Abstract
Rationale
Adenosine receptor stimulation and blockade has been shown to modulate a variety of cocaine related behaviors.
Objectives
These studies identify the direct effects of adenosine receptor stimulation on cocaine seeking during extinction training and the persistent effects on subsequent reinstatement to cocaine seeking.
Methods
Rats self-administered cocaine on a fixed-ratio 1 schedule in daily sessions over 3 weeks. Following 1 week withdrawal, the direct effects of adenosine receptor modulation were tested by administering the adenosine A1 receptor agonist, CPA (0.03 mg/kg and 0.1 mg/kg), the adenosine A2A agonist, CGS 21680 (0.03 mg/kg and 0.1 mg/kg), the presynaptic adenosine A2A receptor antagonist, SCH 442416 (0.3 mg/kg, 1 mg/kg, and 3 mg/kg), or vehicle prior to each of 6 daily extinction sessions. The persistent effects of adenosine receptor modulation during extinction training were subsequently tested on reinstatement to cocaine seeking induced by cues, cocaine, and the dopamine D2 receptor agonist, quinpirole.
Results
All doses of CPA and CGS 21680 impaired initial extinction responding, however only CPA treatment during extinction produced persistent impairment in subsequent cocaine- and quinpirole-induced seeking. Dissociating CPA treatment from extinction did not alter extinction responding or subsequent reinstatement. Administration of SCH 442416 had no direct effects on extinction responding, but produced dose-dependent persistent impairment of cocaine- and quinpirole-induced seeking.
Conclusions
These findings demonstrate that adenosine A1 or A2A receptor stimulation directly impair extinction responding. Interestingly, adenosine A1 receptor stimulation or presynaptic adenosine A2A receptor blockade during extinction produces lasting changes in relapse susceptibility.
Keywords: Adenosine, Dopamine, Extinction, Cocaine, Reinstatement, Self-administration
Introduction
Chronic cocaine use alters the signaling of numerous neurotransmitters throughout the brain, and these changes are thought to, in part, underlie the compulsive drug seeking that characterizes addiction and vulnerability to relapse (Koob and Volkow 2010). Relapse is modeled in rodents using the drug self-administration and reinstatement paradigm where rats are initially trained to perform an operant response to acquire a drug reinforcer. Rats then undergo extinction training resulting in newly learned contextual relationships, culminating in progressive decreases in responding in the previously drug-associated context (Bouton 2004). Following extinction training, reinstatement of lever responding can be induced by a priming injection of the previously self-administered drug, stress, or the reintroduction of a drug-associated cue (Shalev et al. 2002). This model is potentially useful in identifying potential pharmacotherapies that directly reduce reinstatement of drug seeking (Schmidt and Pierce 2010; Shalev et al 2002; Uys and LaLumiere 2008). Unfortunately, it is difficult to exactly replicate this model in human addiction where extinction training is rarely part of abstinence and “reinstatement” is difficult to predict. Therefore, it may be useful to examine the effects of a potential pharmacotherapy co-administered during behavioral interventions, such as extinction training, that could be utilized in humans to produce enduring reductions the relapse susceptibility.
Adenosine is a ubiquitous neuromodulator that influences a variety of neurotransmitters, including dopamine, GABA and glutamate, through its activity at two adenosine receptor subtypes expressed in the brain. Adenosine A1 and adenosine A2A (A1AR and A2AR, respectively) are the primary adenosine receptors in the brain with A1AR having wide expression throughout the brain (e.g. striatal areas, hippocampus, and cortex) and A2ARs having the highest expression with the striatal areas (Dixon et al. 1996). A1AR and A2AR are G protein-coupled receptors that produce inhibitory and stimulatory cellular effects, respectively (Svenningsson et al. 1999b). Postsynaptic A1AR and A2ARs in striatal areas such as the nucleus accumbens (NAc) co-localize with dopamine D1 and D2 receptors, respectively, where they functionally antagonize dopamine receptor-mediated effects (Fink et al. 1992; Svenningsson et al. 1999a). It is widely accepted that A1AR and A2AR stimulation reduces numerous cocaine-related behaviors through their ability to functionally oppose selective dopamine receptor activity (Hack and Christie 2003). For example, stimulation of both A1ARs and A2ARs either systemically or in the NAc blocks the expression of cocaine sensitization (Filip et al. 2006; Hobson et al. 2012). Stimulation of either A1ARs or A2ARs impairs the expression of cocaine conditioned place preference (Poleszak and Malec 2002). Drug discrimination studies demonstrate that antagonism of both A1AR and A2AR substitute for cocaine and produce leftward shifts in cocaine discrimination (Justinova et al. 2003). Interestingly, stimulation of both A1ARS and A2ARs also produces a leftward shift in cocaine discrimination while only stimulation of A2ARs generalizes to cocaine (Justinova et al 2003). In a self-administration paradigm, A2AR stimulation attenuates acquisition of cocaine self-administration (Knapp et al. 2001). Antagonism of A2AR, on the other hand, enhances responding for cocaine on a progressive ratio schedule of reinforcement, but had no effect on fixed ratio responding (Doyle et al. 2012; Justinova et al. 2011). Finally, stimulation of both A1ARs and A2ARs suppresses cocaine reinstatement, while blockade of A2ARs enhances cocaine seeking (Bachtell and Self 2009; Hobson et al. 2013; O’Neill et al. 2012).
In this study, we expand the understanding of the role of adenosine receptors in cocaine seeking by stimulating adenosine receptor subtypes during extinction training. We assess the direct effect of A1AR or A2AR stimulation on extinction responding and identify the persistent effects on subsequent reinstatement induced by cocaine-associated cues and drug priming. We hypothesize that targeting adenosine receptors during extinction training of the self-administration/reinstatement model will enhance the effects of extinction learning and produce lasting inhibitory effects on cocaine seeking during reinstatement testing.
Materials and Methods
Animals and housing conditions
Male Sprague–Dawley rats (Charles River, Wilmington, MA) initially weighing 250–300 g were individually housed with food and water available ad libitum. All experiments were conducted during the light period of a 12 hr light/dark cycle in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Colorado at Boulder.
Drugs
The A2AR agonist, CGS 21680, was purchased from Tocris Bioscience (Ellisville, MO). The A1AR agonist, CPA (N6-cyclopentyladenosine), presynaptic A2AR antagonist, SCH 442416(2-(2-Furanyl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]p yrimidin-5-amine), D2 receptor agonist, quinpirole ((−)-quinpirole hydrochloride), and cocaine hydrochloride were obtained from Sigma-Aldrich (St Louis, MO). All drugs, except SCH 442416, were dissolved in sterile-filtered physiological (0.9%) saline. The presynaptic A2AR antagonist, SCH 442416, was dissolved in 33% DMSO and 66% ddH20.
Cocaine Self-Administration Procedure
Self-administration procedures were performed in operant conditioning chambers (Med-Associates, St Albans, VT) equipped with two response levers and an infusion pump system. Animals were initially trained to lever press for sucrose pellets to facilitate acquisition of cocaine self-administration (O’Neill et al 2012). After lever-press training, animals were fed ad libitum for at least 24 h before catheter implantation surgery, and for the duration of the study. Surgery and cocaine self-administration procedures were similar to those described in O’Neill et al, 2012. Rats were implanted with jugular catheters under halothane anesthesia (1–2.5%). Rats were allowed 3–7 days recovery in their home cage before experimental procedures began. During this time, catheters were flushed daily with 0.1 ml heparinized saline and animals were administered (S)-(+)-ketoprofen (5 mg/kg, s.c.), a non-steroidal anti-inflammatory analgesic (Carabaza et al. 1996). After recovery, animals were allowed to self-administer intravenous cocaine (0.5 mg/kg/100 μl injection) on an FR1 reinforcement schedule in daily 4 h sessions for 5–6 days per week for 3 weeks (15 total sessions). Cocaine injections were delivered over 5 s concurrent with the illumination of a cue light above the active lever and were followed by a 15 s time-out period when the house light remained off and responding produced no consequence. Inactive lever responses produced no consequence throughout testing.
Effects of adenosine receptor stimulation or blockade on extinction responding
Following self-administration, animals remained in their home cages for 6 days of forced abstinence. On days 7–12 following self-administration, animals returned to the operant conditioning chambers for 6 daily 4 h extinction training sessions. Responses on the lever previously paired with cocaine injections during self-administration (active lever) and on the inactive lever were recorded, but had no programmed drug or cue delivery. The effect of adenosine receptor stimulation on responding on both levers was tested in animals counterbalanced for cocaine intake. Five minutes prior to the initiation of the extinction training sessions, animals were treated with vehicle, the A1AR agonist, (CPA: 0.03 mg/kg or 0.1 mg/kg, ip), the A2AR agonist, (CGS 21680: 0.03 mg/kg or 0.1 mg/kg, ip), or the A2AR antagonist (SCH 442416: 0.3 mg/kg, 1 mg/kg or 3 mg/kg, ip). Doses of the adenosine agonists/antagonist were chosen based on previous findings illustrating their effects on lever responding in animals trained to self-administer cocaine with an absence of locomotor suppression or generalized effects on operant behavior (Bachtell and Self 2009; Ferre et al. 2011; Hobson et al 2012; O’Neill et al 2012).
Effects of temporally dissociating adenosine A1 receptor stimulation from extinction training
In order to determine whether the effects of A1AR stimulation during extinction training on subsequent reinstatement are due to an enhancement of extinction learning we temporally dissociated the A1AR receptor stimulation from extinction training. This way we can assess the necessity of coupling extinction learning with A1AR stimulation in decreasing subsequent reinstatement. All cocaine self-administration and extinction procedures were the same except that animals were treated with vehicle or CPA (0.1 mg/kg, ip) 4 h after the end of each 4 h extinction training session with the first treatment administered 4 h after the first extinction training session. We chose to administer the A1AR agonist 4 h post extinction based on the pharmacokinetics of CPA (Mathot et al. 1993) and other experiments examining similar effects (Hammond et al. 2012; Mickley et al. 2012).
Reinstatement procedures
The enduring effects of the adenosine agonist/antagonist treatments administered during extinction training were also tested on reinstatement responding over the subsequent 4 days following extinction training using a repeated testing paradigm. Thus, all animals were tested for cue-, cocaine-, and D2 agonist-induced reinstatement. All reinstatement tests consisted of a 4 h reinstatement session comprised of an initial 2 h extinction phase followed by a 2 h reinstatement test phase. Cue-induced reinstatement was initiated by 5 non-contingent saline infusions paired with the illumination of the cocaine cue light (5 s) administered every 2 min over the first 10 min of the reinstatement phase. Throughout the reinstatement phase, responding at the previously active lever resulted in a 5 s cue light illumination and saline infusion. Responding at the inactive lever resulted in no cue or infusion. Cocaine-induced reinstatement was initiated by a systemic injection of cocaine (15 mg/kg, i.p.) 5 min prior to the reinstatement phase. Reinstatement was initiated by a systemic injection of the dopamine D2 receptor agonist, quinpirole (0.3 mg/kg, s.c.). Quinpirole was used to induce reinstatement because previous studies have shown that dopamine D2 receptors become sensitized following repeated cocaine administration producing behavioral cross-sensitization and robust reinstatement responding in animals extinguished from cocaine self-administration (Bachtell et al. 2005; De Vries et al. 2002; Fontana et al. 1993; Graham et al. 2007; Khroyan et al. 2000; Merritt and Bachtell 2013; Self et al. 1996). Throughout reinstatement testing, responding at both levers was recorded, but resulted in no cues or reward delivery.
Data Analyses
The numbers of animals in each experimental group ranged from 6 to 22. The effect of adenosine modulation (A1AR agonist, A2AR agonist, or the presynaptic A2AR antagonist) on active lever responding during extinction training was analyzed separately by a two-way mixed-design ANOVA with session and treatments as the factors. Likewise, active lever responding during reinstatement testing was analyzed separately by a two-way ANOVA with phase (extinction or reinstatement) and treatment during extinction training serving as the factors. Inactive lever responding was analyzed in separate mixed design ANOVAs in an identical fashion for each experiment (supplemental online material). Significant interactions were followed with simple main effects analyses (one-way ANOVA) and post hoc tests (t-test or Bonferroni’s comparisons). Statistical significance was set at p < 0.05 for all tests.
Results
Adenosine A1 and A2A receptor stimulation decreases initial extinction responding
Prior to extinction training, animals were assigned to treatments groups based on their cocaine intake over the last five self-administration sessions (Figure 1a). Figure 1b illustrates that pretreatment with either CPA or CGS 21680 significantly decreased extinction responding on the first of 6 daily 4 h extinction training sessions. We observed a significant treatment X day interaction (F20,280 = 1.70, p< 0.05) and significant main effects of treatment (F4,280 = 2.91, p< 0.05) and day (F5,280 = 38.94, p< 0.0001). Subsequent analysis of the interaction revealed that pretreatment with CPA (0.3 and 0.1 mg/kg) and CGS 21680 (0.03 and 0.1 mg/kg) significantly reduced active lever responding compared to vehicle during the first extinction training session. Post-hoc analysis revealed a significant reduction in lever responding of all treatment groups compared to vehicle (0.03 mg/kg CPA: t280 = 4.14, p< 0.001; 0.1 mg/kg CPA: t280 = 4.38, p< 0.001; 0.03 mg/kg CGS 21680: t280 = 2.92, p< 0.05; 0.1 mg/kg CGS 21680: t280 = 4.05, p< 0.001). The temporal nature of the effects of adenosine receptor stimulation on lever responding during extinction training is presented in the supplemental results (Supplemental Online Material).
Fig. 1. Stimulating adenosine A1 or adenosine A2A receptors decreases extinction responding during the first extinction session.
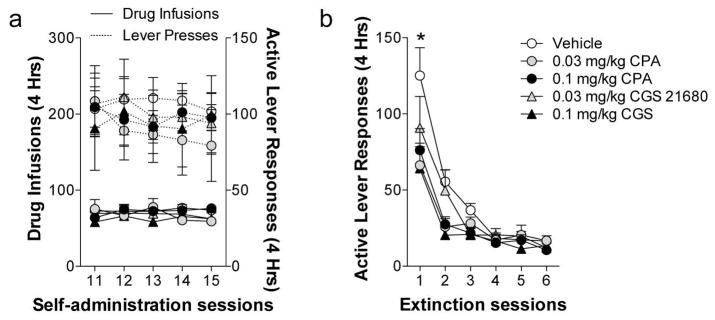
(a) Average number of cocaine infusions and active lever responses for each treatment group in each 4 h session over the last 6 days of the self-administration phase. (b) Systemic administration of the A1AR agonist, CPA, or the A2AR agonist, CGS 21860, reduced extinction responding on the first day of extinction training. * Indicates significant from vehicle pretreatment (t-test, p < 0.05)
Adenosine A1 receptor stimulation during extinction training blunts subsequent cocaine- and quinpirole-induced reinstatement
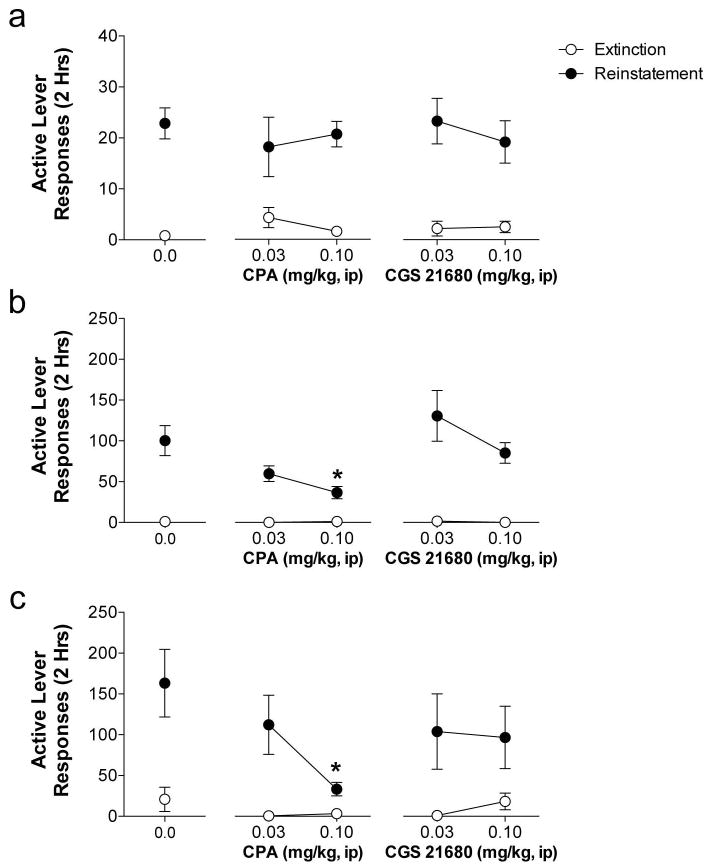
We next assessed the persistent effects of adenosine receptor stimulation during extinction training on subsequent reinstatement testing. CPA administered during extinction training dose-dependently inhibited subsequent reinstatement induced by cocaine and quinpirole, but had no effect on cue-induced reinstatement (Figure 2). CGS 21680 administered during extinction training had no effect on subsequent reinstatement. Analysis of active lever responding during cue-induced reinstatement revealed a significant main effect of reinstatement for all animals (CPA experiment: F1,39 = 72.56, p< 0.0001; CGS 21680 experiment: F1,36 = 69.59, p< 0.0001). No treatment or treatment X reinstatement interaction effects were observed indicating that regardless of treatment during extinction training, all animals reinstated similarly. Analysis of cocaine-induced reinstatement in animals treated with CPA during extinction training revealed a significant treatment X reinstatement interaction (F2,39 = 3.63, p< 0.05) and significant main effects of treatment (F1,39 = 3.62, p< 0.05) and reinstatement (F1,39 = 36.17, p< 0.0001). Subsequent analysis of the interaction revealed that rats treated with 0.1 mg/kg CPA during extinction training showed reduced cocaine-induced reinstatement when compared to vehicle treated rats (t39 = 3.76, p< 0.001). Analysis of quinpirole-induced reinstatement in animals treated with CPA during extinction training revealed a significant treatment X reinstatement interaction (F2,37 = 3.56, p< 0.05) and significant main effects of treatment (F1, 37 = 3.81, p< 0.05) and reinstatement (F1, 37 = 18.84, p < 0.0001). Subsequent analysis of the interaction found that animals treated with 0.1 mg/kg CPA during extinction training showed less D2 agonist-induced reinstatement responding compared to vehicle animals (t37 = 3.80, p< 0.001). Analysis of cocaine- and quinpirole-induced reinstatement in animals treated with CGS 21680 during extinction training revealed a significant main effect of reinstatement (Cocaine: F1,36 = 42.78, p< 0.0001; Quinpirole: F1,34 = 15.56, p< 0.001), but no main effect of treatment or treatment X reinstatement interaction for either drug.
Fig. 2. Persistent effects of stimulating of adenosine A1 receptors during extinction training on subsequent cocaine- and D2 agonist-induced cocaine seeking.
(a) Pretreatment with the A1AR agonist, CPA, or the A2AR agonist, CGS 21680, during extinction training had no effect on subsequent cue-induced reinstatement. Subsequent reinstatement induced by cocaine (b) and quinpirole (c) was significantly blunted by administration of the A1AR agonist, CPA (0.1 mg/kg), but not by the A2AR agonist, CGS 21680, administered during extinction training. * Indicates significant from vehicle pretreatment during extinction training (Bonferroni’s post-test p < 0.001).
Adenosine A1 receptor stimulation temporally dissociated from extinction training has no effect on extinction responding or subsequent reinstatement responding
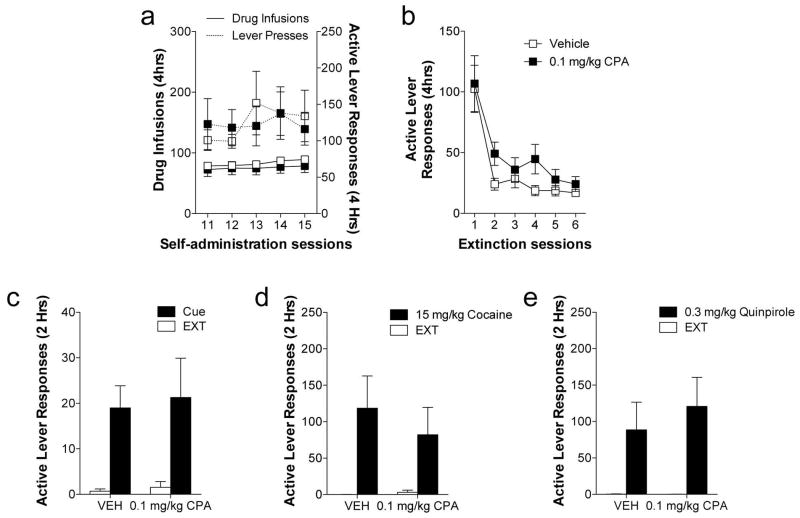
Given the persistent effects of CPA to diminish subsequent reinstatement, we next assessed whether A1AR stimulation temporally dissociated from the extinction training sessions would recapitulate these effects. Animals were separated into balanced treatments groups based on cocaine intake prior to extinction training (Figure 3). Four hours after the end of each extinction training session, animals were administered either vehicle or 0.1 mg/kg CPA, the dose effective in reducing subsequent reinstatement (see above). Analysis of extinction responding at the active lever revealed a significant main effect of session (F5, 60 = 24.49, p< 0.0001), but no effect of treatment or the session X treatment interaction. Following extinction training, animals were tested for cue-, cocaine-, and quinpirole-induced drug seeking (Figure 3). In all reinstatement tests, there was a significant main effect of reinstatement (Cue: F1, 12 = 14.25, p< 0.01; Cocaine: F1, 12 = 11.46, p< 0.01; Quinpirole: F1, 12 = 14.27, p< 0.01), but no significant treatment or treatment X reinstatement interaction suggesting that dissociating adenosine A1 receptor stimulation from the extinction training sessions is not sufficient to produce these persistent effects on reinstatement.
Fig. 3. Dissociating adenosine A1 receptor stimulation from extinction training has no effect on extinction responding or subsequent reinstatement.
(a) Average number of cocaine infusions and active lever responses in each 4 h session over the last 5 days of the self-administration phase. (b) Systemic treatment with the A1AR agonist, CPA (0.1 mg/kg, ip), 4 h after each extinction session has no effect on extinction responding. A1AR stimulation temporally dissociated from extinction training has no effect on (c) cue-, (d) cocaine or (e) D2 agonist-induced reinstatement.
Adenosine A2A receptor blockade has no effect on extinction responding
Prior to extinction training, animals were assigned to treatments groups based on their cocaine intake over the last five self-administration sessions (Figure 4). Lever responding was then extinguished in 6 daily sessions where a pretreatment of vehicle or the A2AR antagonist, SCH 442416 (0.3, 1, or 3 mg/kg) was administered prior to each extinction training session (Figure 4). These doses of SCH 442416 were chosen based on previous work illustrating that low doses (0.3 and 1.0 mg/kg) primarily inhibit presynaptic A2AR receptors decreasing both locomotor activity and evoked glutamate release, while 3.0 mg/kg inhibit postsynaptic A2AR receptors to increase locomotor activity (Orru et al. 2011a; Orru et al. 2011b). Analysis of extinction responding over the entire 4 h session revealed a significant main effect of session (4 h: F5, 135 = 79.04, p < 0.0001; 2 h: F5, 135 = 85.74, p < 0.0001), but no main effect of treatment or treatment X session interaction.
Fig. 4. Blocking adenosine A2A receptors during extinction has no effects on extinction responding.
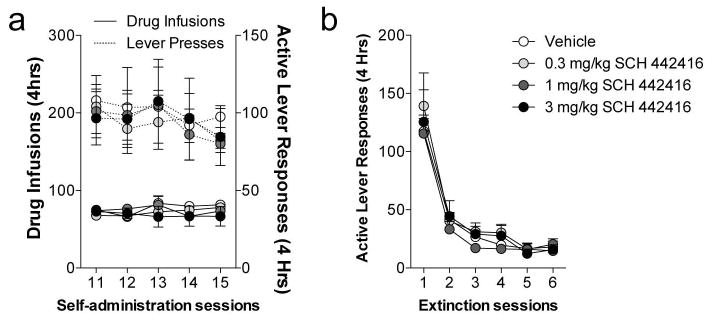
(a) Average number of cocaine infusions and active lever responses in each 4 h session over the last 6 days of the self-administration phase. (b) A2AR antagonism by SCH 442416 (0.3 mg/kg, 1 mg/kg, or 3 mg/kg, i.p.), has no effect on extinction responding when administered immediately prior to the beginning of each 4 h extinction session.
Presynaptic A2A receptor blockade during extinction training decreases subsequent cocaine- and quinpirole-induced reinstatement
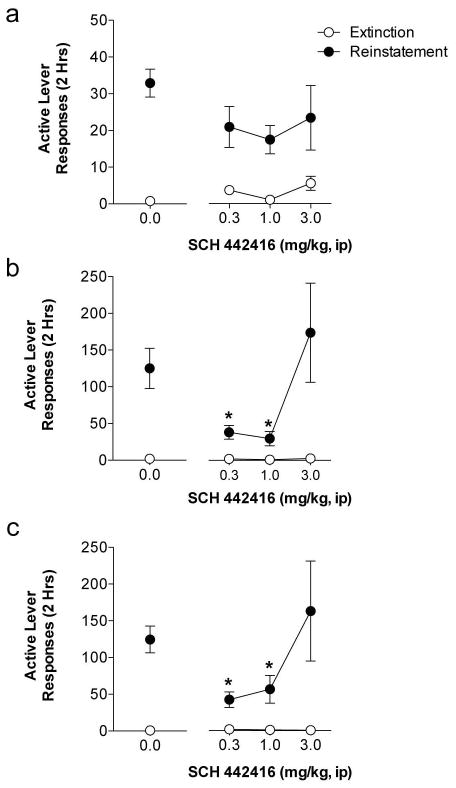
SCH 442416 administered during extinction training dose-dependently inhibited subsequent reinstatement induced by cocaine and quinpirole, but had no effect on cue-induced reinstatement (Figure 5). Analysis of active lever responding during cue-induced reinstatement revealed a significant main effect of reinstatement (F1,26 = 58.12, p< 0.0001), but no main effect of treatment or treatment X reinstatement interaction. Analysis of active lever responding during cocaine-induced reinstatement revealed a significant treatment X reinstatement interaction (F3,27 = 4.02, p < 0.05) and significant main effects of treatment (F3,27 = 3.98, p < 0.05) and reinstatement (F1,27 = 29.42, p < 0.0001). Post-hoc analyses demonstrate that pretreatment with either 0.3 or 1.0 mg/kg SCH 442416 during extinction training significantly reduced cocaine-induced reinstatement compared to vehicle and 3 mg/kg SCH 442416 (Vehicle vs 0.3 SCH 442416: t27 = 2.40, p<0.05, Vehicle vs. 1.0 SCH 442416: t27 = 2.79, p<0.05). Analysis of active lever responding during quinpirole-induced reinstatement revealed a significant treatment X reinstatement interaction (F3,26 = 3.13, p < 0.05) and significant main effects of treatment (F3,26 = 3.05, p < 0.05) and reinstatement (F1,26 = 36.70, p < 0.0001) were observed. Post-hoc analyses demonstrate that pretreatment with either 0.3 or 1.0 mg/kg SCH 442416 during extinction training significantly reduced cocaine-induced reinstatement compared to vehicle and 3 mg/kg SCH 442416 (Vehicle vs. 0.3 SCH 442416: t27 = 2.72, p<0.05, and Vehicle vs. 1.0 SCH 442416:t27 = 2.34, p<0.05).
Fig. 5. Blocking presynaptic, but not postsynaptic, adenosine A2A receptors during extinction produces enduring reductions on reinstatement of cocaine seeking.
(a) Blocking A2ARs during extinction training has no effect on subsequent cue-induced reinstatement. (b) Pretreatment of SCH 442416 during extinction training impaired subsequent reinstatement of cocaine-induced seeking when administered at doses effective at blocking presynaptic A2ARs (0.3 or 1 mg/kg). (c) Similarly, antagonism of presynaptic A2ARs during extinction also impaired subsequent cocaine seeking induced by quinpirole. * Indicates significant from vehicle pretreatment (t-test, p<0.05)
Discussion
Previous studies show that stimulation of adenosine receptors attenuates cocaine seeking induced by pharmacological stimuli (Bachtell and Self 2009; Hobson et al 2013; O’Neill et al 2012). Here, we extend those findings by examining the effect of adenosine receptor stimulation or blockade on extinction responding and subsequent reinstatement. Our findings reveal that stimulation of A1ARs or A2ARs inhibits initial extinction responding paralleling previous work (Bachtell and Self 2009; Hobson et al 2013; Knapp et al 2001; O’Neill et al 2012). Interestingly, we find that only A1AR stimulation produced lasting reductions in cocaine- and quinpirole-induced cocaine seeking. In order to further elucidate the role of adenosine receptors in cocaine seeking we examined the effects of antagonizing presynaptic and postsynaptic A2ARs during extinction training. Neither presynaptic nor postsynaptic A2AR antagonism had a direct effect on extinction responding. Surprisingly, antagonism of presynaptic A2ARs, but not postsynaptic A2ARs, produced persistent decreases in cocaine- and quinpirole-induced cocaine seeking. Together, these results suggest that A1AR receptor stimulation or A2AR receptor antagonism during extinction training produces lasting effects on reinstatement that may be determined by their synaptic localization.
Stimulation of both A1ARs and A2ARs inhibited initial extinction responding, while antagonism of A2ARs had no direct effects on extinction responding. It is unlikely that the stimulation of A1ARs and A2ARs facilitated extinction learning, per se, since these effects occurred nearly immediately in the first extinction training session likely before consolidation of extinction learning occurred. Thus, we suspect that the effects of A1AR and A2AR agonists on extinction responding are a reflection of impaired expression of cocaine seeking upon A1AR or A2AR stimulation as demonstrated in a place conditioning paradigm (Poleszak and Malec 2002), cocaine self-administration (Knapp et al 2001), and non-reinforced lever responding during reinstatement of cocaine seeking in extinguished animals (Bachtell and Self 2009; Hobson et al 2013; O’Neill et al 2012). We suspect that the direct effects of adenosine receptor stimulation on cocaine seeking during extinction training are not associated with more generalized suppression of locomotion that would interfere with lever responding since similar manipulations do not impair sucrose seeking (Bachtell and Self 2009; Hobson et al 2013; O’Neill et al 2012). Together these findings suggest that stimulation of both A1AR and A2AR may reduce the motivation for cocaine seeking during cocaine self-administration, extinction training and reinstatement sessions.
The most interesting finding of these studies was the persistent effect of A1AR stimulation or presynaptic A2AR antagonism during extinction training on subsequent reinstatement of cocaine seeking. The persistent effect of A1AR stimulation required that the administration of the agonist, CPA, be coupled with each extinction training session. Thus, temporally dissociating CPA administration from the extinction training session did not recapitulate the reduction in subsequent reinstatement, suggesting that A1AR stimulation may alter extinction-induced processes occurring during the extinction training sessions. Initial extinction training corresponds with increased extracellular glutamate, and to a lesser extent dopamine, in the NAc when lever pressing is most robust (Suto et al. 2010). However, no increase in either neurotransmitter is observed following consolidation of extinction learning when lever pressing has subsided (Suto et al 2010; Wydra et al. 2013). It is suspected that extinction-induced glutamate release in the NAc results from prefrontal cortex activation since cocaine extinction learning strengthens the connectivity between the infralimbic cortex and the NAc to produce an active inhibitory system that suppresses cocaine seeking (LaLumiere et al. 2010; Peters et al. 2008). Consolidated extinction learning is also associated with several neurobiological changes within the mesocorticolimbic system such as altered expression of tyrosine hydroxylase, glutamate receptors and scaffolding proteins at glutamate synapses (Knackstedt et al. 2010b; Schmidt et al. 2001; Sutton et al. 2003). Both A1ARs and A2ARs are highly localized to brain areas within the mesocorticolimbic system such as the NAc where they may influence extinction-induced neurobiological effects to directly influence extinction responding and subsequent reinstatement.
We hypothesize that A1AR and A2ARs situated on presynaptic terminals in the striatum may contribute to the lasting effects on cocaine seeking. Presynaptic A1ARs and A2ARs are co-localized on cortical glutamate terminals that synapse onto direct pathway neurons where they tightly regulate glutamate release (Ciruela et al. 2006; Corsi et al. 1997; Orru et al 2011b; Quarta et al. 2004; Quiroz et al. 2009). Here, low extracellular adenosine levels result in preferential stimulation of A1ARs inhibiting glutamate release (Fredholm et al. 2001). However, when adenosine concentrations are elevated as would be expected during extinction training or reinstatement testing when glutamate is released, A2AR activation occurs to facilitate glutamate release (Ciruela et al 2006; Corsi et al 1997; Orru et al 2011b; Quarta et al 2004; Quiroz et al 2009). Thus, stimulating A1ARs or antagonizing A2ARs on presynaptic glutamate terminals may impede glutamate transmission and restore glutamate deficiencies.
Several lines of evidence suggest that stimulation of presynaptic A1ARs may be responsible for the lasting effects on reinstatement. First, withdrawal from chronic cocaine impairs adenosine reuptake producing diminished basal adenosine levels in the NAc thereby reducing the ability of A1AR to inhibit glutamate release (Manzoni et al. 1998). This inability of A1AR to sufficiently regulate glutamate release, along with withdrawal-induced increases in A2AR (Frankowska et al. 2013; Marcellino et al. 2007), may in part underlie the enhanced glutamate release observed during cocaine-primed reinstatement (McFarland et al. 2003). Second, administration of an A1AR agonist reverses cocaine-induced impairments in glutamate regulation (Manzoni et al 1998). Thus, combining presynaptic A1AR stimulation during extinction training may restore the ability of A1AR to effectively inhibit glutamate release during subsequent reinstatement testing. Third, we observed a similar lasting effect on reinstatement with administration of a low, but not a high, dose of the A2AR antagonist, SCH 442416. Interestingly, low doses of SCH 442416 impair presynaptic A2AR functions and produce inhibitory effects on glutamate transmission. Thus, systemic administration of low doses SCH 442416 reduces cortically-evoked glutamate release in the striatum and reduces excitatory postsynaptic potentials at glutamate synapses onto direct pathway neurons (Orru et al 2011a; Orru et al 2011b; Quiroz et al 2009). These effects are presumed to facilitate the complementary A1ARs co-localized within the same glutamate terminals. Given the specificity A1AR stimulation had on pharmacologically-induced cocaine seeking, we suspect that A1AR facilitation may exist exclusively at corticoaccumbens glutamate synapses, but not other glutamatergic synapses such as the corticoamygdala synapses that are associated with cue induced reinstatement following extinction training (Grimm and See 2000; McLaughlin and See 2003). Together, these findings suggest that presynaptic A1AR stimulation of cortical terminals in the NAc may produce lasting effects on drug-primed cocaine seeking when concurrent with extinction training.
Alternatively, adenosine receptor expression on astrocytes may influence amino acid reuptake to restore cocaine-induced alterations in basal glutamate and GABA in the NAc (Swanson et al. 1997; Volterra and Meldolesi 2005). Cocaine self-administration reduces the expression of glial glutamate transporter (GLT-1) that contributes to low basal glutamate in the NAc (Knackstedt et al. 2010a). Restoration of GLT-1 deficits during withdrawal corresponds with a persistent attenuation of cocaine- and cue-primed reinstatement (Knackstedt et al 2010a; Reissner et al. 2013). Interestingly, increasing A1AR transmission in the accumbens results in increased expression of GLT-1 mRNA (Wu et al. 2011). Thus, A1AR stimulation may restore cocaine-induced deficits in GLT-1 and basal glutamate to reduce subsequent cocaine seeking, although it is not clear why it would be necessary for A1AR agonists to be administered during extinction training to reveal these lasting effects.
Withdrawal from chronic cocaine administration is also associated with increases in basal GABA in the NAc and ventral pallidum (Wydra et al 2013; Xi et al. 2003). The mechanism underlying this basal GABA alteration is not clear although it may relate to desensitization of GABAB receptors in the striatum (Frankowska et al. 2008; Xi et al 2003). Somewhat paradoxically, artificially increasing GABA transmission through GABA reuptake inhibitors or GABAB receptor stimulation inhibits cocaine-reinforced responding and reinstatement of cocaine seeking (Barrett et al. 2005; Di Ciano and Everitt 2003; Filip et al. 2007; Shoaib et al. 1998; Weerts et al. 2007). Interestingly, stimulation of astrocytic A1ARs also elevates extracellular GABA by decreasing GABA transport into astrocytes (Cristovao-Ferreira et al. 2013; Kirk and Richardson 1994). Thus, astrocytic A1AR stimulation may exacerbate basal GABA elevations that contribute to reductions in drug seeking. Future studies should investigate the role of adenosine receptor modulation on astrocytes to regulate both glutamate and GABA transmission.
Together, these findings build upon evidence demonstrating that adenosine receptor stimulation negatively regulates cocaine seeking in a variety of situations. These findings are novel because they illustrate lasting effects of a pharmacological treatment administered during extinction training on subsequent cocaine seeking. Findings such as these provide the foundation for new treatment strategies aimed at human cocaine addiction, where it is often not feasible to treat an acute relapse episode.
Supplementary Material
Acknowledgments
This work was supported by the University of Colorado Innovative Seed Grant Program and a United States Public Health Service grants (DA029240 & DA033358).
References
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Effects of adenosine A(2A) receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2009;206:469–478. doi: 10.1007/s00213-009-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine- and food-maintained responding and cocaine discrimination in rats. The Journal of pharmacology and experimental therapeutics. 2005;315:858–871. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Carabaza A, Cabre F, Rotllan E, Gomez M, Gutierrez M, Garcia ML, et al. Stereoselective inhibition of inducible cyclooxygenase by chiral nonsteroidal antiinflammatory drugs. J Clin Pharmacol. 1996;36:505–512. doi: 10.1002/j.1552-4604.1996.tb05040.x. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi C, Pazzagli M, Bianchi L, Della Corte L, Pepeu G, Pedata F. In vivo amino acid release from the striatum of aging rats: adenosine modulation. Neurobiol Aging. 1997;18:243–250. doi: 10.1016/s0197-4580(97)00002-x. [DOI] [PubMed] [Google Scholar]
- Cristovao-Ferreira S, Navarro G, Brugarolas M, Perez-Capote K, Vaz SH, Fattorini G, et al. A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes. Purinergic Signal. 2013;9:433–449. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. The GABA(B) receptor agonist baclofen attenuates cocaine- and heroin-seeking behavior by rats. Neuropsychopharmacology. 2003;28:510–518. doi: 10.1038/sj.npp.1300088. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Breslin FJ, Rieger JM, Beauglehole A, Lynch WJ. Time and sex-dependent effects of an adenosine A2A/A1 receptor antagonist on motivation to self-administer cocaine in rats. Pharmacology, biochemistry, and behavior. 2012;102:257–263. doi: 10.1016/j.pbb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Orru M, Guitart X, Navarro G, Cortes A, et al. Adenosine A(2A) Receptors and A(2A) Receptor Heteromers as Key Players in Striatal Function. Front Neuroanat. 2011;5:36. doi: 10.3389/fnana.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Przegalinski E, Muller CE, Agnati L, et al. Involvement of adenosine A2A and dopamine receptors in the locomotor and sensitizing effects of cocaine. Brain Res. 2006;1077:67–80. doi: 10.1016/j.brainres.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E, Vetulani J. Diverse effects of GABA-mimetic drugs on cocaine-evoked self-administration and discriminative stimulus effects in rats. Psychopharmacology. 2007;192:17–26. doi: 10.1007/s00213-006-0694-7. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Fontana D, Post RM, Weiss SR, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor behavior. Behav Pharmacol. 1993;4:375–387. [PubMed] [Google Scholar]
- Frankowska M, Wydra K, Faron-Gorecka A, Zaniewska M, Kusmider M, Dziedzicka-Wasylewska M, et al. Neuroadaptive changes in the rat brain GABA(B) receptors after withdrawal from cocaine self-administration. European journal of pharmacology. 2008;599:58–64. doi: 10.1016/j.ejphar.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Frankowska M, Marcellino D, Adamczyk P, Filip M, Fuxe K. Effects of cocaine self-administration and extinction on D2 -like and A2A receptor recognition and D2 -like/Gi protein coupling in rat striatum. Addiction biology. 2013;18:455–466. doi: 10.1111/j.1369-1600.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochemical pharmacology. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 2007;191:719–730. doi: 10.1007/s00213-006-0473-5. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Hack SP, Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit Rev Neurobiol. 2003;15:235–274. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- Hammond S, Seymour CM, Burger A, Wagner JJ. D-serine facilitates the effectiveness of extinction to reduce drug-primed reinstatement of cocaine-induced conditioned place preference. Neuropharmacology. 2012;64:464–471. doi: 10.1016/j.neuropharm.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson BD, Merritt KE, Bachtell RK. Stimulation of adenosine receptors in the nucleus accumbens reverses the expression of cocaine sensitization and cross-sensitization to dopamine D2 receptors in rats. Neuropharmacology. 2012;63:1172–1181. doi: 10.1016/j.neuropharm.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson BD, O’Neill CE, Levis SC, Monteggia LM, Neve RL, Self DW, et al. Adenosine A and Dopamine D Receptor Regulation of AMPA Receptor Phosphorylation and Cocaine-Seeking Behavior. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Segal PN, Antoniou K, Solinas M, Pappas LA, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307:977–986. doi: 10.1124/jpet.103.056762. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addiction biology. 2011;16:405–415. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: Effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Kirk IP, Richardson PJ. Adenosine A2a receptor-mediated modulation of striatal [3H]GABA and [3H]acetylcholine release. J Neurochem. 1994;62:960–966. doi: 10.1046/j.1471-4159.1994.62030960.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010a;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. Journal of Neuroscience. 2010b;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Foye MM, Cottam N, Ciraulo DA, Kornetsky C. Adenosine agonists CGS 21680 and NECA inhibit the initiation of cocaine self-administration. Pharmacol Biochem Behav. 2001;68:797–803. doi: 10.1016/s0091-3057(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning & memory. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni O, Pujalte D, Williams J, Bockaert J. Decreased presynaptic sensitivity to adenosine after cocaine withdrawal. J Neurosci. 1998;18:7996–8002. doi: 10.1523/JNEUROSCI.18-19-07996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D, Roberts DC, Navarro G, Filip M, Agnati L, Lluis C, et al. Increase in A2A receptors in the nucleus accumbens after extended cocaine self-administration and its disappearance after cocaine withdrawal. Brain research. 2007;1143:208–220. doi: 10.1016/j.brainres.2007.01.079. [DOI] [PubMed] [Google Scholar]
- Mathot RA, Appel S, van Schaick EA, Soudijn W, APIJ, Danhof M. High-performance liquid chromatography of the adenosine A1 agonist N6-cyclopentyladenosine and the A1 antagonist 8-cyclopentyltheophylline and its application in a pharmacokinetic study in rats. Journal of chromatography. 1993;620:113–120. doi: 10.1016/0378-4347(93)80058-c. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Merritt KE, Bachtell RK. Initial d2 dopamine receptor sensitivity predicts cocaine sensitivity and reward in rats. PLoS ONE. 2013;8:e78258. doi: 10.1371/journal.pone.0078258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Remus JL, Ramos L, Wilson GN, Biesan OR, Ketchesin KD. Acute, but not chronic, exposure to d-cycloserine facilitates extinction and modulates spontaneous recovery of a conditioned taste aversion. Physiology & behavior. 2012;105:417–427. doi: 10.1016/j.physbeh.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill CE, LeTendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37:1245–1256. doi: 10.1038/npp.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Bakesova J, Brugarolas M, Quiroz C, Beaumont V, Goldberg SR, et al. Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS ONE. 2011a;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orru M, Quiroz C, Guitart X, Ferre S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 2011b;61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. Journal of Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszak E, Malec D. Adenosine receptor ligands and cocaine in conditioned place preference (CPP) test in rats. Pol J Pharmacol. 2002;54:119–126. [PubMed] [Google Scholar]
- Quarta D, Ferre S, Solinas M, You ZB, Hockemeyer J, Popoli P, et al. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. Journal of neurochemistry. 2004;88:1151–1158. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. The Scientific World Journal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW. Chronic Administration of the Methylxanthine Propentofylline Impairs Reinstatement to Cocaine by a GLT-1-Dependent Mechanism. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21:RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Swanner LS, Beyer CE, Goldberg SR, Schindler CW. The GABAB agonist baclofen modifies cocaine self-administration in rats. Behavioural pharmacology. 1998;9:195–206. [PubMed] [Google Scholar]
- Suto N, Ecke LE, You ZB, Wise RA. Extracellular fluctuations of dopamine and glutamate in the nucleus accumbens core and shell associated with lever-pressing during cocaine self-administration, extinction, and yoked cocaine administration. Psychopharmacology. 2010;211:267–275. doi: 10.1007/s00213-010-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Fourreau L, Bloch B, Fredholm BB, Gonon F, Le Moine C. Opposite tonic modulation of dopamine and adenosine on c-fos gene expression in striatopallidal neurons. Neuroscience. 1999a;89:827–837. doi: 10.1016/s0306-4522(98)00403-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999b;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, et al. Neuronal regulation of glutamate transporter subtype expression in astrocytes. Journal of Neuroscience. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS & neurological disorders drug targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABA B receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug and alcohol dependence. 2007;89:206–213. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lee MR, Kim T, Johng S, Rohrback S, Kang N, et al. Regulation of ethanol-sensitive EAAT2 expression through adenosine A1 receptor in astrocytes. Biochemical and biophysical research communications. 2011;406:47–52. doi: 10.1016/j.bbrc.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K, et al. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addiction biology. 2013;18:307–324. doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. Journal of Neuroscience. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.