resulting in improved adhesion of T cells expressing the corresponding VCAM-1 and ICAM-2 receptors. Together these studies suggest that opportunities may exist for manipulating chemokine expression and tissue adhesion in the tumor microenvironment to allow activated immune cells to cross over the stromal barrier and cause tumor destruction.
Finally, our study11 adds one more reason why blood-based immunomonitoring alone, may not be sufficient to guide our understanding of tumor-specific T cell responses30. And, in our own study in mice, despite high level of T cells circulating in the blood in response to gp100/IFA + covax vaccination, few T cells reached the tumor. On the other hand, vaccination with gp100/saline + covax induced modest T cell level in the blood but the majority of these T cells migrated to the tumor causing significant tumor suppression. Similarly, studies abound where clinical response does not correlate with immune responses in the circulation3,14,39. As a growing number of clinical studies indicate T cell accumulation in the center of the tumor and invading margin to have strong correlation with a favorable clinical outcome in patients with cancer, it would be imperative to actually implement routine tumor sampling during immunotherapy trials. Some accessible cancers, such as cutaneous melanoma, allow for biopsies; for less accessible cancers this can be approached with neoadjuvant trial designs, where immunotherapy is administered before tumor surgery. Identifying the kinds of therapy-induced immune responses, at the tumor site, that correlate with clinical outcome can yield valuable clues for the design of next-generation therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartzentruber DJ, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, et al. Tumor Progression Can Occur despite the Induction of Very High Levels of Self/Tumor Antigen-Specific CD8+ T Cells in Patients with Melanoma. J.Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 4.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bonhoure F, Gaucheron J. Montanide ISA 51 VG as adjuvant for human vaccines. J Immunother. 2006;29:647–648. [Google Scholar]
- 6.Reinhardt RL, Bullard DC, Weaver CT, Jenkins MK. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J Exp Med. 2003;197:751–762. doi: 10.1084/jem.20021690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gnjatic S, Bhardwaj N. Antigen depots: T cell traps? Nat Med. 2013;19:397–398. doi: 10.1038/nm.3113. [DOI] [PubMed] [Google Scholar]
- 8.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hailemichael Y, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ugel S, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell reports. 2012;2:628–639. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Rapoport AP, et al. Combination Immunotherapy after ASCT for Multiple Myeloma Using MAGE-A3/Poly-ICLC Immunizations Followed by Adoptive Transfer of Vaccine-Primed and Costimulated Autologous T Cells. Clin Cancer Res. 2014;20:1355–1365. doi: 10.1158/1078-0432.CCR-13-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezvani K, et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8:351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 16.Bijker MS, et al. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur.J.Immunol. 2008;38:1033–1042. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 17.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N.Engl.J.Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 18.Zwaveling S, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 19.Arens R, van Hall T, van der Burg SH, Ossendorp F, Melief CJ. Prospects of combinatorial synthetic peptide vaccine-based immunotherapy against cancer. Seminars in immunology. 2013;25:182–190. doi: 10.1016/j.smim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nature reviews. Clinical oncology. 2014;11:24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Speiser DE, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J.Clin.Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahonen CL, et al. Combined TLR and CD40 Triggering Induces Potent CD8+ T Cell Expansion with Variable Dependence on Type I IFN. J.Exp.Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diehl L, et al. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat.Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 26.Hailemichael Y, Overwijk W. Peptide-based cancer vaccines: the making and unmaking of a T cell graveyard. OncoImmunology. 2013 doi: 10.4161/onci.24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 28.Gajewski TF, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–276. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 30.Bedognetti D, Wang E, Sertoli MR, Marincola FM. Gene-expression profiling in vaccine therapy and immunotherapy for cancer. Expert Rev Vaccines. 2010;9:555–565. doi: 10.1586/erv.10.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. CXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factors. Journal of clinical pathology. 2007;60:596–599. doi: 10.1136/jcp.2005.032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bedognetti D, et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. British journal of cancer. 2013;109:2412–2423. doi: 10.1038/bjc.2013.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng W, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16:5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 36.Buckanovich RJ, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 37.Calcinotto A, et al. Targeting TNF-alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687–2694. doi: 10.4049/jimmunol.1101877. [DOI] [PubMed] [Google Scholar]
- 38.Bellone M, Calcinotto A. Ways to Enhance Lymphocyte Trafficking into Tumors and Fitness of Tumor Infiltrating Lymphocytes. Frontiers in oncology. 2013;3:231. doi: 10.3389/fonc.2013.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat.Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]