Abstract
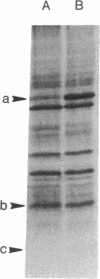
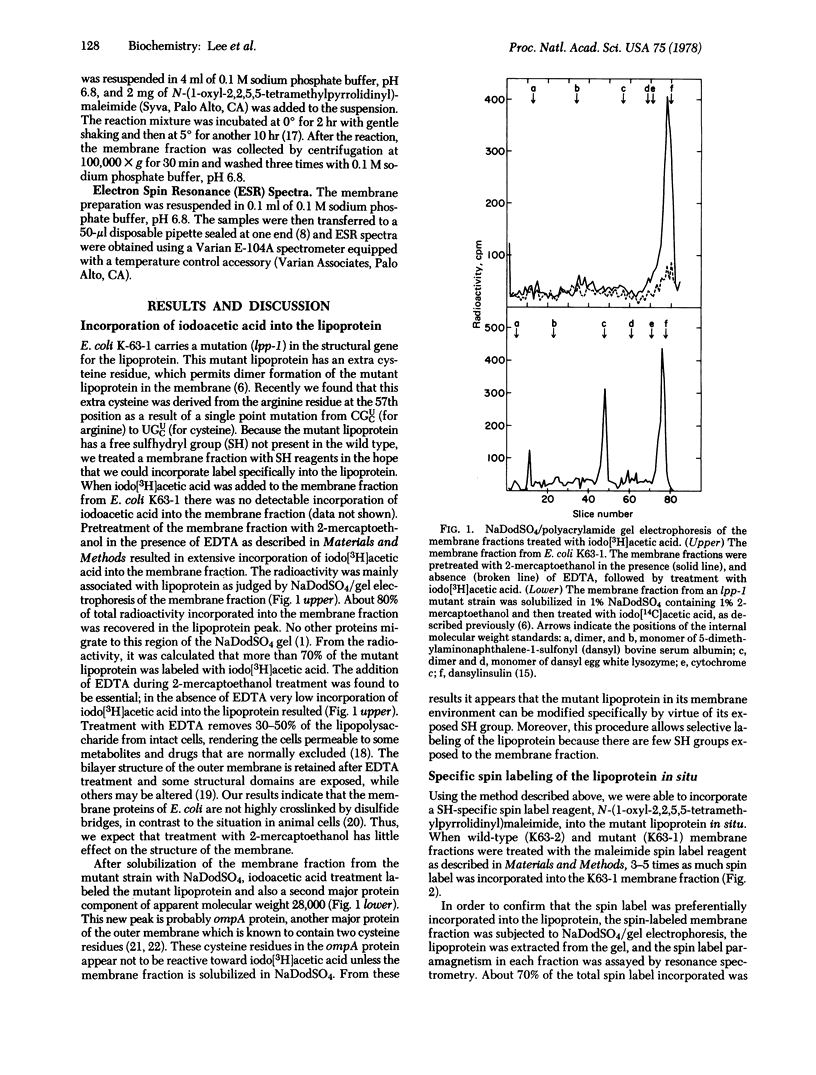
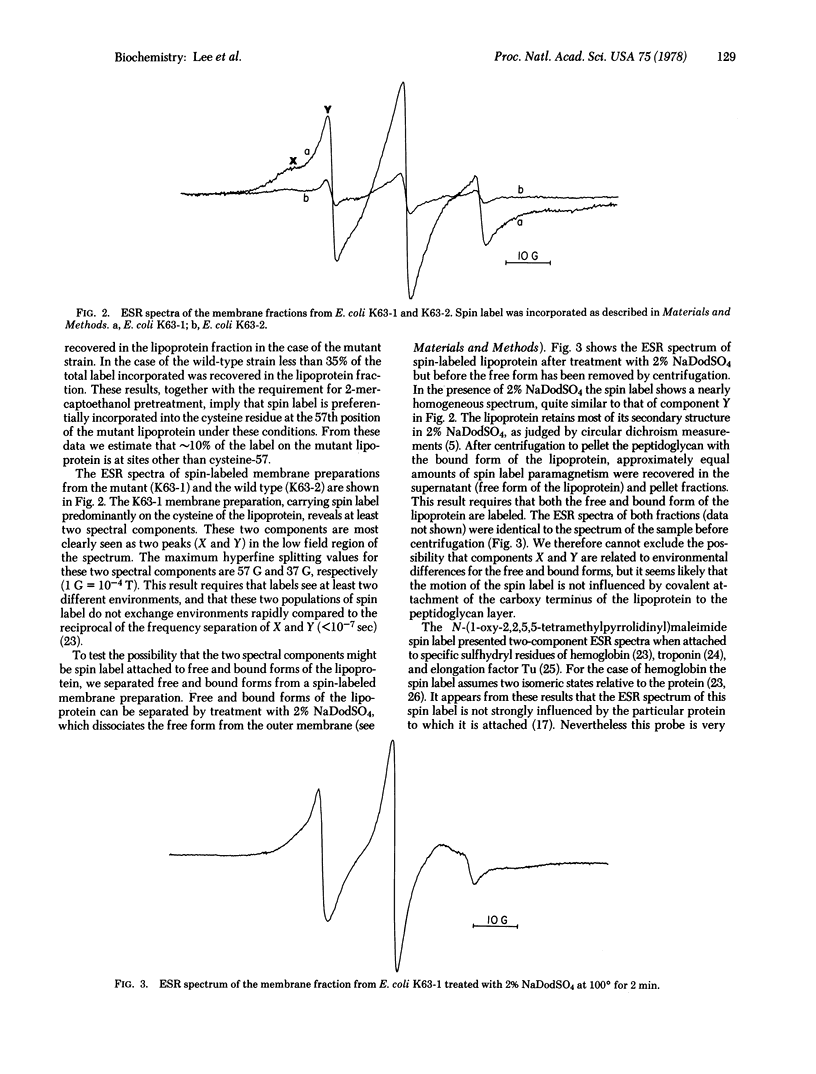
A method was developed to attach a spin label to a specific site on the structural lipoprotein of the Escherichia coli outer membrane in situ. This method takes advantage of the fact that the outer membrane of wild-type E. coli contains few residues reactive towards sulfhydryl reagents. A mutant E. coli strain has been isolated [Suzuki, H., Nishimura, Y., Iketani, H., Campisi, J., Hirashima, A., Inouye, M. & Hirota, Y. (1976) J. Bacteriol. 127, 1494-1501] in which the second position from the carboxy terminus of the lipoprotein is changed from arginine into a cysteine residue. The membrane fraction of this mutant was treated with N-(1-oxyl-2,2,5,5-tetramethylpyrrolidinyl)maleimide in the presence of EDTA and 2-mercaptoethanol. Spin label was found to be preferentially incorporated into the lipoprotein. The spectrum of the spin-labeled membrane shows two components, both arising from spin label at the same site near the carboxy terminus. The strongly immobilized component has a maximum hyperfine splitting value of 53 G, and the weakly immobilized component, 37 G. A fraction of the lipoprotein is covalently bound to the peptidoglycan layer through its carboxy-terminal lysine; the spectrum of the isolated bound form of the lipoprotein was identical to that of the free form. When the matrix protein, the other major outer membrane protein, was removed by mutation, the spectrum of the lipoprotein was altered, suggesting that these two proteins are closely associated.
Keywords: electron spin resonance, peptidoglycan, lipoprotein mutant
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y., Maeda T., Onishi S. I. Conformational transition in polypeptide elongation factor Tu as revealed by electron spin resonance. J Biol Chem. 1974 May 25;249(10):3311–3313. [PubMed] [Google Scholar]
- Bayer M. E., Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Brisson A. D., Scandella C. J., Bienvenüe A., Devaux P. F., Cohen J. B., Changeux J. P. Interaction of a spin-labeled long chain acylcholine with the cholinergic receptor protein in its membrane environment. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1087–1091. doi: 10.1073/pnas.72.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Krämer C., Henning U. Diploidy for a structural gene specifying a major protein of the outer cell envelope membrane from Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):834–841. doi: 10.1128/jb.128.3.834-841.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini M., Inouye S., Inouye M. Ultrastructure of paracrystals of a lipoprotein from the outer membrane of Escherichia coli. J Bacteriol. 1976 Jul;127(1):564–571. doi: 10.1128/jb.127.1.564-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Onishi S., Abe S., Maruyama K. A spin-label study on calcium-induced conformational changes of troponin components. J Biochem. 1974 Jan;75(1):211–213. doi: 10.1093/oxfordjournals.jbchem.a130379. [DOI] [PubMed] [Google Scholar]
- Gaffney B. J., McNamee C. M. Spin-label measurements in membranes. With appendix: a use of computers in EPR spectroscopy. Methods Enzymol. 1974;32:161–198. [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., McConnell H. M. A nitroxide-maleimide spin label. Proc Natl Acad Sci U S A. 1966 Jan;55(1):8–11. doi: 10.1073/pnas.55.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Inouye S., Lee N., Inouye M., Wu H. C., Suzuki H., Nishimura Y., Iketani H., Hirota Y. Amino acid replacement in a mutant lipoprotein of the Escherichia coli outer membrane. J Bacteriol. 1977 Oct;132(1):308–313. doi: 10.1128/jb.132.1.308-313.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Cheng E., Inouye M. Optical properties of an outer membrane lipoprotein from Escherichia coli. Biochim Biophys Acta. 1977 Mar 17;465(3):650–656. doi: 10.1016/0005-2736(77)90280-2. [DOI] [PubMed] [Google Scholar]
- Lee N., Inouye M., Lauterbur P. C. 19F- and 13C-NMR studies of a specifically labelled lipoprotein in the Eschericia coli membrane. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1211–1218. doi: 10.1016/0006-291x(77)91422-x. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Deal W., Ogata R. T. Spin-labeled hemoglobin derivatives in solution, polycrystalline suspensions, and single crystals. Biochemistry. 1969 Jun;8(6):2580–2585. doi: 10.1021/bi00834a048. [DOI] [PubMed] [Google Scholar]
- Moffat J. K. Spin-labelled haemoglobins: a structural interpretation of electron paramagnetic resonance spectra based on X-ray analysis. J Mol Biol. 1971 Jan 28;55(2):135–146. doi: 10.1016/0022-2836(71)90187-2. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Guthmann C., Matricon J., Bienvenue A., Devaux P. F. Study of the transverse diffusion of spin labeled phospholipids in biological membranes. I. Human red bloods cells. Biochim Biophys Acta. 1976 Mar 19;426(3):357–371. doi: 10.1016/0005-2736(76)90382-5. [DOI] [PubMed] [Google Scholar]
- Schmitges C. J., Henning U. The major proteins of the Escherichia coli outer cell-envelope membrane. Heterogeneity of protein I. Eur J Biochem. 1976 Mar 16;63(1):47–52. doi: 10.1111/j.1432-1033.1976.tb10205.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Iketani H., Campisi J., Hirashima A. Novel mutation that causes a structural change in a lipoprotein in the outer membrane of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1494–1501. doi: 10.1128/jb.127.3.1494-1501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]