Abstract
Rationale
Dysregulated reward processing is a hallmark feature of drug addiction; however scant research has evaluated restructuring reward processing in the context of addiction treatment.
Objectives
We examined effects of Mindfulness-Oriented Recovery Enhancement (MORE) on reward responsiveness (RR) and opioid cue-reactivity in a sample of chronic pain patients with opioid use problems. We previously reported that MORE decreased pain, opioid misuse and craving relative to a social support control group (SG). Here we examined whether these outcomes were linked to changes in RR in a subset of participants.
Methods
Participants were chronic pain patients (71% women, age = 46.6±13.9) who received MORE (n=20) or SG (n=29). RR was measured before and after 8 weeks of treatment via heart rate (HR) and heart rate variability (HRV) responses during a dot-probe task that included opioid-, pain-related and natural reward stimuli, as well as craving ratings.
Results
The MORE group, who reported decreased opioid misuse and opioid craving during treatment, evidenced less subjective opioid cue-reactivity, greater HR decelerations, and greater increases in HRV to all cues after treatment compared to the SG; HR and HRV effects were most pronounced for natural reward cues. Within the MORE group, HR deceleration to natural reward cues was correlated with increased subjective arousal to the cues, whereas HR deceleration to opioid cues was correlated with decreased subjective arousal. Effects of MORE on craving were mediated by enhanced RR.
Conclusions
Results suggest that during treatment with MORE, cardiac-autonomic responsiveness to non-drug reward increases, while reactivity to opioid reward decreases. Studies are needed to discern whether changes in RR were a result or a determinant of reductions in opioid misuse and craving. RR may play a role in addiction treatment.
Keywords: mindfulness, chronic pain, opioid misuse, addiction, reward, heart rate variability, attentional bias, positive affect, allostasis, cue-reactivity
Introduction
Reward responsiveness to salutary objects and events is fundamental for flexible adaptation to changing environmental contexts. The perceived reward value of such stimuli, encoded in dopaminergic activations of mesocorticolimbic brain circuits, motivates approach behaviors which preserve the physical and psychological well-being of the individual. However, the processing of natural rewards may be usurped by psychoactive drugs which capitalize on the mesocorticolimbic dopamine system to drive addictive behavior (Volkow et al. 2004). Theory suggests that addiction occurs when motivation to obtain natural rewards is re-organized around seeking drug-induced reward and the desire to alleviate dysphoria induced by withdrawal and aversive experiences (e.g., stress and pain) (Alcaro and Panksepp 2011). Due to dysregulation of neural circuitry subserving stress and reward, individuals with substance use disorders may become insensitive to natural reward from healthful and socially affiliative stimuli, while becoming dependent on drugs to preserve a sense of well-being (Koob and Le Moal 2008). Thus, the neurobiological effects of chronic drug use may foster impulsive selection of immediate, drug-induced reward over both delayed and immediate non-drug rewards (de Wit 2009).
Research with animals and humans links drug addiction-related changes in dopamine transmission to altered reward function across multiple drugs of abuse including cocaine, methamphetamine, nicotine, alcohol, and opioids (Heinz et al. 2004; Kalivas and Volkow 2005; Lee et al. 2009; Lintas et al. 2012; Gipson et al. 2013). Further, compared to healthy controls, drug-dependent individuals exhibit significantly less reward responsiveness and attenuated brain response to natural rewards (Volkow et al. 2010; Augustus Diggs et al. 2013). Decreased responsiveness to images depicting immediate natural rewards as indicated by cue-elicited event-related brain potentials has been observed among opiate dependent individuals and is robustly predictive of future opiate consumption (Lubman et al. 2007, 2008, 2009).
Dyregulated reward responsiveness may link chronic pain with the development of opioid misuse and addiction (Garland et al. 2013a). Chronic pain coupled with opioid dose escalation results in neuroadaptations to limbic-striatal circuits in the brain, resulting in increased sensitivity to pain and decreased reward derived from healthful objects and events. The motivation to allay the resultant dysphoria manifests as craving, an attentional bias (AB) towards opioid-related cues (Garland et al. 2012b), and ultimately, compulsive opioid use. This conceptual model yields the following prediction: if dysregulated reward processing links chronic pain to opioid misuse, then restructuring reward responsiveness may be a means of ameliorating this pathogenic process. Thus, treatments that target reward processing are needed. Cognitive training regimens centered on restoring sensitivity to naturally rewarding stimuli may increase reward responsiveness and counter processes at the heart of addictive behavior.
We recently conducted an early-stage randomized controlled trial (RCT) of Mindfulness-Oriented Recovery Enhancement (MORE) (Garland et al., 2010; 2014), a new multimodal intervention designed to address chronic pain, craving, and opioid misuse behaviors, which integrates training in savoring natural rewards with training in mindfulness (Kabat-Zinn 1982) and cognitive reappraisal (Kober et al. 2011) techniques. In this RCT, MORE significantly decreased pain severity and functional impairment, as well as opioid craving and opioid misuse (Garland et al. 2014). While the mindfulness and cognitive reappraisal components of this intervention may be useful for reducing chronic pain and negative affect (Rosenzweig et al. 2010; Ochsner & Gross, 2005), training in savoring, i.e., intentionally focusing attention on pleasant events, may help to increase responsiveness to non-drug rewards (Garland et al. 2010; Geschwind et al. 2011), thus reducing appetitive drive for opioids.
The primary aim of the current study was to examine psychophysiological data to explore the effects of MORE on reward responsiveness and opioid cue-reactivity in a sample of opioid (mis)using chronic pain patients. We examined whether the observed decreases in opioid craving and misuse following MORE were linked with changes in responsiveness to cues representing opioids and non-drug rewards. We used a biological marker, heart rate (HR), to index reward responsiveness. While overall HR reflects summation of parasympathetic and sympathetic influences, heart rate variability (HRV) in the high frequency range reflects parasympathetic control of the heart (Berntson et al. 1997) and is thought to index integrative activity in a network of central (e.g., prefrontal and cingulate cortices) and autonomic nervous system structures (e.g., vagus nerve) involved in cognitive control over attention, emotion, and reward responses (Thayer et al. 2009; Garland et al. 2012a). Thus, to probe whether cue-elicited cardiac responses were driven by parasympathetic modulation of the central-autonomic network during reward processing, in the present study we examined high frequency HRV in addition to HR.
Because parasympathetic influences on the myocardium tend to decrease HR, we hypothesized that (a) compared to participation in a support group (SG), participation in MORE would be associated with enhanced reward responsiveness as indicated by greater decreases in HR and increases in HRV during emotional attention to cues representing natural rewards. We also hypothesized that (b) such reward responsiveness would be associated with opioid craving. Lastly, we hypothesized that (c) enhanced responsiveness to natural rewards would statistically mediate the effects of MORE on opioid craving.
Materials and Methods
Participants and Procedure
The current study examined data from a subset of individuals (14 men and 35 women, mean age = 46.6, SD = 13.9) from a previously published study of MORE vs a SG for chronic pain and prescription opioid misuse (Garland et al. 2014). Individuals who had complete HR data (MORE n = 20; SG n = 29) from a psychophysiological assessment conducted one week before, and one week after, the 8-week treatments were selected for the present study. Participants were recruited from primary care, pain, and neurology clinics, and met study inclusion criteria if they reported recurrent pain on more days than not stemming from chronic non-cancer-related pain conditions and had taken opioids analgesics daily or nearly every day for at least the past 90 days (Chou et al. 2009). At each assessment point, participants completed self-report measures of generalized opioid craving, opioid misuse, and opioid-related pain relief, and then participated in a lab protocol which involved measurement of HR during completion of a dot probe task. The protocol was approved by the Florida State University IRB, and all procedures complied with the standards set forth in the Helsinki Declaration of 1975. Participants were assessed for comorbid psychiatric disorders with the Mini-International Neuropsychiatric Interview 6.0 (MINI) (Sheehan et al. 1998) and excluded if they were suicidal or psychotic. There were no significant between-groups differences in clinical characteristics (see Table 1). Although all participants reported symptoms of physiological dependence (i.e., tolerance and withdrawal) on the MINI resulting from regular and prolonged use of opioids, a smaller percentage (28.6%) met opioid use disorder criteria. However, most (87.5%) participants reported opioid analgesic misuse as defined by a validated cut-point on the Current Opioid Misuse Measure (COMM; Butler et al. 2007). Participants were paid $200 for the study.
Table 1.
Baseline demographic and clinical characteristics of prescription opioid using chronic pain patients, by treatment group (N = 49).
| Measure | MORE (n = 20) | Support Group (n = 49) |
|---|---|---|
| Female, N (%) | 15 (75%) | 20 (69%) |
| Age | 46.0 ± 13.6 | 46.9 ± 14.4 |
| Primary pain condition, N (%) | ||
| Low back pain | 10 (50%) | 18 (62%) |
| Fibromyalgia | 5 (25%) | 7 (24%) |
| Arthritis | 0 (0%) | 1 (3%) |
| Cervicalgia | 1 (5%) | 2 (7%) |
| Other | 4 (20%) | 1 (3%) |
| Opioid use disorder dx, N (%) | 4 (20%) | 10 (35%) |
| Opioid misuse (COMM), N (%) | 18 (90%) | 24 (83%) |
| Comorbid psychiatric dx, N (%) | ||
| Major depressive disorder | 8 (40%) | 18 (62%) |
| Generalized anxiety disorder | 5 (29%) | 9 (38%) |
| Post-traumatic stress disorder | 1 (5%) | 3 (10%) |
| Substance use disorder (non-opioid) | 4 (20%) | 1 (3%) |
| Alcohol use disorder | 4 (20%) | 4 (14%) |
Note. There were no significant between-groups differences on any of these variables on t-tests and chi-square tests. MORE = Mindfulness-Oriented Recovery Enhancement; SG = Support Group. COMM = Current Opioid Misuse Measure (Butler et al., 2007)
Self-Report Measures
A single item “How much do you want your opioids right now?” anchored on a 10-point scale (1 = not at all, 10 = extremely) assessed current generalized opioid craving once during the assessment session in the week prior to intervention and once during the assessment session in the week following intervention. Participants were asked to rate their pain “right now” from 0 to 10 scale and their degree of opioid-induced pain relief on a 0 – 100% scale with two question items drawn from the Brief Pain Inventory (BPI; Cleeland 1994) once at the beginning of each pre- and post-treatment laboratory assessment session.
Dot Probe Task
HRV was measured during a dot probe task (E-Prime: PST Inc., Pittsburgh, PA). Each trial began with a fixation cross (i.e., crosshair) presented for 500 ms. Next, two images matched for visual complexity, composition, and figure-ground relationships appeared side by side on the computer screen for either 200 or 2000ms. Pairs of photos containing one emotionally-salient image and one neutral image were presented. Three blocks of cues (opioid-related, pain-related, and pleasure-related) were presented in randomized, counterbalanced order across participants. A set of 12 photographs representing each type of cue were selected from the International Affective Picture System (IAPS) (Lang et al. 1997a) and media libraries on the Internet. For instance, opioid-related cues included images of pills and pill bottles. Pain-related cues included images of severe injuries, painful medical procedures, and human faces grimacing in pain. Pleasure-related cues included images of babies, beautiful landscapes, and romantic couples. A set of 36 neutral images were selected from the IAPS and each was paired with an emotionally-salient image that matched the photos for visual features such as color, figure-ground relationships, and presence of human faces. Presentation duration and left/right position of the images was randomized and counterbalanced within each block of 64 trials. Both pictures disappeared, and a target probe replaced one of the images after a 50 ms inter-stimulus interval. Probes appeared for 100 ms, and probe location was counterbalanced. Participants indicated the location of the target by responding with a button press, and the reaction time was recorded (presented here as Supplementary Material). Subjective opioid cue-reactivity relative to pre-stimulus baseline was assessed immediately after the opioid cue block on the dot probe task with a single question “How much do you want your opioids right now?” anchored on a 10-point scale (1 = not at all, 10 = extremely). Participants rated the arousal elicited by the opioid-, pain-, and pleasure-related images on a 10-point scale (Lang et al. 1997) .
HRV Measurement
Disposable Ag-AgCl electrodes were attached to participants' right and left pectoral muscles. Electrocardiogram (ECG) data were sampled at 1000 Hz and recorded continuously throughout the protocol on a Biopac MP150 (Biopac Systems, Goleta, CA). Next, participants were instructed to remain motionless and silent for a 5-minute baseline, after which they participated in the dot probe task. R-R intervals were detected in the ECG using automated routines in Acqknowledge 4.1 (BIOPAC, Inc.) and then visually inspected to correct artifacts.
Study interventions
The manualized MORE intervention (Garland 2013) involved group training in mindfulness, cognitive reappraisal, and savoring skills integrated into a manualized 8-session group intervention designed to address pathogenic factors involved in chronic pain and prescription opioid misuse. Sessions were 2 hours in length, and administered by a Masters-level social worker who had practiced mindfulness for over a decade and had clinical experience delivering mindfulness training to persons with psychiatric disorders. This clinician was supervised by the developer of MORE (an experienced, licensed psychotherapist). The first author reviewed video/audio-recordings of the sessions to monitor therapist adherence to the MORE treatment manual. MORE participants were asked to engage in daily 15 minute mindfulness practice sessions at home guided by a CD. Germane to the present study, participants were taught a savoring practice, which involved using mindfulness to intentionally orient and sustain attention on the sensory features (visual, auditory, olfactory, kinesthetic, proprioceptive) of a pleasant experience or object (e.g., a beautiful nature scene like a sunset) while attending to and appreciating any positive emotions arising in response to the pleasant event. For example, in a 20-minute long meditation session, participants were instructed to mindfully attend to the colors, textures, and scents of a bouquet of fresh flowers, and to absorb and appreciate the emotions of contentment and joy arising from this savoring practice. Similar savoring techniques using different sensory targets were discussed across multiple treatment sessions and given as homework practice. The active control condition in this study consisted of 8 weekly, 2-hour support group sessions, in which a Master's-level clinical social worker (different from the MORE facilitator) facilitated discussion and disclosure of emotions on topics pertinent to chronic pain and long-term opioid use. This support group format was based on the evidence-based Matrix Model intensive outpatient treatment manual (Rawson and McCann 2006). The first author reviewed video/audio-recordings of the sessions to monitor therapist adherence to the support group treatment manual. Support group participants were asked to engage in 15 minutes of journaling a day on chronic pain-related themes at home.
Statistical Analysis
With regard to the analysis of HR, Kubios 2.0 (Biosignal Analysis and Medical Imaging Group, University of Finland) was used to calculate beats-per-minute (BPM) and for spectral analysis of HR, applying a fast Fourier transform to extract normalized high frequency HRV from a de-trended, end-tapered interbeat interval time series. HRV in the respiratory frequency band (0.15 – 0.40 Hz) was selected as our estimate of vagally-mediated HRV. HR and HRV indices were averaged across the 5-minute baseline and each of the dot probe cue conditions. HRV data were skewed and log-transformed for subsequent analyses.
For hypothesis testing, we used the following multistage analytic approach. First, we conducted repeated-measures ANOVA (RM-ANOVA) to test whether MORE led to greater reductions in subjective opioid cue-reactivity than the SG. Second, we conducted a RM-ANOVA to examine whether participants in MORE and SG significantly differed on pre- and post-treatment HR values at rest and during each of the dot probe cue conditions. We were particularly interested in HR cue responsiveness; that is, the planned contrast between cue-elicited levels of HR and resting state HR. Third, we computed Pearson correlations to examine associations between: HR responses to opioid, pain, and pleasure cues; stimulus arousal ratings; current pain; and opioid craving. Fourth, we conducted a series of path analyses to examine natural reward responsiveness (as indexed by HR responsiveness to pleasure cues) as a potential mediator of the effects of MORE on reductions in opioid craving, while simultaneously testing for alternative mediational pathways. Changes in HR responsiveness to opioid cues and changes in opioid-induced pain relief might also mediate the effects of MORE on opioid craving. To simultaneously test these alternative hypotheses, all three potential mediators were included in a multivariate path model predicting change in generalized opioid craving and controlling for confounding by baseline opioid use disorder status. We conducted a sensitivity analysis on an additional path model that controlled for changes in opioid misuse as measured by the COMM and changes in current pain levels on the BPI. The Sobel test assessed the significance of indirect effects (Baron and Kenny 1986). Adequacy of model fit was determined via fit indices (Kline 1998), including a χ2/df ratio of between 1 and 3 (Carmines and McIver 1981), comparative fit index (CFI) > .90 and the root-mean square error of approximation (RMSEA) ≤ 0.08 (Browne and Cudeck 1993). Fifth, we conducted a path analysis with the same mediators listed above to examine effects of reward responsiveness on subjective opioid cue-reactivity. Finally, to determine the autonomic source of the observed cue-elicited changes in HR, we conducted a RM-ANCOVA to examine whether MORE and SG participants differed on post-treatment HRV cue responses from resting state levels of HRV, controlling for pre-treatment resting HRV.
Results
Effects of MORE on Subjective Opioid Cue-Reactivity
MORE led to significantly greater pre-post intervention reductions in subjective opioid cue-reactivity on the dot probe task (M = -.68, SE = .45) than the SG (M = .84, SE = .48), F(1,43) = 4.98, p = .03, η2partial = .11.
Effects of MORE on HR Responses During Attention to Emotional Information
RM-ANOVA was conducted on mean HR values (see Table 2) with treatment group, condition (rest, opioid, pain, and pleasure cue), and time (pre- and post-treatment) as factors. While neither the main effect of time nor condition were significant, there was a significant group X time effect, F(1,46) = 4.47, p = .04, η2partial = .09, indicating that, on average, the MORE group experienced significantly greater reductions in HR from pre-post treatment (see Figure 1). There was a significant group X time X condition effect, F(1,46) = 5.20, p = .005, η2partial = .10. Planned comparisons revealed that relative to participants in the SG, participants in the MORE intervention exhibited significantly greater pre-post changes in HR response (i.e., deceleration) during attention to pleasure, F(1,46) = 9.80, p = .003, η2partial = .18, opioid , F(1,46) = 9.25, p = .004, η2partial = .17, and pain cues, F(1,46) = 6.42, p = .02, η2partial = .12. Importantly, there was no significant change in resting state HR, indicating that the effects of MORE on HR were specific to attention to emotional information and not due to a generalized relaxation response.
Table 2.
HR and stimulus arousal responses during each of three cue conditions and at rest (baseline) on a dot probe task as evidenced by patients participating in Mindfulness-Oriented Recovery Enhancement (MORE, n = 20) or a Support Group (SG, n = 29) treatment for chronic pain and opioid misuse.
| Pre-Treatment | Post-Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline | Opioid Cue | Pain Cue | Pleasure Cue | Baseline | Opioid Cue | Pain Cue | Pleasure Cue | |
| Heart Rate | ||||||||
| MORE | 76.31 (18.48) | 79.81 (19.81) | 79.50 (19.63) | 79.45 (18.87) | 75.58 (10.58) | 72.81 (12.61) | 72.30 (13.69) | 69.87 (18.64) |
| SG | 79.00 (13.02) | 77.64 (14.76) | 77.20 (14.84) | 77.85 (13.85) | 81.74 (14.31) | 82.30 (12.93) | 81.07 (13.42) | 82.58 (13.54) |
| Arousal | ||||||||
| MORE | --- | 4.76 (1.59) | 5.16 (1.53) | 4.90 (1.38) | --- | 4.79 (1.13) | 5.81 (1.34) | 5.20 (0.79) |
| SG | --- | 4.34 (2.10) | 5.21 (1.99) | 4.33 (1.72) | --- | 4.37 (1.85) | 5.52 (2.11) | 4.22 (1.29) |
Note: MORE = Mindfulness-Oriented Recovery Enhancement. SG = Support Group. HR = Heart Rate (in beats per minute). Stimulus arousal was rated on a 10-point Likert-type scale, with higher scores indicating greater arousal. No resting state arousal ratings were obtained.
Fig.1.
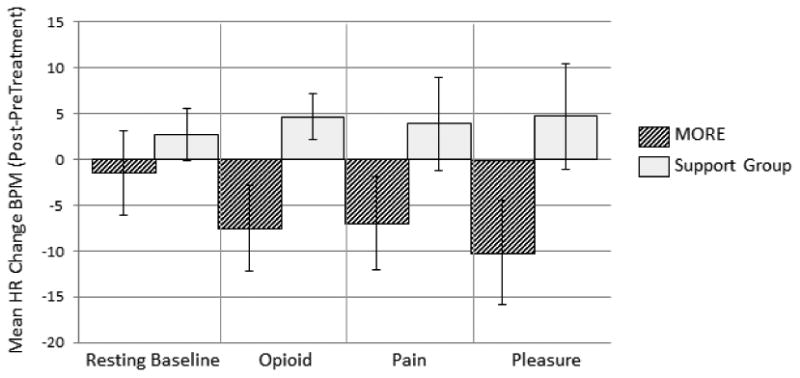
Effects of Mindfulness-Oriented Recovery Enhancement (MORE) and Support Group (SG) interventions on changes in heart rate (HR) from pre- to post-treatment during a 5-minute resting baseline and during three blocks of cues on a dot probe task. The HR change score value on the Y-axis was calculated by subtracting pre-treatment HR during each of the four conditions of the task (resting baseline, opioid cue, pain cue, and pleasure cue) from post-treatment HR during each of the four conditions of the task, such that a negative value indicates a decrease in HR at post-treatment from pre-treatment levels. Relative to the SG, the MORE group evidenced significantly greater pre-post treatment decreases in HR to all three cue types; however, there was no significant pre-post treatment change in HR during the resting baseline. Group X time X condition effect, F(1,46) = 5.20, p = .005, η2partial = .10. BPM = beats per minute.
Zero-order correlations
Within the MORE group, HR decelerations to pleasure cues were significantly correlated with increased arousal ratings for such cues, r = -.51, p = .04. In contrast, within the MORE group, HR decelerations to opioid and pain cues were significantly correlated with decreased arousal ratings for such cues, r = .52, p = .038, and r = .50, p < .05, respectively. Stimulus arousal and HR responses were uncorrelated in the SG. Across both groups at pre- and post-treatment, current pain levels were not significantly correlated with resting HR, nor were they significantly correlated with cue-elicited HR. In contrast, across both treatment groups, individuals who exhibited larger decreases in HR to pleasure cues experienced greater reductions in opioid craving over the course of treatment, r = -.34, p = .02.
Test of Enhanced Reward Responsiveness as a Mediator
To test our third hypothesis: that the effects of MORE on opioid craving would be statistically mediated by enhanced natural reward processing, formal mediation analyses were appropriate. Our multivariate path model predicting change in generalized opioid craving (see Figure 2) exhibited excellent fit, χ 2/df = .92; CFI = 1.00; RMSEA = .00 (.00, .13), R2 = .26. According to the Sobel test, the indirect effect of MORE on reducing opioid craving by enhancing physiological (HR) responsiveness to pleasure cues was statistically significant, z = 2.43, p = .015. Thus, we found that increased reward responsiveness as indicated by HR significantly mediated the effect of MORE on opioid craving. In contrast, neither HR responses to opioid cues nor changes in pain relief over the course of treatment were significant mediators of the craving-reducing effects of MORE. In alternate path models, including indirect paths to HR responses to pain cues, change in current pain levels, and change in self-reported opioid misuse on the COMM did not change the valence or significance of the direct and indirect effects above, but did significantly worsen model fit, so these variables were omitted in the final models. The supplementary multivariate path model predicting change in subjective opioid cue-reactivity also exhibited good fit, χ2/df = .90; CFI = 1.00; RMSEA = .00 (.00, .11), R2 = .81; however, the indirect effect of MORE on subjective opioid cue-reactivity by enhancing physiological (HR) responsiveness to pleasure cues was not statistically significant.
Fig. 2.
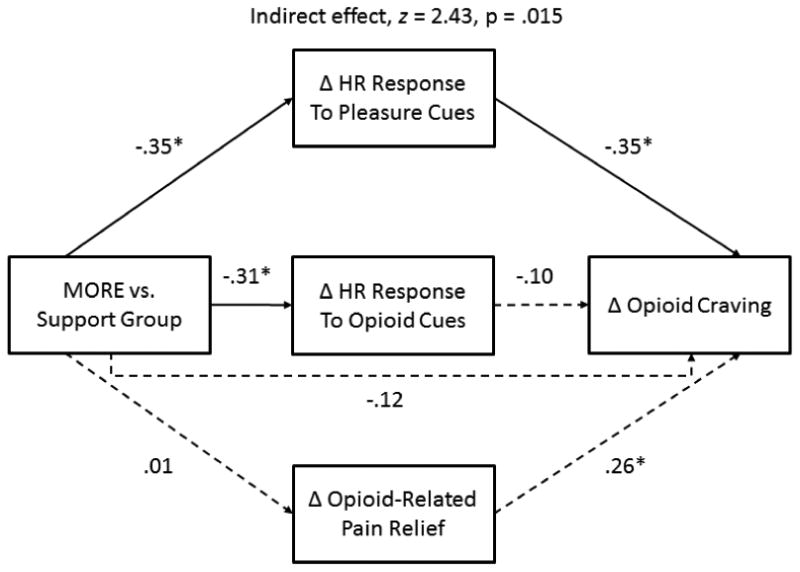
Multivariate path model (N = 49) depicting statistical mediation of the craving-reducing effects of Mindfulness-Oriented Recovery Enhancement by heart rate responsiveness to pleasure cues. This model controls for pre-randomization group differences in opioid use disorder status (not depicted for visual parsimony). *p < 0.05
Between-Groups Differences in HRV During Attention to Emotional Information
To determine the source of the HR decelerations observed at post-treatment, RM-ANCOVA was conducted on post-treatment HRV values with treatment group and condition as factors, and pre-treatment resting state HRV as a covariate. The group X condition interaction was significant, F(1,49) = 4.31, p = .04, η2partial = .09; planned Helmert contrasts indicated that across all cue conditions, participants in MORE experienced significantly greater increases in HRV from rest than the SG participants. Post-hoc tests revealed that this effect was largely driven by natural reward responsiveness; participants in MORE exhibited significantly greater increases in HRV from rest during emotional attention to pleasure cues, F(1,49) = 5.28, p = .026, η2partial = .10, but did not differ significantly with regard to HRV response to opioid or pain cues.
Discussion
Study findings indicate that MORE reduces opioid cue-reactivity and enhances natural reward processing. These results provide preliminary support for the hypothesis that therapeutic enhancement of reward responsiveness is linked with reduced opioid craving in a drug-dependent sample, though causal directionality of this relation cannot be conclusively established with the present data. The current study has implications for understanding of the role of allostatic dysregulation of natural reward in addiction treatment (Koob and LeMoal 2001).
The allostatic model posits that prolonged exposure to drugs and stressful experiences results in an upward shift in basal reward threshold, leading to a reward deficit and dysphoric mood which encourages increased consumption of drugs as a means of achieving an overall positive affective balance. Ironically, this attempt to reach a positive affective state comes with a cost: the continued use of drugs further increases brain reward thresholds, making the individual increasingly insensitive to naturally-rewarding experiences while becoming increasingly sensitive to stress and pain (Koob and Le Moal 2001). Thus, addiction results in progressive deviation of brain reward systems from their normal homeostatic operating level that fuels increasing dependence on drugs as a means of maintaining dwindling positive affect (Koob and LeMoal 2008). In the case of chronic pain patients who are dependent on prescription opioid analgesics, recurrent episodes of pain may result in stress and negative affect, which when prolonged, dysregulates mesocorticolimbic-striatal reward circuitry and promotes addictive behaviors (Garland et al. 2013a). While evidence exists to support this theory, there is a dearth of data regarding whether this allostatic process can be reversed by restoring reward processing.
In the present study, we observed a significant association between mindfulness-based enhancement of natural reward processing and reduction in opioid craving; formal mediation analysis indicated that the effects of MORE on opioid craving were statistically mediated by increased reward responsiveness. One interpretation of these findings suggests that allostatic processes in addiction may be countered by a behavioral intervention designed to enhance savoring of natural rewards. Alternatively, changes in opioid misuse as a result of behavioral treatment may result in reduced opioid craving and increased responsiveness to natural reward. Due to the fact that HR data was only collected pre- and post-treatment in the present study, there is no way to conclusively determine whether changes in reward responsiveness led to decreased opioid misuse and craving or whether changes in opioid use and craving caused changes in reward responsiveness. Indeed, if chronic exposure to opioids blunts responsiveness to non-drug rewards via opioidergic-dopaminergic interactions, then reduction in opioid dose as a result of treatment may have led to the restoration of reward responsiveness observed in the present sample. Although path analysis indicated the presence of a statistically significant indirect effect of MORE on opioid craving through enhanced responsiveness to natural reward, due to study design limitations, the causal order of these effects cannot be disentangled.
In contrast to the observed mediational relationship between reward responsiveness and generalized opioid craving, changes in HR response to pleasure cues did not mediate the effect of MORE on subjective opioid cue-reactivity. This incongruity accords with the observation that cue-reactivity elicited in a laboratory environment, i.e., phasic cue-specific craving, is a distinct construct from generalized craving in everyday life, i.e., tonic craving (Sayette and Tiffany 2012; Tiffany and Wray 2013). Further, our findings accord with a meta-analysis of cue-reactivity in addiction research conducted by Carter and Tiffany (1999) which showed that physiological and subjective cue responses were largely uncorrelated. Our results suggest that although MORE reduces both general opioid craving and subjective opioid cue-reactivity, these treatment-related reductions are mediated by different processes, and enhanced reward responsiveness seems to be most closely linked with decreases in tonic appetitive drive towards opioids.
As evidence of our hypothesis that MORE enhances reward responsiveness, following treatment we observed robust HR decelerations from resting, baseline levels during exposure to natural reward cues. Attention to emotional cues has been shown to consistently result in significantly greater decreases from resting, baseline HR than attention to neutral cues (Kreibig 2011). Thus, HR decelerations indicate a robust response to emotional stimuli, rather than a lack of response. In support of this notion, prior research indicates that highly arousing, pleasant visual cues elicit greater HR decelerations than less arousing cues (Bradley et al. 2001). Such decreases in HR may be coordinated by activation of the central-autonomic network in response to emotional stimuli (Lane et al. 2009). Analyses indicated that the HR decelerations observed in this study resulted from enhanced parasympathetic activation (high frequency HRV). Preclinical models of appetitive conditioning indicate that increases in HRV elicited by conditioned stimuli are associated with a reward expecting state (Inagaki et al. 2005). Concomitantly, cue-elicited increases in HRV are associated with appetitive responses to food cues (Udo et al. 2013; Nederkoorn et al. 2000) and addictive drugs such as nicotine and amphetamine (Culbertson et al. 2010; Erblich et al. 2011), and increases in alcohol cue-elicited HRV predicted the time-course of relapse up to six months following alcohol treatment (Garland et al. 2012a). While increased autonomic responsiveness to drug-related stimuli may signal heightened appetitive drive for the drug and relapse vulnerability during a quit attempt, increased autonomic responsiveness to healthful and socially-affiliative stimuli may buffer such risk and represent a reversal of the usurpation of reward learning processes previously recruited by addictive drugs.
The primary limitation of this study was the lack of a quantitative measure of opioid dosing. We were unable to obtain accurate opioid dosing data for the whole sample due to non-responses and ambiguous responses (e.g., reporting the opioid dose without reporting number of pills taken per day) on our self-report opioid dosing measure. Consequently, we do not know whether enhancing reward responsiveness reduced opioid use, or whether reduced opioid use led to the observed increases in reward responsiveness. Additional research is needed to discern whether changes in reward responsiveness were a result or a determinant of reductions in opioid misuse and craving. To that end, future studies should carefully track opioid dosing and other psychotropic medication usage via a multi-pronged approach including self-report, prescription history, pill count, and urine and serum toxicology screens. It should also be noted that the observed relations between stimulus arousal and HR were inconsistent across cue types. Following treatment with MORE, individuals who reported the greatest increases in arousal to pleasure cues experienced the largest HR decelerations to those cues, whereas those who reported the greatest decreases in arousal to opioid and pain cues experienced the largest HR decelerations to those cues. The apparent paradox presented by the opposing directionality of the HR/arousal correlations for different stimulus types may relate to the fact that parasympathetic nervous activity can be elicited during appetitive responding (Inagaki et al. 2005), as well as during self-regulatory effort – thereby increasing HRV and decreasing HR (Segestrom and Nes 2007). Thus, one speculative interpretation of the discrepant findings in the current study is that MORE may have up-regulated parasympathetic responsiveness to natural reward stimuli while enhancing parasympathetic regulation of arousal elicited by opioid and pain cues. With regard to this latter point, MORE significantly modulated opioid cue-reactivity, as evidenced by decreased HR responses to opioid cues and phasic cue-specific opioid craving. To further resolve this discrepancy and elucidate the neural mechanisms underlying changes in reward responsiveness, future studies should employ neuroimaging paradigms designed to probe reward processing.
These limitations notwithstanding, this study provides support for a recently proposed theoretical model of the neurocognitive mechanisms of mindfulness-based treatment of addiction (Garland et al. 2013c), and may have important clinical implications. Because addiction biases selection toward immediate drug-induced reward over non-drug reward, enhancing responses to immediate non-drug rewards may be a therapeutically crucial step towards restoring the valuation of delayed non-drug rewards so important to addiction recovery, such as preservation of physical or social function. More research is needed to determine whether learning to savor the immediate and momentary sensory pleasure inherent in healthful objects and experiences can provide the motivation necessary to sustain addiction recovery (Davidson and White 2007).
In sum, the current study suggests that MORE can reduce opioid cue-reactivity while restructuring natural reward processing, and provides preliminary support for the hypothesis that behavioral interventions may ameliorate craving by enhancing reward responsiveness. If neural reward circuits provide a basic logic for goal selection (Shizgal and Hyman 2013), training in selective attentive processing of natural rewards over drug rewards may provide the learning signal necessary to undo addiction and switch reward processing back toward its intrinsic valuation of adaptive and pro-regulatory objects and behaviors.
Supplementary Material
Acknowledgments
This work was supported by grant numbers DA032517 and DA037005 from the National Institutes of Health awarded to E.L.G.; and a grant from the Fahs Beck Fund for Research and Experimentation, also awarded to E.L.G.
Footnotes
Conflict of Interest: None
Contributor Information
Eric L. Garland, Huntsman Cancer Institute.
Brett Froeliger, Medical University of South Carolina.
Matthew O. Howard, University of North Carolina at Chapel Hill.
References
- Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011;35:1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Augustus Diggs H, Froeliger B, Carlson JM, Gilbert DG. Smoker–nonsmoker differences in neural response to smoking-related and affective cues: An fMRI investigation. Psychiatry Res: Neuroimaging. 2013;211:85–87. doi: 10.1016/j.pscychresns.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufman PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Subst Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136–136. [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmines EG, McIver JP. Analyzing models with unobserved variables: Analysis of covariance structures. Social measurement. 1981 Current issues:65–115. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS. Brief Pain Inventory–Short Form (BPI–SF) Houston, TX: 1994. [Google Scholar]
- Cultbertson C, Nicholas S, Zaharovits I, London ED, De La Garza R, Brody AL, Newton TF. Methamphetamine craving induced in an online virtual reality environment. Pharmacol Biochem Behav. 2010;96:454–460. doi: 10.1016/j.pbb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, White W. The concept of recovery as an organizing principle for integrating mental health and addiction services. J Beh Health Services. 2007;34:1094–3412. doi: 10.1007/s11414-007-9053-7. [DOI] [PubMed] [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Bio Psychol. 2007;74:286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos A, Sarstedt M, Fuchs C, Wilczynski P, Kaiser S. Guidelines for choosing between multi-item and single-item scales for construct measurement: a predictive validity perspective. J Acad Marketing Sci. 2012;40:434–449. [Google Scholar]
- Donchin E. Cognitive psychophysiology: Event-related potentials and the study of cognition. Lawrence Erlbaum & Associates; Hilldale, New Jersey: 1984. [Google Scholar]
- Erblich J, Bovbjerg DH, Sloan RP. Exposure to smoking cues: Cardiovascular and autonomic effects. Addict Behav. 2011;36:737–742. doi: 10.1016/j.addbeh.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL. Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain. NASW Press; Washington, D.C: 2013. [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013a doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams J, Howard MO. Mindfulness-Oriented Recovery Enhancement for chronic pain and prescription opioid misuse: Results from an early stage randomized controlled trial. J Consult Clin Psychol. 2014 doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in Psychiatry. 2013c;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Franken IH, Howard MO. Cue-elicited heart rate variability and attentional bias predict alcohol relapse following treatment. Psychopharmacol. 2012a;222:17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Passik S, Howard M. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J Behav Med. 2012b doi: 10.1007/s10865-012-9455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. 2012c;35:591–602. doi: 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord SA, Palsson OS, Garland EL, Faurot KR, Coble RS, Mann JD, Frey W, Leniek K, Whitehead WE. Mindfulness training reduces the severity of irritable bowel syndrome in women: Results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678–1688. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Fredrickson BL, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clin Psychol Rev. 2010a;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. J Psychoactive Drugs. 2010b;42:177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Drukker M, van Os J, Wichers M. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: A randomized controlled trial. J Consult Clin Psychol. 2011;79:618–628. doi: 10.1037/a0024595. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci USA. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cog Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Inagaki H, Kuwahara M, Tsubone H. Changes in autonomic control of heart associated with classical appetitive conditioning in rats. Exp Animals. 2005;54:61–69. doi: 10.1538/expanim.54.61. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical consideratoins and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Amer J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kirsh KL, Jass C, Bennett DS, Hagen JE, Passik SD. Initial development of a survey tool to detect issues of chemical coping in chronic pain patients. Palliat Support Care. 2007;5:219–226. doi: 10.1017/s1478951507000387. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford Press; New York: 1998. [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA. 2011;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Phil Trans Royal Soc B: Bio Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GD, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kreibig SD. Autonomic nervous system activation in emotion: A review. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, Thayer JF. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213–22. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention; Gainesville, FL: 1997a. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: PJ Lang, Simons RF, Balaban MT., editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997b. pp. 97–135. [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas A, Chi N, Lauzon NM, Bishop SF, Sun N, Tan H, Laviolette SR. Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. Eur J Neurosci. 2012;35:279–290. doi: 10.1111/j.1460-9568.2011.07943.x. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychol Med. 2007;37:1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. J Psychopharm. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch Gen Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- McGrath RE. Conceptual complexity and construct validity. J Pers Assess. 2005;85:112–124. doi: 10.1207/s15327752jpa8502_02. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FTY, Jansen A. Cephalic phase responses, craving, and food intake in normal subjects. Appetite. 2000;35:45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cog Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ratcliffe R. Methods of dealing with reaction-time outliers. Psychol Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Rawson R, McCann MJ. Counselor's Treatment Manual: Matrix Intensive Outpatient Treatment for People With Stimulant Use Disorders. DHHS Publication No. (SMA); 2006. [Google Scholar]
- Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Tiffany ST. Peak-provoked craving: An alternative to smoking cue-reactivity. Addiction. 2013;108:1030–1031. doi: 10.1111/j.1360-0443.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol Sci. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Schoth DE, Liossi C. Attentional bias toward pictorial representations of pain in individuals with chronic headache. Clin J Pain. 2010;26:244–250. doi: 10.1097/AJP.0b013e3181bed0f9. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shizgal P, Hyman SP. Motivational and addictive states. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum S, Hudspeth J, editors. Principles of Neural Science. 5th. McGraw-Hill; New York: 2013. [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Ann Rev Clin Psychol. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose ES, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JW. The clinical significance of drug craving. Ann NY Acad Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Weinberger AH, Grilo CM, Brownell KD, DiLeone RJ, Lampert R, Matlin SL, Yanagisawa K, McKee SA. Heightened vagal activity during high-calorie food presentation in obese compared with non-obese individuals—Results of a pilot study. Obes Res Clin Practice. 2013 doi: 10.1016/j.orcp.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: A longitudinal outcomes trial. J Pain. 2012;13:146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


