Abstract
Background
While animal study and cadaveric study have demonstrated an association between knee joint loading rate and joint degeneration, the relationship between knee joint loading rate during walking and osteoarthritis has not yet been sufficiently studied in humans.
Methods
Twenty-eight participants (14 transfemoral amputees and 14 age and body mass matched controls) underwent knee MRI with subsequent assessment using the semiquantitative Whole-Organ Magnetic Resonance Image Score. Each subject also underwent gait analysis in order to determine knee adduction moment loading rate, peak, and impulse and an exploratory measure, knee adduction moment rate*magnitude.
Findings
Significant correlations were found between medial tibiofemoral joint degeneration and knee adduction moment peak (slope = 0.42 [SE 0.20]; P=.037), loading rate (slope = 12.3 [SE 3.2]; P=.0004), and rate*magnitude (slope = 437 [SE 100]; P<.0001). These relationships continued to be significant after adjusting for body mass or subject type. The relationship between medial knee semiquantitative MRI score and knee adduction moment loading rate and rate*magnitude continued to be significant even after adjusting for peak moment (P<.0001), however, the relationship between medial knee semiquantitative MRI score and peak moment was no longer significant after adjusting for either loading rate or rate*magnitude (P>.2 in both cases).
Interpretation
This study suggests an independent relationship between knee adduction moment loading rate and medial tibiofemoral joint degeneration. Our results support the hypothesis that rate of loading, represented by the knee adduction moment loading rate, is strongly associated with medial tibiofemoral joint degeneration independent of knee adduction moment peak and impulse.
Introduction
Knee osteoarthritis (OA) is a common cause of mobility-related disability. This can be especially problematic in specific populations with other primary mobility-related disabilities such as in individuals with lower extremity amputation (Morgenroth et al., 2012, Royer and Koenig, 2005, Royer and Wasilewski, 2006). Mechanical loading is thought to play an important role in the etiology of OA in both the amputee and the general population. However, there is uncertainty regarding which biomechanical measure of joint loading is most associated with the natural history of knee OA. The medial tibiofemoral compartment is affected much more commonly than the lateral tibiofemoral compartment (Wise et al., 2012). This discrepancy is thought to be primarily due to medial joint contact forces that are significantly larger than the lateral joint forces during walking (Schipplein and Andriacchi, 1991). This distribution of contact forces depends largely on the knee joint frontal plane moment (Schipplein and Andriacchi, 1991). More specifically, the knee adduction moment (KAM) is a reliable indicator of medial compartment compressive loading during walking (Birmingham et al., 2007, Zhao et al., 2007), and it has been shown to be an important predictor of the presence (Baliunas et al., 2002), severity (Sharma et al., 1998, Thorp et al., 2006), and progression of radiographic knee OA (Miyazaki et al., 2002).
A number of different features of the KAM may be important to the etiopathology of knee OA. The peak KAM is the loading variable associated with knee OA that has been studied most extensively, in both cross sectional (Thorp et al., 2006, Sharma et al., 1998, Baliunas et al., 2002, Creaby et al., 2010) and longitudinal studies (Miyazaki et al., 2002, Bennell et al., 2011). The KAM impulse, representing the overall loading exposure, has more recently been shown to have an association with knee OA severity as well (Creaby et al., 2010, Thorp et al., 2006). The KAM loading rate, a variable that was introduced in a recent study assessing knee extensor strength (Lloyd et al., 2010), has not been explored in relation to knee OA. While KAM loading rate has not yet been studied in the context of knee joint degeneration, the rate of loading of a joint has been suggested as an important factor in the development of OA (Radin and Paul, 1971) due to the viscoelastic nature of articular cartilage and underlying subchondral bone.
Increased loading rates have been associated with the initiation (Ewers et al., 2002) and propagation (Kerin et al., 2003) of articular cartilage surface fissuring during an in vivo animal study and in cadaveric in vitro study respectively. While suggesting an association between rate of loading and tissue degeneration, these studies were performed with externally applied loads under non-physiologic conditions. The effects of loading rate have also been studied under more physiologic conditions in humans in vivo. Human subjects with knee OA have been shown to have an increased vertical ground reaction force (GRF) loading rate during walking when compared with matched healthy controls (Mundermann et al., 2005, Messier et al., 1992) and while walking at faster self-selected speeds (Hunt et al., 2010). However, GRF loading rate is not necessarily an accurate predictor of knee joint loading rate since GRF and rate of force application can be modulated through muscle contraction and limb segment motion in order to dampen joint contact force loading rate (Cole et al., 1996). Individuals with knee OA have also been shown to have a trend toward increased intersegmental axial loading rate at the knee compared with healthy controls (Mundermann et al., 2005). While this is more indicative of overall knee joint contact forces, intersegmental knee joint forces are not specific to the medial tibiofemoral compartment where a majority of knee OA occurs, and does not have as strong a basis in the literature regarding predictive association with knee OA. Since the KAM has been shown to be a proxy for medial knee joint loading associated with OA, KAM loading rate may be a more specific means of exploring rate of loading at the medial tibiofemoral joint.
Although in vitro and animal in vivo studies suggest that loading rate is an important factor in joint degeneration associated with knee OA, in vivo human study has been limited with existing studies primarily focused on non-specific measures of lower extremity loading. We are not aware of any prior studies exploring the relationship between KAM loading rate and medial tibiofemoral joint degeneration. This study therefore aims to determine if there is a relationship between the KAM loading rate during walking and the severity of medial knee joint degenerative changes on MRI. A secondary aim was to assess whether KAM loading rate and an exploratory measure termed KAM rate*magnitude (RM) correlate more strongly with medial tibiofemoral joint degeneration than KAM peak and KAM impulse.
Methods
Subjects
This cross sectional cohort study utilized data from subjects that were part of a research project comparing transfemoral amputees with an age and body mass matched control group of non-amputees. Given that there were no significant differences in age, body mass or walking speed (see Results Section for details), amputee and control group data were pooled in this study in order to perform overall regression analyses that were not dependent on whether or not a subject had an amputation. For subjects with an amputation, all biomechanical and MRI outcome measures pertain to the intact limb. Subjects were recruited through bulletin board advertisements and local medical clinics, and all subjects signed informed consent prior to participation as approved by the University of Washington and VA Puget Sound Health Care System Institutional Review Boards in compliance with the Helsinki Declaration. Subjects were excluded if they had a history of any prior knee injury that either impeded weight bearing for more than one week, led to medical imaging, or led to a medical evaluation; if they had any other neurologic, rheumatologic, or musculoskeletal disorders (other than knee pain or amputation) that limited their ability to walk; if they had any contraindications to MRI; and if they were younger than 35 or older than 65. Amputee subjects were also excluded if their amputation was within the past five years; if they were not regular community ambulators with a properly fitting prosthetic limb; if the etiology of their amputation was related to diabetes or vascular disease; or if they used an assistive device such as a cane. Control subjects were also excluded if they were experiencing any current or recent knee pain. Knee pain in the amputee group was assessed using the Western Ontario and McMaster University Rating (WOMAC).
Magnetic resonance imaging (MRI) acquisition and interpretation
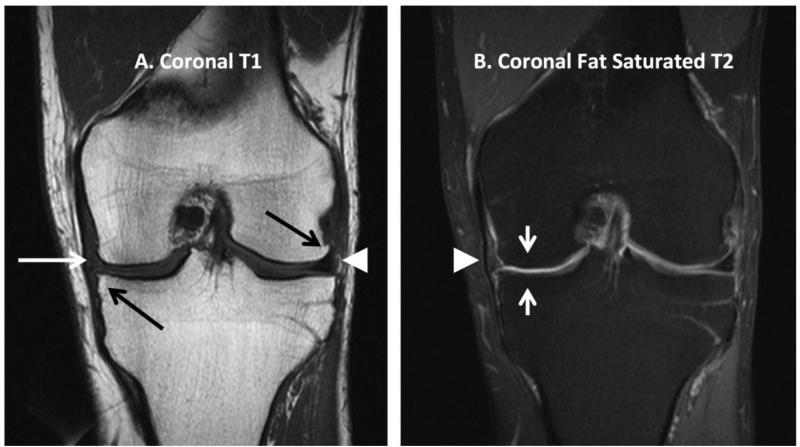
Each subject underwent unilateral knee MRI with subsequent Whole-Organ Magnetic Resonance Image Score (WORMS) (Peterfy et al., 2004) scoring by a board-certified radiologist (JRM) with 11 years experience with musculoskeletal imaging. The radiologist was blinded to participant study group assignment, biomechanical gait measurements and historical information. All knee MRIs were performed in a supine, non-weight bearing position with a 1.5 Tesla Intera MR system (Philips Medical Systems, Best, The Netherlands) using a quadrature knee/foot transmit-receive SENSE coil (IGC Medical Advances, Milwaukee, Wisconsin, USA). Scanning protocol was identical for all participants and is summarized in Table 1. Representative MR images are included for illustration in Figure 1. MR sequence selection for this study was based upon those used by Peterfy et al (Peterfy et al., 2004), with minor adaptations employed to improve conspicuity of anatomic variables to be scored. MR images were analyzed on picture archiving and communication system workstations (AGFA Healthcare, Greenville, SC, USA; or, Brit Systems, Dallas, TX, USA) using the previously validated WORMS semiquantitative scoring methodology (Peterfy et al., 2004). WORMS scoring has been described in detail by Peterfy et al (Peterfy et al., 2004), but a summary follows. All image sequences were used to score with respect to 14 independent articular features (cartilage signal & morphology, subarticular bone marrow abnormality, subarticular cysts, subarticular bone attrition, marginal osteophytes, medial & lateral meniscal integrity, anterior & posterior cruciate ligament integrity, medial & lateral ligament integrity, synovitis, loose bodies and periarticular cysts/bursae). The five features concerning cartilage and bone characteristics were evaluated in 15 anatomical regions corresponding to sub-compartments of the patellofemoral and tibiofemoral joints as well as the subspinous region of the tibia. Integer scores were assigned for cartilage signal & morphology between 0 – 6 (which also included solitary exception scale point of 2.5); subarticular bone marrow abnormality 0 – 3; subarticular cysts 0 – 3; subarticular bone attrition 0 – 3; and marginal osteophytes 0 – 7. Maximal knee joint compartment total score attainable was 308. A maximum of 18 additional points could be scored from meniscal, ligamentous or synovial abnormalities, and a maximum of 6 additional points could be scored from subspinous region abnormalities for a maximum grand total score of 332. Thus, the sum of tricompartmental joint abnormality scores will not always equal the total WORMS score for the entire joint. Since medial knee joint loading is expected to primarily affect degenerative changes in the medial joint, the medial tibiofemoral compartment overall sub-score (medial WORMS score) was calculated as described previously (Peterfy et al., 2004).
Table 1.
Imaging parameters of the MRI sequences
| Sequence | TR (msec) | TE (msec)/Flip Angle | Slice Thickness/Interslice Gap (mm) | Matrix | Scan Percentage (%) | NSA | FOV (mm2) | ETL |
|---|---|---|---|---|---|---|---|---|
| Sag Oblq PD | 1725 | 10 | 4/0.4 | 304 × 512 | 80 | 2 | 160 | 4 |
| Sag T2 SPIR | 4359 | 70 | 4/0.4 | 256 × 512 | 75 | 4 | 160 | 14 |
| Sag 3D GRE WATSc | 24 | 9.7/20° | 1.3/0 | 256 × 256 | 85 | 2 | 160 | - |
| Cor T1 | 574 | 14 | 4/0.4 | 272 × 512 | 80 | 2 | 160 | 0 |
| Cor PD SPIR | 2397 | 13 | 4/0.4 | 256 × 512 | 80 | 3 | 160 | 5 |
| Ax PD SPIR | 2557 | 10 | 4/0.4 | 256 × 2 | 80 | 4 | 150 | 9 |
MRI = magnetic resonance imaging; TR = repetition time; TE = echo time; NSA = number of excitations; FOV = field of view; ETL = echo train length; Sag = sagittal; Oblq = oblique; PD = proton density; SPIR = spectral inversion recovery fat saturation; 3D = 3-dimensional; GRE = gradient echo; WATSc = water selective cartilage Cor = coronal; Ax = axial
Figure 1.
Representative coronal plane MR images of the knee with examples of pathologic findings and normal morphologic findings. On both images, the left side is medial and the right side is lateral. A. Coronal T1-weighted image with medial tibial plateau osteophyte and lateral femoral condyle osteophyte (black arrows), non-displaced tear of the medial meniscus (white arrow), and normal morphology lateral meniscus (white arrowhead). B. Coronal fat saturated T2-weighted image of the same subject with severe thinning of articular cartilage in the central region of the medial femoral condyle and medial tibial plateau (short white arrows); and normal morphology medial collateral ligament (white arrowhead).
Gait analysis
Kinematic and GRF data were measured while subjects walked over ground across force plates embedded along a 10-meter walkway at a controlled speed of 1.10 m/s (+/- 10%) as monitored by two photoelectric beams. Gait kinematics were collected with a 12-camera motion capture system (Vicon, Centennial, CO) sampled at 120 Hz. Thirty-five 14 mm reflective markers were placed on each subject at locations consistent with a modified Vicon Plug-in-Gait full-body model. Anthropometric measurements were taken for each individual according to the Vicon Plug-in-Gait requirements for static and dynamic modeling. Ground reaction forces were collected with one Kistler plate (Winterthur, Switzerland), two Bertec force plates (Columbus, OH) and two AMTI force plates (Watertown, MA), each sampled at 1200 Hz. A minimum of five successful over-ground trials was collected for each condition.
Knee joint moments were calculated using a standard inverse dynamics approach. The magnitude of the peak KAM was quantified using Vicon's event analyzer. In order to determine the exposure to load, the net positive impulse of the KAM was calculated by utilizing the trapezoid rule (Robbins et al., 2009, Robbins and Maly, 2009) without normalizing to stride time in a custom written Matlab software program (Mathworks, Natick, MA). The KAM loading rate (Lloyd et al., 2010) and a variable that we termed KAM rate*magnitude (RM) were also calculated in Matlab from initial foot contact to the first peak of the KAM curve. KAM loading rate was calculated as the maximum instantaneous slope of the KAM curve from initial foot contact to the first peak of the KAM curve. Since the maximum loading rate occurred at variable points along the rise of each subject's KAM curve, RM was explored as an additional outcome measure that takes both loading magnitude and loading rate into account simultaneously. This variable was developed to account for the possibility that loading rate at higher loading magnitudes may have greater influence than an equal loading rate at lower loading magnitudes. KAM RM was calculated as the average of the sum of the products of the rate of rise multiplied by magnitude of the KAM across each set of three adjacent sampling points (based on a sampling frequency of 120 Hz) from initial foot contact to the first peak of the KAM curve as described by the following:
where
Since it has been shown that non-body mass normalized KAM is a more sensitive indicator of knee OA disease progression (Robbins et al., 2011), knee joint moments were not weight normalized in this study. Gait assessment and MRI acquisition were collected on the same day whenever possible (12 cases). For the other 16 cases, MRI data was collected on average 10.1 days after gait assessment.
Statistical analysis
To test for a significant relationship between KAM measures and medial tibiofemoral joint degeneration measured by the medial WORMS score, linear mixed effects regression was carried out with KAM measure as the dependent variable, medial WORMS score as the independent fixed effect and subject as a random effect, the latter to account for repeated KAM measures within subject. To ensure that body mass was not a significant confounder, additional regression analyses were carried out adding and adjusting for body mass as an independent covariate. To further assess whether the relationships between biomechanical loading variables and medial WORMS score were affected by amputee vs. control group status, additional models were also carried out adjusting for subject type (amputee or non-amputee). Finally, additional models were carried out adjusting for select KAM measures by adding these variables as independent covariates to the above model. Results are presented as slope of change in KAM per increase in medial WORMS score. To determine if the slopes differed by subject type, an interaction term between WORMS score and amputee/non-amputee status was added to the model. Differences in age and weight by subject type (amputee or non-amputee) were assessed using 2 sample t-tests. Differences in KAM measure by subject type was determined using linear mixed effects regression as above with subject type as the independent fixed effect and with additional adjustments for age and speed. Analyses were carried out using R 2.14.0 statistical software (2011) and the package lme4: Linear mixed-effects models using S4 classes (Bates, 2011) to carry out the linear mixed effects regression.
Results
Twenty-eight subjects (mean age: 56.0 [SD 8.7] years; mean weight: 83.3 [SD 10.5] kg) including a combination of 14 male unilateral transfemoral amputee subjects and 14 age and body-weight matched non-amputee control subjects were studied. Average duration since amputation was 32 (SD 12.3) years. Amputee subjects had a relatively low average WOMAC pain score (3.6 (SD 4.9) out of a possible 20), and the WOMAC pain question specific to walking indicated that 8/14 amputee subjects had no pain during walking and the remaining 6 had minimal pain during walking (0.64 (SD 0.93) out of a possible 4.0). There were no significant differences between amputee and control subjects in age or body mass. Although there were no statistically significant differences in walking speed or in any of the biomechanical loading variables, the amputee group walked with a trend toward a slightly slower speed and trended toward somewhat lower peak KAM, higher KAM loading rate and higher KAM RM (Table 2).
Table 2.
Mean (SE) of demographic and biomechanical measures by subject group
| Control | Amputee | P-value† | |
|---|---|---|---|
| Age (years) | 55.6 (2.2) | 56.4 (2.6) | .83 |
| Weight (kg) | 84.6 (3.2) | 82.2 (2.5) | .57 |
| Walking speed (m/s) | 1.14 (0.01) | 1.11 (0.01) | .10 |
| Peak KAM (Nm) | 51.8 (3.8) | 46.6 (3.8) | .33 |
| KAM Impulse (Nm) | 21.2 (1.9) | 20.1 (1.9) | .66 |
| KAM loading rate (Nm/s) | 762 (68) | 926 (68) | .083 |
| KAM RM (Nm2/s) | 12300 (2300) | 15600 (2300) | .30 |
2 sample t-test for differences in groups by age and weight, and linear mixed effects regression for difference in biomechanical measures by group
Subjects had a wide range of WORMS scores, with distribution of medial versus lateral tibiofemoral degeneration consistent with prior literature (Wise et al., 2012) (Table 3). The KAM throughout the stance phase had a typical two-peak shape, consistent with prior studies (Mundermann et al., 2005, Baliunas et al., 2002, Hurwitz et al., 2002).
Table 3.
MRI WORMS* scores for each subject (overall, medial, lateral and patellofemoral scores)
| Overall | Medial | Lateral | Patellofemoral | |
|---|---|---|---|---|
| Subject 1 | 1 | 0 | 0 | 0 |
| Subject 2 | 19 | 13 | 0 | 3 |
| Subject 3 | 22 | 6 | 10 | 5 |
| Subject 4 | 25.5 | 2 | 13.5 | 10 |
| Subject 5 | 21 | 2 | 1 | 15 |
| Subject 6 | 36 | 17.5 | 4 | 11.5 |
| Subject 7 | 49.5 | 20.5 | 0 | 28 |
| Subject 8 | 19 | 9 | 0 | 6 |
| Subject 9 | 51.5 | 36 | 2 | 8.5 |
| Subject 10 | 61 | 11.5 | 9.5 | 38 |
| Subject 11 | 2 | 0 | 0 | 2 |
| Subject 12 | 6 | 5 | 0 | 1 |
| Subject 13 | 48 | 27 | 1 | 17 |
| Subject 14 | 98 | 55 | 15 | 19 |
| Subject 15 | 3 | 0 | 0 | 2 |
| Subject 16 | 35.5 | 16 | 5 | 10.5 |
| Subject 17 | 12 | 4 | 1 | 5 |
| Subject 18 | 3 | 0 | 0 | 1 |
| Subject 19 | 10 | 2 | 0 | 2 |
| Subject 20 | 16 | 5 | 1 | 4 |
| Subject 21 | 19.5 | 0 | 8.5 | 9 |
| Subject 22 | 47.5 | 21 | 12 | 9.5 |
| Subject 23 | 11 | 3 | 0 | 8 |
| Subject 24 | 9 | 6 | 0 | 1 |
| Subject 25 | 1 | 0 | 0 | 1 |
| Subject 26 | 21 | 5 | 2 | 12 |
| Subject 27 | 2 | 2 | 0 | 0 |
| Subject 28 | 5 | 0 | 0 | 5 |
WORMS = whole-organ magnetic resonance image score
Subjects 1-14 are transfemoral amputee subjects and subjects 15-28 are non-amputee subjects
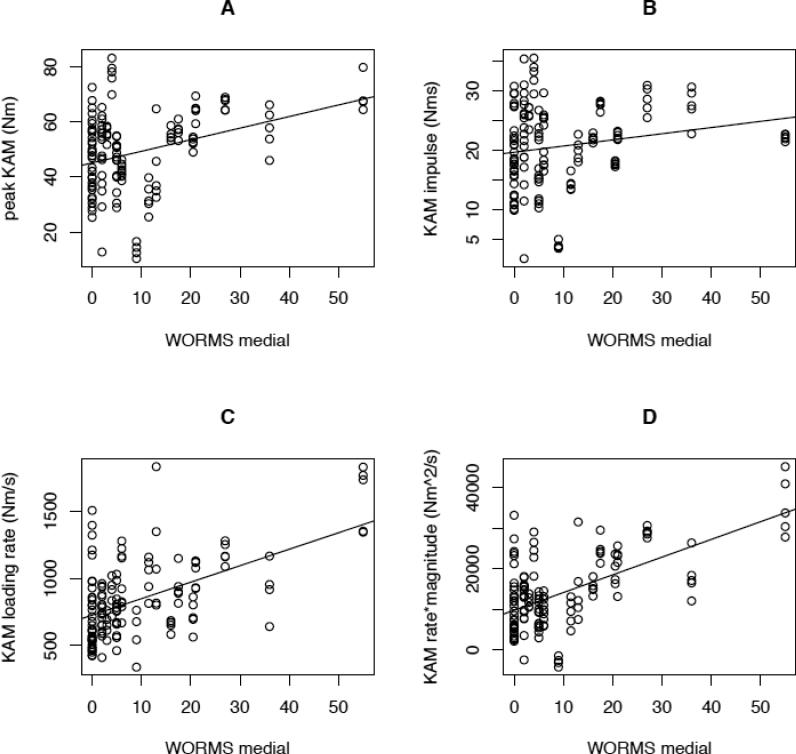
Overall, there were statistically significant correlations between medial tibiofemoral joint degeneration (as measured by medial WORMS score) and peak KAM (slope = 0.42 [SE 0.20]; P=.037), KAM loading rate (slope = 12.3 [SE 3.2]; P=.0004), and KAM RM (slope = 437 [SE 100]; P<.0001) (Figure 2). These relationships continued to be significant after adjusting for body mass (respective P-values of .019; .0004; and <.0001) or amputee versus non-amputee subject classification (respective P-values of .004; .008; and .0001). The slopes representing the regression relationship between each of the biomechanical loading variables and medial WORMS score were almost identical in the amputee vs. control group (P > .9). The relationship between medial WORMS score and both KAM loading rate and KAM RM continued to be significant even after adjusting for peak KAM (P=.014; P<.0001 respectively). However, the relationship between medial WORMS score and peak KAM was no longer significant after adjusting for either KAM loading rate or KAM RM (P>.2 in both cases).
Figure 2.
Scatterplots, each with linear regression line, for the relationship between medial tibiofemoral WORMS score and KAM loading variables (A. KAM peak; B. KAM impulse; C. KAM loading rate; and D. KAM rate*magnitude).
The relationship between medial WORMS score and KAM impulse was not statistically significant, even prior to adding covariates to the regression analysis (slope = 0.10 [SE 0.10]; P=.3).
Discussion
Dynamic loading is believed to play a significant role in the progression of knee OA. While the rate of loading has been suggested as an important mechanical component associated with joint degeneration, it has received limited study in humans. This study shows that the rate of dynamic loading at the knee, as represented by the KAM loading rate and RM, were associated with increased medial tibiofemoral joint degenerative changes on MRI, even after adjusting for body mass and subject classification. The peak KAM was also associated with increased degenerative changes, but this relationship was no longer significant after adjusting for KAM loading rate or RM. The relationship between KAM loading rate and RM with medial knee degenerative changes continued to be significant after adjusting for peak KAM. These findings suggest that dynamic loading rate, as represented by KAM loading rate and RM, is an important variable deserving further exploration in the prevention and treatment of knee OA.
Although the relationship between KAM loading rate and knee joint degenerative changes has not received prior study in humans, there have been numerous in vitro studies and in vivo animal studies suggesting the importance of the rate of loading in relation to the development of OA. Articular cartilage and underlying subchondral bone are viscoelastic structures that become less deformable when subjected to faster loading rates (Radin and Paul, 1971). When articular cartilage is rapidly loaded, internal stresses can become quite large due to lack of fluid flow, and this can lead to fracture of the collagen matrix (Radin et al., 1991a). Subchondral bone can sustain microscopic damage with repetitive impulsive loads, which accumulate and can lead to subchondral stiffening (Radin et al., 1978, Radin and Rose, 1986), increasing shear stresses within articular cartilage thus leading to further degenerative changes (Anderson et al., 1993, Radin et al., 1991a). Rapidly applied repetitive loading has been shown to cause joint damage as shown in vitro (Lukoschek et al., 1988) and in vivo animal models (Radin et al., 1984), even when load amplitude is within physiologic limits. For instance, when tested in rabbits in vivo, higher rate of loading led to cartilage degeneration more often than in animals with lower loading rate even though the latter had higher magnitudes of load (Yang, 1989).
While direct force measurements or repetitive loading models are generally not feasible in human study, kinetic gait laboratory analysis has provided important clues in associations with knee OA. Humans with radiographic evidence of knee OA have been shown to walk with a more extended knee at heel strike and have an increased GRF rate of rise during early stance when compared with healthy matched controls (Mundermann et al., 2005). Similarly, individuals with “pre-osteoarthritic” knee pain demonstrated a more rapid rise of the GRF (with no significant difference in the peak GRF) and less knee flexion during early stance compared to age-matched controls (Radin et al., 1991b). A reduction in knee flexion during early stance has been suggested as a primary cause of increased loading rate since force cannot be dissipated as readily in eccentric loading of the knee extensor musculotendon unit with a more extended knee (Radin et al., 1991b). While GRF as measured in these prior studies is an important contributor to KAM, the current study noted a more specific relationship between KAM loading rate and medial knee joint degenerative changes on MRI.
In addition to studying KAM loading rate in isolation, we developed a variable termed KAM RM that incorporates both loading rate and loading magnitude (as described in more detail in the Methods). Similar to KAM loading rate, RM also demonstrated a significant and independent relationship with medial tibiofemoral degenerative changes on MRI. While degenerative changes at the knee were associated with KAM loading rate, RM and peak, our study did not demonstrate a similar relationship with KAM impulse. This finding is consistent with prior study showing a lack of association between KAM impulse with signs of structural damage such as medial tibial cartilage defects or bone marrow lesions (Bennell et al., 2011). However, this finding differs from prior studies that have found that KAM impulse was able to accurately differentiate between radiographic severity of OA (Thorp et al., 2006), and to predict increased medial tibial cartilage loss at 12-month follow up (Bennell et al., 2011). Given the relatively smaller number of subjects in this study compared to some prior studies of KAM impulse, type II error cannot be ruled out as a reason for an apparent lack of statistical relationship with respect to KAM impulse.
While a majority of studies examining the KAM have assessed knee OA using radiographs, there are potential limitations to their use in the research setting in isolation. Radiographs are insensitive to early knee OA degenerative changes, and while advanced disease is typically readily visible, the Kellgren and Lawrence grading system (Kellgren and Lawrence, 1963) that is typically used to quantify disease severity is non-linear and has other inherent limitations (Hunter and Guermazi, 2012). A number of more recent studies have used MRI, which is more sensitive and specific than radiographs and can directly visualize cartilage and other structures that are thought to play an important role in OA given the contemporary understanding of OA as a disease of the entire joint. Studies utilizing MRI have demonstrated an association between KAM and cartilage deficits (Creaby et al., 2010) and bone marrow lesions (Bennell et al., 2010), and can demonstrate early macroscopic changes to articular cartilage and other soft tissue structures seen in OA (Eckstein et al., 2006). Semiquantitative scoring of MRIs such as the Whole Organ Magnetic Resonance Imaging Score (WORMS) (Peterfy et al., 2004) allow for the quantification of the spectrum of structures and features that are thought be important in the global pathophysiology of knee OA, and has been shown to demonstrate adequate reliability, sensitivity and specificity (Hunter et al., 2011). WORMS also allows for subscore analysis of degeneration in specific regions such as the medial tibiofemoral compartment (Peterfy et al., 2004). We are not aware of any prior studies using a semiquantitative MRI score summing overall degenerative joint changes to assess the relationship between dynamic loading and knee OA. Similar to Peterfy et al (Peterfy et al., 2004) who developed WORMS scoring and demonstrated high inter-observer reliability, we used a 1.5T magnet. Although 3T allows for higher spatial resolution, we are not aware of any studies comparing WORMS assessment with a 3T versus a 1.5T scanner.
There are potential limitations to this study. The subject population included both amputees and non-amputees, however, there were no significant differences in the potentially confounding variables of age or body mass between these groups. Although some differences between transfemoral amputee intact limb kinematics and kinetics and those of the general population have been documented in prior literature (Seroussi et al., 1996), the biomechanical loading variable outcome measures between these two sub-populations were not significantly different in this study (Table 2). Although type II error cannot be ruled out with respect to the lack of differences in biomechanical variables between groups, the relationship between knee loading variables and medial WORMS score continued to be significant even after statistically adjusting for subject classification (amputee versus non-amputee), and perhaps more importantly, there were no significant differences (P >.9) between the slopes in the amputee group and the control group representing the relationship between biomechanical loading variables with medial WORMS score. Amputee subjects in this study also demonstrated a distribution of medial and lateral tibiofemoral degenerative changes similar to that exhibited in the general population (Wise et al., 2012). Amputee subjects were studied since they are a population with a higher prevalence of knee OA thought to be related primarily to mechanical causes (Morgenroth et al., 2012, Norvell et al., 2005). While the population studied was not defined by a specific K-L grade, our goal was to study a group of individuals with a range of degenerative changes and assess the relationship with features of the KAM. While the heterogeneous nature of the study population precludes definitive conclusions regarding the importance of KAM loading rate in all knee OA populations, the findings are strongly suggestive of the importance of KAM loading rate in association with degenerative changes at the medial tibiofemoral joint. Future study in a larger cohort of individuals with knee OA will be beneficial.
As with any cross sectional study, another potential limitation is that the demonstration of association between variables does not allow for definitive conclusions regarding causation. While there is prior in vitro and animal study literature to support loading rate as a potentially important causative factor in development of OA, we cannot rule out that knee OA causes gait changes that could lead to increased KAM loading rate. Future longitudinal study is therefore warranted to confirm whether KAM loading rate is a true causative factor in the development or progression of medial knee OA.
Conclusions
This study provides further insight into the dynamic loading characteristics associated with degenerative changes at the knee. Prior studies have established the relationship between dynamic knee loading and knee OA, however, to date there has been limited exploration of the importance of the rate of loading during walking with respect to knee OA. This study suggests an independent relationship between KAM loading rate and medial knee degeneration on MRI. Our results support the hypothesis that rate of loading, represented by the KAM loading rate, is strongly associated with medial tibiofemoral joint degeneration, independent of KAM peak and impulse. Further large-scale and prospective designed studies are warranted to explore the potentially causal link between KAM loading rate and knee OA.
Acknowledgement
This material is based on work supported in part by the U.S. Department of Veterans Affairs, Office of Research and Development (RR&D Center Grant A4843C). Additionally, David Morgenroth's work on this project was supported by the National Institutes of Health (NIH grant K12 HD001097). None of the authors have any conflicts of interest related to this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflict of interest related to this work.
References
- R: A language and environment for statistical computing [Online] R Foundation for Statistical Computing; Vienna, Austria: 2011. Available: http://www.R-project.org. [Google Scholar]
- ANDERSON DD, BROWN TD, RADIN EL. The influence of basal cartilage calcification on dynamic juxtaarticular stress transmission. Clin Orthop Relat Res. 1993:298–307. [PubMed] [Google Scholar]
- BALIUNAS AJ, HURWITZ DE, RYALS AB, KARRAR A, CASE JP, BLOCK JA, ANDRIACCHI TP. Increased knee joint loads during walking are present in subjects with knee osteoarthritis. Osteoarthritis Cartilage. 2002;10:573–9. doi: 10.1053/joca.2002.0797. [DOI] [PubMed] [Google Scholar]
- BATES DM, MARTIN, BOLKER BEN. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42. 2011 [Google Scholar]
- BENNELL KL, BOWLES KA, WANG Y, CICUTTINI F, DAVIES-TUCK M, HINMAN RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–4. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- BENNELL KL, CREABY MW, WRIGLEY TV, BOWLES KA, HINMAN RS, CICUTTINI F, HUNTER DJ. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann Rheum Dis. 2010;69:1151–4. doi: 10.1136/ard.2009.118182. [DOI] [PubMed] [Google Scholar]
- BIRMINGHAM TB, HUNT MA, JONES IC, JENKYN TR, GIFFIN JR. Test-retest reliability of the peak knee adduction moment during walking in patients with medial compartment knee osteoarthritis. Arthritis Rheum. 2007;57:1012–7. doi: 10.1002/art.22899. [DOI] [PubMed] [Google Scholar]
- COLE GK, NIGG BM, VAN DEN BOGERT AJ, GERRITSEN KG. The clinical biomechanics award paper 1995 Lower extremity joint loading during impact in running. Clin Biomech (Bristol, Avon) 1996;11:181–193. doi: 10.1016/0268-0033(96)00008-3. [DOI] [PubMed] [Google Scholar]
- CREABY MW, WANG Y, BENNELL KL, HINMAN RS, METCALF BR, BOWLES KA, CICUTTINI FM. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage. 2010;18:1380–5. doi: 10.1016/j.joca.2010.08.013. [DOI] [PubMed] [Google Scholar]
- ECKSTEIN F, BURSTEIN D, LINK TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–54. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- EWERS BJ, JAYARAMAN VM, BANGLMAIER RF, HAUT RC. Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. J Biomech. 2002;35:747–55. doi: 10.1016/s0021-9290(02)00019-2. [DOI] [PubMed] [Google Scholar]
- HUNT MA, HINMAN RS, METCALF BR, LIM BW, WRIGLEY TV, BOWLES KA, KEMP G, BENNELL KL. Quadriceps strength is not related to gait impact loading in knee osteoarthritis. Knee. 2010;17:296–302. doi: 10.1016/j.knee.2010.02.010. [DOI] [PubMed] [Google Scholar]
- HUNTER DJ, GUERMAZI A. Imaging techniques in osteoarthritis. PM R. 2012;4:S68–74. doi: 10.1016/j.pmrj.2012.02.004. [DOI] [PubMed] [Google Scholar]
- HUNTER DJ, ZHANG W, CONAGHAN PG, HIRKO K, MENASHE L, REICHMANN WM, LOSINA E. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19:589–605. doi: 10.1016/j.joca.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ DE, RYALS AB, CASE JP, BLOCK JA, ANDRIACCHI TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20:101–7. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- KELLGREN J, LAWRENCE J. Atlas of Standard Radiographs. Department of Rheumatology and Medical Illustrations; Oxford, Blackwell: 1963. [Google Scholar]
- KERIN AJ, COLEMAN A, WISNOM MR, ADAMS MA. Propagation of surface fissures in articular cartilage in response to cyclic loading in vitro. Clin Biomech (Bristol, Avon) 2003;18:960–8. doi: 10.1016/j.clinbiomech.2003.07.001. [DOI] [PubMed] [Google Scholar]
- LLOYD CH, STANHOPE SJ, DAVIS IS, ROYER TD. Strength asymmetry and osteoarthritis risk factors in unilateral trans-tibial, amputee gait. Gait Posture. 2010;32:296–300. doi: 10.1016/j.gaitpost.2010.05.003. [DOI] [PubMed] [Google Scholar]
- LUKOSCHEK M, SCHAFFLER MB, BURR DB, BOYD RD, RADIN EL. Synovial membrane and cartilage changes in experimental osteoarthrosis. J Orthop Res. 1988;6:475–92. doi: 10.1002/jor.1100060403. [DOI] [PubMed] [Google Scholar]
- MESSIER SP, LOESER RF, HOOVER JL, SEMBLE EL, WISE CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- MIYAZAKI T, WADA M, KAWAHARA H, SATO M, BABA H, SHIMADA S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–22. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGENROTH DC, GELLHORN AC, SURI P. Osteoarthritis in the disabled population: a mechanical perspective. PM R. 2012;4:S20–7. doi: 10.1016/j.pmrj.2012.01.003. [DOI] [PubMed] [Google Scholar]
- MUNDERMANN A, DYRBY CO, ANDRIACCHI TP. Secondary gait changes in patients with medial compartment knee osteoarthritis: increased load at the ankle, knee, and hip during walking. Arthritis Rheum. 2005;52:2835–44. doi: 10.1002/art.21262. [DOI] [PubMed] [Google Scholar]
- NORVELL DC, CZERNIECKI JM, REIBER GE, MAYNARD C, PECORARO JA, WEISS NS. The prevalence of knee pain and symptomatic knee osteoarthritis among veteran traumatic amputees and nonamputees. Arch Phys Med Rehabil. 2005;86:487–93. doi: 10.1016/j.apmr.2004.04.034. [DOI] [PubMed] [Google Scholar]
- PETERFY CG, GUERMAZI A, ZAIM S, TIRMAN PF, MIAUX Y, WHITE D, KOTHARI M, LU Y, FYE K, ZHAO S, GENANT HK. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- RADIN EL, BURR DB, CATERSON B, FYHRIE D, BROWN TD, BOYD RD. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum. 1991a;21:12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
- RADIN EL, EHRLICH MG, CHERNACK R, ABERNETHY P, PAUL IL, ROSE RM. Effect of repetitive impulsive loading on the knee joints of rabbits. Clin Orthop Relat Res. 1978:288–93. [PubMed] [Google Scholar]
- RADIN EL, MARTIN RB, BURR DB, CATERSON B, BOYD RD, GOODWIN C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res. 1984;2:221–34. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- RADIN EL, PAUL IL. Response of joints to impact loading. I. In vitro wear. Arthritis Rheum. 1971;14:356–62. doi: 10.1002/art.1780140306. [DOI] [PubMed] [Google Scholar]
- RADIN EL, ROSE RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- RADIN EL, YANG KH, RIEGGER C, KISH VL, O'CONNOR JJ. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991b;9:398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- ROBBINS SM, BIRMINGHAM TB, MALY MR, CHESWORTH BM, GIFFIN JR. Comparative diagnostic accuracy of knee adduction moments in knee osteoarthritis: a case for not normalizing to body size. J Biomech. 2011;44:968–71. doi: 10.1016/j.jbiomech.2010.12.021. [DOI] [PubMed] [Google Scholar]
- ROBBINS SMK, BIRMINGHAM TB, JONES GR, CALLAGHAN JP, MALY MR. Developing an estimate of daily cumulative loading for the knee: Examining test–retest reliability. Gait & posture. 2009;30:497–501. doi: 10.1016/j.gaitpost.2009.07.118. [DOI] [PubMed] [Google Scholar]
- ROBBINS SMK, MALY MR. The effect of gait speed on the knee adduction moment depends on waveform summary measures. Gait & posture. 2009;30:543–546. doi: 10.1016/j.gaitpost.2009.08.236. [DOI] [PubMed] [Google Scholar]
- ROYER T, KOENIG M. Joint loading and bone mineral density in persons with unilateral, trans-tibial amputation. Clin Biomech (Bristol, Avon) 2005;20:1119–25. doi: 10.1016/j.clinbiomech.2005.07.003. [DOI] [PubMed] [Google Scholar]
- ROYER TD, WASILEWSKI CA. Hip and knee frontal plane moments in persons with unilateral, trans-tibial amputation. Gait Posture. 2006;23:303–6. doi: 10.1016/j.gaitpost.2005.04.003. [DOI] [PubMed] [Google Scholar]
- SCHIPPLEIN OD, ANDRIACCHI TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- SEROUSSI RE, GITTER A, CZERNIECKI JM, WEAVER K. Mechanical work adaptations of above-knee amputee ambulation. Arch Phys Med Rehabil. 1996;77:1209–14. doi: 10.1016/s0003-9993(96)90151-3. [DOI] [PubMed] [Google Scholar]
- SHARMA L, HURWITZ DE, THONAR EJ, SUM JA, LENZ ME, DUNLOP DD, SCHNITZER TJ, KIRWAN-MELLIS G, ANDRIACCHI TP. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–40. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- THORP LE, SUMNER DR, BLOCK JA, MOISIO KC, SHOTT S, WIMMER MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis Rheum. 2006;54:3842–9. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- WISE BL, NIU J, YANG M, LANE NE, HARVEY W, FELSON DT, HIETPAS J, NEVITT M, SHARMA L, TORNER J, LEWIS CE, ZHANG Y. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken) 2012;64:847–52. doi: 10.1002/acr.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG KH, BOYD RD, KISH VL, et al. Differential Effect of Load Magnitude and Rate on the Initiation and Progresion of Osteoarthritis. Trans Orthop Res Soc. 1989;14:148. [Google Scholar]
- ZHAO D, BANKS SA, MITCHELL KH, D'LIMA DD, COLWELL CW, JR., FREGLY BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25:789–97. doi: 10.1002/jor.20379. [DOI] [PubMed] [Google Scholar]