Abstract
Mortality is highest in the first months of maintenance hemodialysis (HD). In many Western countries, patients who transition to kidney replacement therapy usually begin thrice-weekly HD regardless of their level of residual kidney function (RKF). RKF is a major predictor of survival. RKF may decline more rapidly with more thrice-weekly HD treatments, is associated with a reduced need for dialytic solute clearance, and is an important factor in the prescription of peritoneal dialysis. In this paper we review the concept of incremental HD, in which weekly dialysis dose, in particular HD treatment frequency, is based on a variety of clinical factors such as RKF (including urine output >0.5 L/day), volume status, cardiovascular symptoms, body size, potassium and phosphorus levels, nutritional status, hemoglobin, comorbid conditions, hospitalizations, and health related quality of life. These ten clinical criteria may identify which patients might benefit from beginning maintenance HD twice-weekly. Periodic monitoring of these criteria will determine the timing for increasing dialysis dose and frequency. We recognize that twice-weekly HD represents a major paradigm shift for many clinicians and jurisdictions. Therefore, we propose conducting randomized controlled trials of twice-weekly vs. thrice-weekly HD to assess the potential of twice-weekly HD to improve survival and health related quality of life while simultaneously reducing costs, protecting fragile vascular accesses, and optimizing resource use. Such incremental and individualized HD therapy may prove to be the most appropriate approach for transitioning to dialytic therapy.
Keywords: Transition of care, residual kidney function, incremental dialysis, incremental hemodialysis, end-stage renal disease, kidney replacement therapy, renal replacement therapy
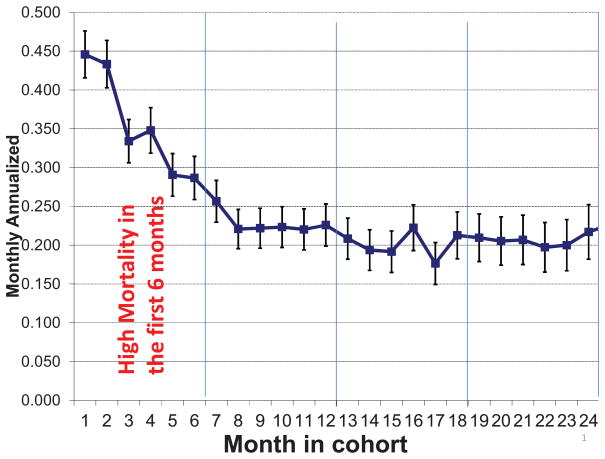
Currently, there are over 400,000 people who receive maintenance hemodialysis (HD) in the United States. The prevalence of HD patients is increasing, with approximately 100,000 new starts and 80,000 deaths each year.1 While HD is life-sustaining, maintenance dialysis patients have marked decrements in health-related quality of life (HRQOL) and a seven-fold increase in mortality compared to the general population.1 Mortality during the first year of conventional thrice-weekly HD is unacceptably high (see Figure 1) even though 45% of patients have an eGFR ≥10 ml/min/1.73 m2 when they initiate dialysis therapy.2 Despite multiple studies, the cause of the exceedingly high mortality in the first year of transition to conventional HD treatment remains unclear.3, 4 Whereas there are many potential risk factors for this exceptional death rate in the first months of dialysis therapy,5, 6 the most obvious change is the abrupt transition from non-dialysis dependent CKD to thrice-weekly HD, although no study has examined whether a gradual and incremental transition to HD therapy with less HD frequency would have improved the dismal first year survival rate. The observed high mortality in the first year of HD may also be the result of confounding by indication as a result of clinical deterioration that may prompt the physician to initiate thrice-weekly HD in very sick patients or may be caused by the initiation of HD, including a truly causal effect of thrice-weekly HD treatment. Therefore, the relationship may be complex and similar to the association of high doses of erythropoiesis stimulating agents (ESA) and thromboembolic events and mortality, which encompasses both confounding by indication (as sicker patients with ESA hyporesponsiveness usually need higher ESA doses) and true harm of ESAs, including harm due to relative thrombocytosis.7
Figure 1.
High mortality rate during the first 6 months of hemodialysis therapy. Reproduced Lukowsky et al4 with permission from S. Karger AG, Basel.
Residual Kidney Function
Progressive loss of RKF in incident dialysis patients is associated with increased death risk over time.8–10 Nevertheless, mortality is the highest in the first several months of dialysis therapy4 when most patients starting kidney replacement therapy have their highest RKF. The loss of RKF is faster in patients on HD than those receiving peritoneal dialysis (PD).10, 11 HD may cause episodic ischemic damage to the kidneys, leading to repetitive bouts of ischemic events similar to acute kidney injury (AKI).8, 9 Notwithstanding the ongoing debate about the causal link between AKI and subsequent CKD12 and that the use of the term “AKI” may not be appropriate for the mechanism of the faster loss of RKF in HD patients, the cumulative effect of repetitive ischemic events may accelerate the decline in RKF.8, 9 Consistent with this hypothesis, the recent Frequent Hemodialysis Network (FHN) study showed that frequent nocturnal HD may accelerate loss of RKF.13 Intense dialysis therapy may also remove the stimulus for the hyperfunctioning of the remaining nephrons, which would be consistent with Bricker’s “intact nephron hypothesis”.14
Higher RKF in dialysis patients has been associated with improved HRQOL, a decrease in pro-inflammatory mediators, and improved survival.15 The rate of loss of RKF over time may correlate with mortality risk.16 Higher RKF may be related to greater survival through different mechanisms, including better fluid and salt removal, more effective phosphorus excretion, better middle molecule clearance, more endogenous vitamin D and erythropoietin production, reduced left ventricular hypertrophy, and less inflammation.8–11, 17 Indeed a survival advantage of diuretic therapy in dialysis patients may be related to RKF.18
Consistent observations suggest that RKF declines more rapidly in patients on HD than PD. In a recent study, 617 of 734 (84%) HD patents reported good urine output at baseline, but only 28% had acceptable RKF after one year.15 Preserved RKF was independently associated with lower mortality (HR, 0.70; 95% CI, 0.52–0.93) and better HRQOL, lower C-reactive protein (p=0.02) and IL-6 (p=0.03) levels and, 12,000 U/wk lower erythropoietin doses (p<0.001).15 Incremental dialysis has been used successfully in the initiation of PD. Therefore, it seems prudent to revisit the topic of incremental HD and plan a randomized controlled trial to explore the hypothesis that transitioning to kidney replacement therapy with incremental HD may preserve RKF longer and decrease mortality during the first year of HD. Several investigators, including Termorshuizen et al,19 Bargman et al.20 and Szeto et al21 have reported that among patients with substantial RKF, dialysis dose did not have a significant impact on outcomes.
Relationship of Twice-Weekly HD to Outcomes
In the United States, twice-weekly HD with longer duration of each treatment22 was replaced by thrice-weekly HD as the standard of care some 30 years ago.23 Unfortunately, however, no clinical trials have compared twice-weekly vs thrice-weekly HD. Currently, in some countries, including India and China, over half of HD patients receive ≤2 treatments per week. Some patients are dialyzed even less frequently, e.g. once weekly to even once a month. This practice pattern may largely reflect limited resources and financial constraints. Nevertheless it may be associated with improved outcomes in some patients.11, 24 In a seven-year observational study in Taiwan,11 patients dialyzed twice (n= 23) versus thrice (n=51) weekly had a slower decline of RKF, as indicated by higher urine output and better creatinine clearance. Patients dialyzed twice weekly also had lower levels of serum beta-2-microglobulin, fewer intra-dialytic hypotensive episodes, and fewer hospitalizations.11
Hanson et al.25 reported that among 15,067 adult HD patients, the 570 patients who were receiving twice-weekly HD were more likely to be older, White, and women and to have shorter dialysis vintage (<1 year), higher serum albumin, lower serum creatinine, lower BMI, and higher eGFR upon transition to maintenance dialysis therapy.25 Interestingly, twice-weekly HD patients had a 24% lower adjusted mortality (RR=0.76, p=0.02), although among incident patients the survival advantage of infrequent HD was not significant when adjusted for RKF (RR=0.85, p=0.31).25 Notably, outcomes were no worse with the more convenient, less intrusive, and less costly schedule. The observed survival advantage among patients receiving twice-weekly HD may have reflected higher baseline RKF and longer preservation of RKF. Unfortunately, however, data on RKF were not collected in this study.25 Therefore, a carefully planned and adequately powered randomized controlled trial is needed to determine if the apparent survival advantage of infrequent HD is mediated, at least in part, from higher baseline RKF or improved preservation of RKF.
Several historical reports and reviews have advocated the use of incremental or infrequent (including twice-weekly) HD upon the first transition to kidney replacement therapy in the United States.26, 27 Some of these recommendations stem from the 1997 KDOQI Peritoneal Dialysis Adequacy Work Group report published in AJKD in 1998 that suggested using incremental PD to maintain the RKF, 28 as well as from the European Best Practice Guidelines Expert Group on Hemodialysis.29 According to some of the authors of this Perspective, nephrologists caring for US veterans with chronic kidney failure often encounter patients who insist on initiating hemodialysis twice-weekly instead of the routine thrice-weekly protocol. Some nephrologists may not be adverse to this arrangement as long as patients are clearly informed that their choice is not considered the standard of care in the US. Many patients may choose less frequent HD because of reluctance to travel inconvenient distances three times a week, skepticism about their need for more frequent dialysis (or dialysis at all), involvement with infirm family members and children requiring time and attention, or post dialysis fatigue, among other reasons. Also, some patients are elderly or debilitated with cancer and other comorbid conditions, and thus their HRQOL or life expectancy are unlikely to benefit from more frequent dialysis. For such patients, end-of-life concerns and palliative considerations including less frequent HD are more important, and more frequent dialysis could adversely affect their and their family members’ quality of life. Some patients have fragile or otherwise tenuous dialysis vascular accesses that would benefit from infrequent puncture and smaller needles, and less frequent, incremental HD could be viewed as a form of breaking-in the access.
While the target for urea reduction ratio (URR) in patients dialyzed twice a week is uncertain, we suggest that a URR of ≥65% should be achieved with each treatment, or alternatively target equivalent eKt/V of >1.2 adjusted for HD frequency. This, combined clearance of urea and creatinine, can be used to monitor RFK.30 In 1997, KDOQI articulated in detail how standard dialysis dosing could account for the intensity and frequency of HD and RKF. 28 Many patients who start twice-weekly HD may eventually transition to thrice-weekly HD when urine output declines, interdialytic weight gain increases over time, or hypertension becomes more difficult to control; nevertheless, many patients may continue twice-weekly HD for extended periods and with acceptable outcomes. According to coauthors KKZ and CPK, in some Veterans Affairs medical centers in the United States, until recently, twice-weekly HD was the standard transition to kidney replacement therapy in many veterans undergoing maintenance hemodialysis treatment. Incident twice-weekly HD patients are likely healthier and have higher RKF than thrice-weekly HD patients. Similarly, twice-weekly HD was the typical initial prescription in the United Kingdom (UK) until the past decade, when the British Renal Association was able to pressure the National Health Services to ensure thrice-weekly HD as the standard of care in the UK.31 However, it should be reiterated that to date no controlled trial has been conducted to examine superiority or non-inferiority of twice-weekly HD upon transition to kidney replacement therapy.6
There are other important reasons to perform twice-weekly HD upon initiating dialysis therapy. First, having two sessions a week means less frequent cannulations of a new ateriovenous fistula or graft, which may prolong its longevity. In addition, incremental HD may offer a compromise and reconciliation between the two camps of early vs. late dialysis initiation32, 33 in lieu of the traditional approach of initiating thrice-weekly HD. A potential issue, however, is the report of increased mortality on Mondays and Tuesdays after longer interdialytic intervals,34 which may reflect higher interdialytic weight gains and fluid gains35 and hyperkalemia.36–38 With a twice-weekly HD regimen, the patients would be subjected to 3-day and 4-day intervals, assuming patients receive HD on Monday-Wednesday, Tuesday-Friday, or Wednesday-Saturday. Indeed that is why we suggest a threshold of 2.5 L fluid gain, as well as the potassium eligibility cutoffs (see Box 1 and the discussion below).
Box 1. Proposed criteria for twice weekly hemodialysis.
Treatment Criteria for 2x/wk HD
|
Implementation Strategies
|
The proposed criteria are general rather than specific and to should be refined for use in clinical trials and clinical decision making.
Lack of systolic dysfunction (EF, >40%) and no major coronary intervention over the previous 3 months
Abbreviations: K: Potassium, P: phosphorus, Hb: hemoglobin; 2x/wk, twice-weekly; HD, hemodialysis; RKF, residual kidney function; EF, ejection fraction; 3x/wk, thrice-weekly
Decision Support for Incremental HD
We suggest developing and implementing a clinically relevant decision support for incremental HD upon transition to kidney replacement therapy. Our proposed approach is based upon 10 clinical criteria that, if proven useful, can guide initial HD dose and frequency as well as the timing of changes in the dialysis prescription (Box 1). The proposed criteria are meant to be inclusive rather than specific and should be refined for use in future controlled trials and clinical decision making. These metrics should be reexamined periodically, e.g. weekly, monthly, or quarterly, and entered into a paper-based or computerized protocol to determine the dose and frequency of HD treatment. The RKF, including urine volume >0.5 L/day, is perhaps the most important determinant of HD frequency. We do not imply that our suggestion of >0.5 L/day of urine output being the core requirement for the twice-weekly HD should replace the KDOQI suggestion of 2 ml/min/1.73 m2 BSA urea clearance;28 however, our goal here is to advance pragmatic criteria that can be more consistently pursued under all conditions especially given the simplicity of measuring urine output as compared to the known challenges of accurately calculating urea or creatinine clearance. In India and China, twice-weekly HD therapy is practiced widely with little to no systematically defined selection and maintenance criteria; hence, Chinese and Indian programs can use our suggested criteria listed in Box 1 until the results of comparative effectiveness studies or controlled trials are available.
Why to Examine Incremental and Twice-Weekly HD
Although physiologic considerations suggest that patients starting HD with significant RKF should require lower dialysis doses than anuric patients, it is common and routine practice in the US and most Western countries to prescribe the same dialysis dose and frequency in both groups of patients. This practice may stem from beliefs that: (1) it will be difficult to make patients adhere to the inevitable increase in dialysis time and/or frequency; (2) RKF declines rapidly and is not generally monitored, and the overlooked loss of RKF will result in inadequate HD; and (3) more frequent HD than is required can only benefit patients. A close examination of these considerations suggests that the uniform dosing approach may not be optimal for a variety of reasons. Although twice-weekly HD is not recognized as the standard of care in most countries, it is commonly practiced in many regions of the world and under circumstances where financial constraints and resource scarceness appear to prevail. Occasionally even in the US patients may choose twice-weekly HD for convenience and perceived superior quality of life and end-of-life constellation.
The innovative concept of reviving incremental HD including twice-weekly HD upon transition to kidney replacement therapy may lead to an important paradigm shift in our current nephrology practice. It offers a novel approach to the unanswered question of timing the initiation of maintenance dialysis, as it offers an alternative to the abrupt transition to the usual thrice-weekly HD treatments. Doing so may better preserve RKF11 and decrease the degree of inflammation. It may also improve patient centered outcomes including health related quality of life, satisfaction,25 and end-of-life concerns while significantly containing costs and resources. This topic and the associated new approach to “Transition of Care in CKD” is the focus of one of the new special study centers of the United States Renal Data System (www.USRDS.org) and also is responsive to the novel NIH Roadmap Initiative PROMIS measures of physical function, sleep39 and fatigue40 as health-related quality of life outcomes.
We envision a series of randomized controlled trials of incremental versus thrice-weekly HD that characterizes the relationship between dialysis dose and frequency, RKF, and patient-related outcomes. We feel that VA medical center dialysis clinics are excellent sites for these trials. In order to ensure generalizability, future trials should recruit a demographically diverse and representative population sample with respect to race/ethnicity, age, gender, location and cause of chronic kidney failure. These studies should employ state of art methods for assessing RKF, episodes of AKI, and patient centered outcomes.
Conclusions
The authors appreciate the important challenge of their proposal to the current paradigm of thrice weekly HD treatments upon transitioning to kidney replacement therapy. The long-term goals are to provide personalized and incremental HD to patients at the start of transition to dialysis therapy in order to optimize survival, health-related quality of life, and other pertinent outcomes. The design of future studies should permit a rapid scaling-up of the interventions in larger clinical trials since it takes advantage of resources and personnel that are readily available in many centers including most VA medical centers in the United States. Finally, notwithstanding the potentially favorable aspects of twice-weekly or incremental HD upon transition to kidney replacement therapy, the majority of the authors of this perspective feel that the “PD first”41 should continue to be highlighted as the preferred mode of transition given consistent data demonstrating an association between preservation of RKF and PD. Moreover, the individualized choice of transition of care to a kidney replacement therapy versus more conservative and palliative options should not be ignored for certain patients with terminal conditions.5
Acknowledgments
Drs. Kalantar-Zadeh, Unruh, Kovesdy, Shah, and Goldfarb serve as physicians in US Department of Veterans Affairs medical centers with or without compensation or are part- or full-time employees of a US Department of Veterans Affairs medical centers. Opinions expressed in this paper are those of the authors and do not represent the official opinion of the US Department of Veterans Affairs.
Support: This work was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R01-DK078106, R21-DK077341 and K24-DK091419 and a philanthropic grant from Mr. Harold C. Simmons and Mr. Louis Chang.
Footnotes
Financial Disclosure: Dr. Kalantar-Zadeh has received honoraria and/or grants from Abbott, Abbvie, Alexion, Amgen, DaVita, Fresenius, Genzyme, Keryx, Otsuka, Shire, Rockwell, and Vifor, the manufacturers of drugs or devices and/or providers of services for CKD patients. Dr. Goldfarb has received honoraria from Mission Pharmacal, been a consultant for Keryx and Takeda, and is or has been the site principal investigator for research supported by Amgen, Daxor, Reata, and Hospira. The remaining authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Renal Data System (USRDS) Usrds 2012 annual data report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012 www.usrds.org.
- 2.Molnar MZ, Ojo AO, Bunnapradist S, Kovesdy CP, Kalantar-Zadeh K. Timing of dialysis initiation in transplant-naive and failed transplant patients. Nat Rev Nephrol. 2012;8:284–292. doi: 10.1038/nrneph.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW. Predictors of early mortality among incident us hemodialysis patients in the dialysis outcomes and practice patterns study (dopps) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]
- 4.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: New insights. Am J Nephrol. 2012;35:548–558. doi: 10.1159/000338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee CM, Kalantar-Zadeh K. Transition to dialysis:Controversies in its timing and modality. Introduction Semin Dial. 2013;26:641–643. doi: 10.1111/sdi.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee CM, Unruh M, Chen J, Kovesdy CP, Zager P, Kalantar-Zadeh K. Infrequent dialysis: A new paradigm for hemodialysis initiation. Semin Dial. 2013;26:720–727. doi: 10.1111/sdi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streja E, Kovesdy CP, Greenland S, Kopple JD, McAllister CJ, Nissenson AR, Kalantar-Zadeh K. Erythropoietin, iron depletion, and relative thrombocytosis: A possible explanation for hemoglobin-survival paradox in hemodialysis. Am J Kidney Dis. 2008;52:727–736. doi: 10.1053/j.ajkd.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial. 2011;24:487–494. doi: 10.1111/j.1525-139X.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 9.Hyodo T, Koutoku N. Preservation of residual renal function with hdf. Contrib Nephrol. 2011;168:204–212. doi: 10.1159/000321762. [DOI] [PubMed] [Google Scholar]
- 10.Diao Z, Zhang D, Dai W, Ding J, Zhang A, Liu W. Preservation of residual renal function with limited water removal in hemodialysis patients. Ren Fail. 2011;33:875–877. doi: 10.3109/0886022X.2011.605535. [DOI] [PubMed] [Google Scholar]
- 11.Lin YF, Huang JW, Wu MS, Chu TS, Lin SL, Chen YM, Tsai TJ, Wu KD. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology (Carlton) 2009;14:59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 12.Rifkin DE, Coca SG, Kalantar-Zadeh K. Does aki truly lead to ckd? J Am Soc Nephrol. 2012;23:979–984. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, Chertow GM, Ornt DB, Raimann JG, Larive B, Kliger AS. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013 doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bricker NS, Morrin PA, Kime SW., Jr The pathologic physiology of chronic bright’s disease. An exposition of the “intact nephron hypothesis”. Am J Med. 1960;28:77–98. doi: 10.1016/0002-9343(60)90225-4. [DOI] [PubMed] [Google Scholar]
- 15.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The choices for healthy outcomes in caring for end-stage renal disease (choice) study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Wal WM, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT, Korevaar JC, Geskus RB. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26:2978–2983. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, You L, Li H, Lin Y, Zhang Z, Hao C, Chen J. Association of circulating fibroblast growth factor-23 with renal phosphate excretion among hemodialysis patients with residual renal function. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.00230112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, Cruz JM, Akiba T, Kurokawa K, Ramirez S, Young EW. Diuretic use, residual renal function, and mortality among hemodialysis patients in the dialysis outcomes and practice pattern study (dopps) Am J Kidney Dis. 2007;49:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: An analysis of the netherlands cooperative study on the adequacy of dialysis (necosad )-2. Am J Kidney Dis. 2003;41:1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 20.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the canusa study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 21.Szeto CC, Wong TY, Chow KM, Leung CB, Law MC, Li PK. Independent effects of renal and peritoneal clearances on the mortality of peritoneal dialysis patients. Perit Dial Int. 2004;24:58–64. [PubMed] [Google Scholar]
- 22.Curtis JR, Eastwood JB, Smith EK, Storey JM, Verroust PJ, de Wardener HE, Wing AJ, Wolfson EM. Maintenance haemodialysis. Q J Med. 1969;38:49–89. [PubMed] [Google Scholar]
- 23.Blagg CR. Hemodialysis 1991. Blood Purif. 1992;10:22–29. doi: 10.1159/000170070. [DOI] [PubMed] [Google Scholar]
- 24.Lin X, Yan Y, Ni Z, Gu L, Zhu M, Dai H, Zhang W, Qian J. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33:66–72. doi: 10.1159/000334634. [DOI] [PubMed] [Google Scholar]
- 25.Hanson JA, Hulbert-Shearon TE, Ojo AO, Port FK, Wolfe RA, Agodoa LY, Daugirdas JT. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19:625–633. doi: 10.1159/000013533. [DOI] [PubMed] [Google Scholar]
- 26.Golper TA. Incremental dialysis. J Am Soc Nephrol. 1998;9:S107–111. [PubMed] [Google Scholar]
- 27.Burkart JM, Golper TA. Should we treat patients with incremental dialysis prescriptions? Blood Purif. 2000;18:298–303. doi: 10.1159/000014452. [DOI] [PubMed] [Google Scholar]
- 28.National Kidney Foundation I. Kidney Disease Dialysis Outcome Quality Initiative,. K/doqi clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 1998 doi: 10.1016/s0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 29.European best practice guidelines expert group on haemodialysis. Nephrol Dial Transplant. 2002;17(Suppl 7):S16–S31. [PubMed] [Google Scholar]
- 30.Kalantar-Zadeh K, Casino F. Let’s give twice-weekly hemodialysis a chance: Revisiting the taboo. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu096. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alston H. Conservative care for end-stage kidney disease: Joint medical conference with the renal association, british geriatrics society and association for palliative medicine. Clinical medicine. 2013;13:383–386. doi: 10.7861/clinmedicine.13-4-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosansky SJ, Cancarini G, Clark WF, Eggers P, Germaine M, Glassock R, Goldfarb DS, Harris D, Hwang SJ, Imperial EB, Johansen KL, Kalantar-Zadeh K, Moist LM, Rayner B, Steiner R, Zuo L. Dialysis initiation: What’s the rush? Semin Dial. 2013;26:650–657. doi: 10.1111/sdi.12134. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra R, Rivara M, Himmelfarb J. Initiation of dialysis should be timely: Neither early nor late. Semin Dial. 2013;26:644–649. doi: 10.1111/sdi.12127. [DOI] [PubMed] [Google Scholar]
- 34.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 37.Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: The role of race. Nephron Clin Pract. 2012;120:c8–16. doi: 10.1159/000329511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torlen K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272–1284. doi: 10.2215/CJN.00960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky-Cade MA, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ow YL, Thumboo J, Cella D, Cheung YB, Yong Fong K, Wee HL. Domains of health-related quality of life important and relevant to multiethnic english-speaking asian systemic lupus erythematosus patients: A focus group study. Arthritis care & research. 2011;63:899–908. doi: 10.1002/acr.20462. [DOI] [PubMed] [Google Scholar]
- 41.Ghaffari A, Kalantar-Zadeh K, Lee J, Maddux F, Moran J, Nissenson A. Pd first: Peritoneal dialysis as the default transition to dialysis therapy. Semin Dial. 2013;26:706–713. doi: 10.1111/sdi.12125. [DOI] [PubMed] [Google Scholar]