Abstract
Background
Nutrition is strongly linked with health outcomes in chronic kidney disease (CKD). However, few studies have examined relationships between dietary patterns and health outcomes in persons with CKD.
Study Design
Observational cohort study.
Setting & Participants
3,972 participants with CKD (defined as an estimated glomerular filtration rate < 60 ml/min/1.73 m2 or an albumin-creatinine ratio ≥30 mg/g at baseline) from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a prospective cohort study of 30,239 black and white adults at least 45 years of age.
Predictors
Five empirically derived dietary patterns identified via factor analysis: “Convenience” (Chinese and Mexican foods, pizza, other mixed dishes), “Plant-Based” (fruits, vegetables), “Sweets/Fats” (sugary foods), “Southern” (fried foods, organ meats, sweetened beverages), and “Alcohol/Salads” (alcohol, green-leafy vegetables, salad dressing).
Outcomes
All-cause mortality and end-stage renal disease (ESRD).
Results
A total of 816 deaths and 141 ESRD events were observed over approximately 6 years of follow-up. There were no statistically significant associations of Convenience, Sweets/Fats or Alcohol/Salads pattern scores with all-cause mortality after multivariable adjustment. In Cox regression models adjusted for sociodemographic factors, energy intake, co-morbidities, and baseline kidney function, higher Plant-Based pattern scores (indicating greater consistency with the pattern) were associated with lower risk of mortality (HR comparing fourth to first quartile, 0.77; 95%CI, 0.61–0.97) whereas higher Southern pattern scores were associated with greater risk of mortality (HR comparing fourth to first quartile, 1.51; 95%CI, 1.19–1.92). There were no associations of dietary patterns with incident ESRD in multivariable-adjusted models.
Limitations
Missing dietary pattern data, potential residual confounding from lifestyle factors.
Conclusions
A Southern dietary pattern rich in processed and fried foods was independently associated with mortality in persons with CKD. In contrast, a diet rich in fruits and vegetables appeared to be protective.
INDEX WORDS: dietary pattern, kidney failure, chronic kidney disease (CKD), disease progression, nutrition, mortality risk, modifiable risk factor
Nutrition plays a vital role in chronic kidney disease (CKD) outcomes. Dietary interventions focused on reducing the consumption of nutrients such as protein, sodium and phosphorus have been linked to improved outcomes in CKD patients, particularly those with moderate to severe disease.1 Additionally, anemia, metabolic acidosis and disorders of bone and mineral metabolism—all common complications of CKD associated with excess risk of mortality 2–7—can be mechanistically linked, either directly or indirectly, with metabolic or nutritional disturbances related to kidney disease.
Despite the strong link between nutrition and health in CKD, relatively few studies examined relationships between dietary intake and clinical outcomes in persons with CKD. Further, the studies that do exist almost exclusively focused on the intake of individual macronutrients (e.g., protein)8 or micronutrients (e.g., sodium, phosphorus),9, 10 whereas much less is known about the associations of dietary patterns with clinical outcomes in CKD. This is noteworthy in that individuals do not typically eat macro- or micronutrients in isolation, but instead consume foods consisting of a wide variety of nutrients that interact biologically and have important synergistic effects.11
Dietary patterns can be examined using a priori dietary scores derived from predefined patterns of eating behavior (e.g., Healthy Eating Index, Mediterranean Diet Score) or by using a posteriori, data-driven methods.12 This latter methodology has the advantage of not making any assumptions about diet quality based upon contemporary notions of diet-disease relationships but instead describes patterns of food consumption based on actual foods consumed within a particular population.12, 13 To our knowledge, no prior studies have examined associations of dietary patterns with risk of death or development of end-stage renal disease (ESRD) in a large prospective cohort of individuals with CKD. Accordingly, we examined associations of empirically derived dietary patterns with risk of mortality and incident ESRD in persons with CKD from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study, a prospective cohort of black and white US adults. Our primary hypothesis was that patterns of eating consistent with Westernized diets (high intake of processed meats, saturated fats, refined grains, and sweetened beverages) would be associated with increased risk of all-cause mortality and ESRD, whereas patterns of eating characterized by high intake of nutrient-rich items such as fruits, vegetables, lean proteins, and whole grains would be associated with lower risk of all-cause mortality and ESRD.
METHODS
Study Population and Participants
The REGARDS study is a population-based investigation of stroke incidence in black and white US adults aged 45 years or older. Details of the study design have been reviewed elsewhere.14 Briefly, the study was designed to provide approximately equal representation of men and women and oversampled individuals who were black as well as individuals living in eight Southeastern US states that have disproportionately higher stroke mortality than the rest of the United States, termed the “stroke buckle” (coastal plain regions of Georgia, North Carolina and South Carolina) and “stroke belt” (the remaining regions of Georgia, North Carolina and South Carolina and the states of Tennessee, Mississippi, Alabama, Louisiana, and Arkansas). Potential participants were identified from commercially available lists of residents and recruited through an initial mailing followed by telephone contacts. Trained personnel conducted computer-assisted telephone interviews to obtain information including participants’ sociodemographics, cardiovascular risk factors, cigarette smoking, physical activity, and use of medications. Following this call, trained health professionals conducted an in-home study visit that included an electrocardiographic recording, inventory of medications and collection of fasting blood and urine samples. Several questionnaires, including the 1998 Block food frequency questionnaire (Block 98 FFQ, NutritionQuest, Berkeley, CA), were left with participants to be completed after the in-home visit and mailed back to the study center. Overall, 30,239 black and white adults were enrolled between January 2003 and October 2007. The REGARDS study protocol was approved by the Institutional Review Boards governing research in human subjects at participating centers and all participants provided informed consent.
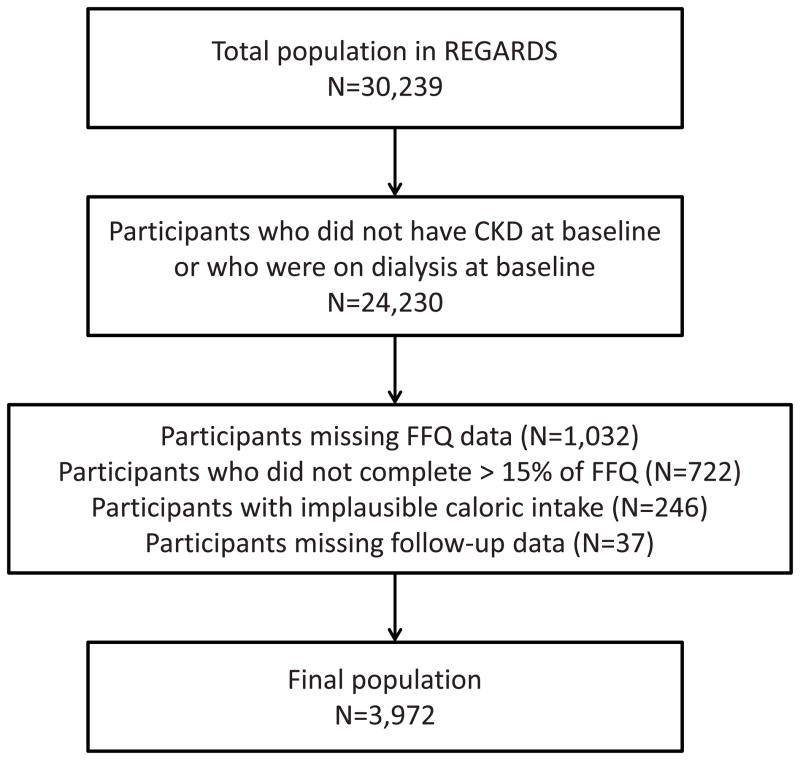
For this study, we limited the analysis to participants with CKD (defined as an estimated glomerular filtration rate [eGFR] < 60 ml/min/1.73 m2 or a urine albumin-creatinine ratio [ACR] ≥ 30 mg/g) who were not receiving renal replacement therapy at baseline (n= 6,009) (Figure 1). Of these participants, we excluded those missing follow-up data (n=37), or who did not return the FFQ (1,032), did not complete >15% of the FFQ (722) or had caloric intakes outside of the range deemed plausible (defined as 500–4500 kcal/day for women and 800–5000 kcal/day for men; n=246). This left 3,972 participants in the analyzed sample.
Figure 1.
Flow diagram indicating derivation of final analyzed study sample.
Dietary Assessment
Dietary data were collected using the Block 98 FFQ, a semi-quantitative, 110-item FFQ that assesses usual dietary intake over the past year, including frequency of consumption (average times per day, week, or month) and the portion size of specific foods or beverages (e.g., ½ cup of carrots, 2 slices of bacon), as described in detail elsewhere.15 The FFQs were completed by participants at home and mailed to the study center, where they were checked for completeness and scanned. Scanned files were then sent to NutritionQuest for analysis of nutrient contents using proprietary algorithms.
Primary Predictors
The predictors of interest were empirically derived dietary pattern scores. Food and beverage questions from the FFQ were collapsed into 56 investigator-defined individual food groups (delineated in Table S1, available as online supplementary material). A principal components analysis was used to derive dietary patterns and factor loadings for each of the 56 individual food groups, applying varimax rotation to maintain uncorrelated factors and improve interpretability. On the basis of the scree test and eigenvalues ≥1.5, a five-factor solution was retained. In total, the five retained patterns explained ~24% of the total variance in the study population. All other factors explained less than 3% of the variance each and were not included in this analysis. Congruence by race, region and gender was confirmed across the five patterns by deriving the patterns separately in each sub-population. We calculated congruence (vector cosine) using the congruence coefficient as an index of similarity for the factors derived in factor analysis. We calculated this value as the sum of the products of the loadings divided by the square root of the sum of the squared products of the loadings. To further test the fit of each pattern, we initially derived the patterns in half the population using factor analysis and then confirmed the fit using confirmatory factor analysis in the other half. The final pattern scores were derived in the whole population.
The retained patterns (Convenience, Plant-Based, Sweets/Fats, Southern, Alcohol/Salads) were named according to the highest food group loadings within each factor (Table S1). In general, the Convenience dietary pattern was characterized by high factor loadings for Chinese and Mexican food, pasta dishes, pizza, soup and other mixed dishes including frozen or take-out meals; the Plant-Based pattern, by fruits, vegetables, and fish; the Sweets/Fats pattern, by desserts and carbohydrate-heavy items; the Southern dietary pattern, by organ meats, fried foods, sugar-sweetened beverages and greens typical of southern cuisines; and the Alcohol/Salads pattern, by alcohol, green-leafy vegetables, and salad dressing. A factor score for each of the patterns was calculated for each study participant by summing observed intakes of component food groups weighted by their respective factor loadings.
Ascertainment of Outcome
The outcomes of interest were all-cause mortality and incident ESRD. Mortality was ascertained from contact with proxies provided by the participant upon recruitment or during follow-up. The REGARDS study staff confirmed dates of death through the Social Security Death Index, death certificates, or the National Death Index. Incident ESRD was assessed via linkage with the US Renal Data System, a registry of ESRD that captures over 95% of all incident cases in the United States, through September 30, 2011. Follow-up time for each participant was calculated from the date of the in-home visit to the date of death, ESRD or last telephone follow-up, updated through September 30, 2011.
Covariates of Interest
Age, race, sex, smoking history, annual family income, and educational attainment were determined by self-report. Physical activity was assessed through a single question: “How many times per week do you engage in intense physical activity, enough to work up a sweat?” Waist circumference (in centimeters) was measured during the in-home visit using a tape measure midway between the lowest rib and the iliac crest with the participant standing. Hypertension was defined as a systolic blood pressure ≥ 140 mm Hg or a diastolic blood pressure ≥ 90 mm Hg, or a self-report of a prior diagnosis of hypertension or current use of anti-hypertensive medications. History of coronary artery disease was defined as having any of the following: electrocardiographic evidence of myocardial infarction, prior history of a cardiac procedure (coronary artery bypass surgery or percutaneous angioplasty), or self-reported history of myocardial infarction. Diabetes was defined as self-reported use of insulin or oral hypoglycemic agents, fasting blood glucose concentration of 126 mg/dL or higher, or a non-fasting blood glucose concentration of 200 mg/dL or higher. Serum creatinine was measured using isotope dilution mass spectrometry–traceable methods. The CKD Epidemiology Collaboration creatinine equation was used to calculate eGFR.16 Urine albumin was measured by nephelometry using the BNII ProSpec nephelometer (now Siemens AG), and urine creatinine was measured by the rate Jaffé method using the Modular-P chemistry analyzer (Roche/Hitachi, Basel, Switzerland).
Statistical Analyses
Descriptive statistics were used to examine baseline characteristics of participants with CKD across quartiles of each dietary pattern. Next, linear regression was used to determine trends in mean energy intake and energy-adjusted mean dietary nutrient intakes across quartiles of each dietary pattern, using tests for trend based upon linear regression with dietary pattern score as the continuous independent variable. Nutrient intakes were energy adjusted using general linear models.
Mortality and ESRD incidence rates were calculated by quartile of each dietary pattern. After confirming the proportionality of hazards, Cox regression models were used to estimate the hazard ratio (HR) of mortality as a function of each dietary pattern, separately, in sequential models. Model 1 was adjusted for age, race, sex, geographic region of residence (stroke belt, stroke buckle or other), and total energy intake. Model 2 was adjusted for variables in Model 1 plus lifestyle factors (self-reported frequency of exercise per week, current smoking), comorbidities (history of heart disease and hypertension), educational achievement (< vs. ≥ high school diploma), annual family income (< vs. ≥ $20,000/year), and natural log-transformed ACR and eGFR. In all Cox models, dietary patterns were analyzed in quartiles (with the lowest quartile serving as the referent group) and on a continuous scale. We used an identical analytical strategy to examine the association of dietary patterns with incident ESRD. In sensitivity analyses, we examined the association of dietary patterns with incident ESRD treating all-cause death as a competing risk. In addition, we examined for effect modification by age, race, gender and diabetes on risk of mortality and incident ESRD by testing the statistical significance (P < 0.1) of multiplicative interaction terms.
A two-tailed P value < 0.05 was considered statistically significant for all analyses other than the tests for interaction. All analyses were conducted using SAS software version 9.2 (SAS Institute Inc, Cary, NC).
RESULTS
Study Participants
In general, higher consumption of the Convenience and Alcohol/Salads dietary patterns (defined by greater proportion of participants in the fourth quartile as compared to the first) was associated with younger age, white race, male sex, higher income and residence outside the Southeastern United States (Table 1). Higher consumption of the Plant-Based dietary pattern was associated with older age, black race, female sex, and residence within the Southeastern United States. Higher consumption of the Southern pattern was associated with younger age, lower income, black race, male sex, residence in the Southeastern United States, current smoking, and diabetes. Higher consumption of the Sweets/Fats pattern was associated with white race, male sex, current smoking and lower prevalence of diabetes. With respect to indices of kidney function and inflammation at baseline, greater consumption of the Convenience, Southern and Alcohol/Salads patterns was associated with higher eGFR. Higher Southern pattern scores were also associated with higher median urinary ACR values.
Table 1.
Participant characteristics by quartiles of dietary pattern scores.
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Convenience | ||||
| Age (y) | 71.1 (0.3) | 69.6 (0.3) | 69.2 (0.3) | 66.3 (0.3)† |
| Black race | 49 | 39 | 31 | 28† |
| Male sex | 40 | 42 | 52 | 55† |
| Residence in Southeast|| | 60 | 57 | 55 | 49† |
| Income <$20,000/y | 28 | 26 | 20 | 21† |
| Current Smoker | 14 | 13 | 15 | 17* |
| Abdominal obesity‡ | 54 | 54 | 51 | 57 |
| Physical activity, none | 41 | 41 | 40 | 40 |
| Diabetes | 34 | 33 | 29 | 35 |
| eGFR (ml/min/1.73 m2) | 64.6 (0.7) | 67.8 (0.8) | 68.1 (0.8) | 72.2 (0.8) † |
| ACR (mg/g) | 43.2 [13.0–115.8] | 39.3 [10.5–87.6] | 41.8 [11.4–100.0] | 44.1 [15.5–103.4] |
| Plant Based | ||||
| Age (y) | 67.1 (0.3) | 69.5 (0.3) | 69.8 (0.3) | 69.8 (0.3)† |
| Black race | 30 | 37 | 38 | 43† |
| Male sex | 55 | 50 | 45 | 39† |
| Residence in Southeast|| | 54 | 57 | 54 | 55 |
| Income <$20,000/y | 19 | 21 | 24 | 20 |
| Current Smoker | 25 | 12 | 12 | 9† |
| Abdominal obesity‡ | 53 | 56 | 52 | 55 |
| Physical activity, none | 49 | 42 | 39 | 32† |
| Diabetes | 30 | 33 | 34 | 33 |
| eGFR (ml/min/1.73 m2) | 68.1 (0.8) | 67.3 (0.8) | 66.4 (0.8) | 70.1 (0.8) |
| ACR (mg/g) | 42.4 [12.9–103.1] | 42.9 [11.0–107.6] | 39.4 [11.0–94.5] | 43.3 [15.8–101.8] |
| Sweets/Fats | ||||
| Age (y) | 67.9 (0.3) | 69.8 (0.3) | 69.7 (0.3) | 68.7 (0.3) |
| Black race | 49 | 39 | 30 | 31† |
| Male sex | 41 | 44 | 51 | 53† |
| Residence in Southeast|| | 53 | 54 | 55 | 58 |
| Income <$20,000/y | 21 | 21 | 21 | 22 |
| Current Smoker | 14 | 12 | 14 | 18* |
| Abdominal obesity‡ | 58 | 53 | 53 | 51* |
| Physical activity, none | 38 | 41 | 39 | 45* |
| Diabetes | 36 | 35 | 31 | 28† |
| eGFR (ml/min/1.73 m2) | 69.6 (0.8) | 67.5 (0.8) | 65.8 (0.8) | 69.7 (0.8) |
| ACR (mg/g) | 43.4 [13.8–114.3] | 40.9 [12.1–98.3] | 39.4 [9.9–102.9] | 43.9 [14.2–96.1] |
| Southern | ||||
| Age (y) | 70.2 (0.3) | 70.0 (0.3) | 69.3 (0.3) | 66.6 (0.3)† |
| Black race | 11 | 31 | 44 | 62† |
| Male sex | 42 | 41 | 50 | 55† |
| Residence in Southeast|| | 49 | 53 | 57 | 61† |
| Income <$20,000/y | 14 | 18 | 22 | 31† |
| Current Smoker | 9 | 11 | 18 | 20† |
| Abdominal obesity‡ | 44 | 56 | 54 | 64† |
| Physical activity, none | 40 | 41 | 41 | 41 |
| Diabetes | 24 | 33 | 33 | 41† |
| eGFR (ml/min/1.73 m2) | 67.1 (0.7) | 66.2 (0.8) | 67.6 (0.8) | 71.8 (0.9)† |
| ACR (mg/g) | 36.9 [9.6–70.7] | 37.1 [10.3–90.8] | 45.3 [13.9–112.5] | 53.3 [20.6–146.6]† |
| Alcohol/Salads | ||||
| Age (y) | 69.8 (0.3) | 69.6 (0.3) | 68.9 (0.3) | 67.8 (0.3)† |
| Black race | 53 | 42 | 31 | 22† |
| Male sex | 38 | 42 | 51 | 56† |
| Residence in Southeast|| | 58 | 56 | 56 | 49† |
| Income <$20,000/y | 29 | 24 | 19 | 12† |
| Current Smoker | 12 | 14 | 15 | 18† |
| Abdominal obesity‡ | 54 | 54 | 55 | 54 |
| Physical activity, none | 42 | 42 | 42 | 38 |
| Diabetes | 33 | 33 | 34 | 30 |
| eGFR (ml/min/1.73 m2) | 67.1 (0.8) | 65.9 (0.8) | 68.0 (0.8) | 71.4 (0.7)† |
| ACR (mg/g) | 41.2 [11.6–96.6] | 40.2 [11.0–108.2] | 41.1 [11.9–96.9] | 43.3 [15.8–105.1] |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range]. For each pattern, Q1 represents least consistency with pattern whereas Q4 represents most consistency with pattern. Tests for trend based upon Cochran-Armitage trend test for categorical variables and linear regression with dietary pattern score (continuous) as the independent variable for continuous variables.
Abbreviations: eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; Q, quartile
P for trend < 0.05
P for trend < 0.001
Abdominal obesity defined as a waist circumference ≥ 88 cm for women and ≥ 102 cm for men.
Indicates persons living in US stroke belt or stroke buckle states.
Dietary Patterns and Nutrient Characteristics
Participants with higher scores for the Convenience, Sweets, Southern and Alcohol/Salads patterns consumed higher amounts of total fat and saturated fat as a percentage of total energy intake (Table 2). Higher scores for the Convenience and Alcohol/Salads patterns were also associated with higher intake of protein, whereas higher scores for the Southern and Sweets patterns were associated with the lower protein intake. Participants with higher scores for the Plant-Based pattern consumed lower amounts of total fat, saturated fats, mono-unsaturated fats and trans fats, and higher amounts of fiber than participants with lower scores.
Table 2.
Nutrient intakes by quartile of dietary pattern scores.
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Convenience | ||||
| Energy (kJ) | 1428 (18) | 1405 (18) | 1630 (19) | 2200 (25) † |
| % from Total fat | 36.2 (0.3) | 36.5 (0.2) | 37.4 (0.2) | 38.2 (0.2) † |
| % from Saturated Fat | 10.5 (0.1) | 10.6 (0.1) | 10.7 (0.1) | 11.1 (0.1) † |
| % from Protein | 13.0 (0.1) | 14.2 (0.1) | 14.9 (0.1) | 15.9 (0.1) † |
| % from Carbohydrates | 52.4 (0.3) | 49.1 (0.3) | 47.9 (0.3) | 46.1 (0.2) † |
| % from Alcohol | 1.6 (0.1) | 2.8 (0.2) | 2.7 (0.2) | 2.5 (0.2)† |
| Polyunsaturated fatty acid (g) | 18.6 (0.2) | 18.1 (0.2) | 18.2 (0.2) | 17.3 (0.2) † |
| Monounsaturated fatty acid (g) | 25.4 (0.2) | 26.1 (0.2) | 26.5 (0.2) | 26.9 (0.2) † |
| Trans Fat (g) | 5.7 (0.1) | 5.9 (0.1) | 5.9 (0.1) | 5.7 (0.1) |
| Fiber (g) | 15.1 (0.2) | 14.8 (0.2) | 15.4 (0.2) | 16.0 (0.2) † |
| Plant Based | ||||
| Energy (kJ) | 1490 (22) | 1484 (19) | 1635 (20) | 2049 (25) † |
| % from Total fat | 38.5 (0.2) | 37.5 (0.2) | 37.1 (0.2) | 35.4 (0.2) † |
| % from Saturated Fat | 11.7 (0.1) | 11.0 (0.1) | 10.5 (0.1) | 9.7 (0.1) † |
| % from Protein | 13.5 (0.1) | 14.3 (0.1) | 14.7 (0.1) | 15.4 (0.1) † |
| % from Carbohydrates | 46.8 (0.1) | 48.6 (0.2) | 49.5 (0.3) | 51.2 (0.3) † |
| % from Alcohol | 4.0 (0.2) | 2.4 (0.2) | 1.8 (0.1) | 1.3 (0.1)† |
| Polyunsaturated fatty acid (g) | 17.6 (0.2) | 18.1 (0.2) | 18.7 (0.2) | 17.8 (0.2) |
| Monounsaturated fatty acid (g) | 27.8 (0.2) | 26.9 (0.2) | 26.2 (0.2) | 23.9 (0.2) † |
| Trans Fat (g) | 6.6 (0.1) | 6.3 (0.1) | 5.8 (0.1) | 4.6 (0.1) † |
| Fiber (g) | 10.8 (0.2) | 13.3 (0.2) | 15.9 (0.2) | 21.3 (0.2) † |
| Sweets/Fats | ||||
| Energy (kJ) | 1250 (18) | 1395 (15) | 1679 (16) | 2337 (23) † |
| % from Total fat | 34.7 (0.3) | 36.7 (0.2) | 37.9 (0.2) | 39.2 (0.2) † |
| % from Saturated Fat | 9.9 (0.1) | 10.6 (0.1) | 10.9 (0.1) | 11.4 (0.1) † |
| % from Protein | 15.9 (0.1) | 14.6 (0.1) | 14.2 (0.1) | 13.2 (0.1) † |
| % from Carbohydrates | 48.4 (0.3) | 49.5 (0.3) | 49.6 (0.3) | 48.9 (0.3) * |
| % from Alcohol | 4.6 (0.3) | 2.3 (0.2) | 1.4 (0.1) | 1.2 (0.1) † |
| Polyunsaturated fatty acid (g) | 17.0 (0.2) | 17.9 (0.2) | 18.2 (0.2) | 19.1 (0.2) † |
| Monounsaturated fatty acid (g) | 25.4 (0.2) | 25.9 (0.2) | 26.6 (0.2) | 27.1 (0.2) † |
| Trans Fat (g) | 4.5 (0.1) | 5.2 (0.1) | 5.9 (0.1) | 7.6 (0.1) † |
| Fiber (g) | 16.4 (0.2) | 16.2 (0.2) | 15.6 (0.2) | 13.2 (0.2) † |
| Southern | ||||
| Energy (kJ) | 1567 (19) | 1377 (17) | 1584 (19) | 2144 (26) † |
| % from Total fat | 35.3 (0.2) | 36.9 (0.2) | 37.5 (0.2) | 38.8 (0.2) † |
| % from Saturated Fat | 10.0 (0.1) | 10.6 (0.1) | 10.9 (0.1) | 11.4 (0.1) † |
| % from Protein | 15.0 (0.1) | 14.4 (0.1) | 14.1 (0.1) | 14.4 (0.1) * |
| % from Carbohydrates | 50.7 (0.3) | 49.6 (0.3) | 48.9 (0.3) | 47.0 (0.3) † |
| % from Alcohol | 2.7 (0.2) | 2.3 (0.2) | 2.4 (0.2) | 2.2 (0.2) * |
| Polyunsaturated fatty acid (g) | 17.4 (0.2) | 18.4 (0.2) | 18.3 (0.2) | 18.1 (0.2) † |
| Monounsaturated fatty acid (g) | 25.5 (0.2) | 26.6 (0.2) | 26.6 (0.2) | 26.3 (0.2) † |
| Trans Fat (g) | 5.7 (0.1) | 5.9 (0.1) | 5.9 (0.1) | 5.6 (0.1) |
| Fiber (g) | 18.3 (0.2) | 16.1 (0.2) | 14.7 (0.2) | 12.2 (0.2) † |
| Alcohol/Salads | ||||
| Energy (kJ) | 1595 (23) | 1483 (19) | 1610 (19) | 1969 (25) † |
| % from Total fat | 33.2 (0.2) | 36.4 (0.2) | 38.1 (0.2) | 40.8 (0.3) † |
| % from Saturated Fat | 9.5 (0.1) | 10.5 (0.1) | 10.9 (0.1) | 11.8 (0.1) † |
| % from Protein | 14.0 (0.1) | 14.2 (0.1) | 14.7 (0.1) | 14.9 (0.1) † |
| % from Carbohydrates | 54.9 (0.3) | 51.1 (0.3) | 47.9 (0.3) | 42.4 (0.3) † |
| % from Alcohol | 0.5 (0.1) | 0.9 (0.1) | 2.5 (0.2) | 5.5 (0.3) † |
| Polyunsaturated fatty acid (g) | 15.8 (0.2) | 17.7 (0.2) | 18.6 (0.2) | 20.1 (0.2) † |
| Monounsaturated fatty acid (g) | 24.0 (0.2) | 25.9 (0.2) | 26.6 (0.2) | 28.4 (0.2) † |
| Trans Fat (g) | 6.3 (0.1) | 6.1 (0.1) | 5.7 (0.1) | 4.9 (0.1) † |
| Fiber (g) | 16.4 (0.2) | 15.2 (0.2) | 15.2 (0.2) | 14.6 (0.2) † |
Note: Values are given as mean ± standard error. Intakes of each nutrient are adjusted for total caloric intake except for percent energy intake from fat, protein, carbohydrates, and saturated fat. For each individual pattern, quartile (Q)1 represents least consistency with pattern whereas Q4 represents most consistency with pattern. Tests for trend based upon linear regression with dietary pattern score (continuous) as the independent variable.
P for linear trend < 0.05
P for linear trend < 0.001;
Dietary Pattern Scores and Mortality
A total of 816 deaths were observed over a mean 6.5 years of follow-up. There were no statistically significant associations between Convenience, Sweets/Fats or Alcohol/Salads pattern scores and HRs of mortality in models adjusted for age, gender, race, geographic region of residence, energy intake, lifestyle factors, comorbidities, education, income, ACR, and eGFR (Table 3). As compared to the lowest quartile of Plant-Based pattern scores, the highest quartile was associated with lower risk of mortality in the fully-adjusted model (HR, 0.77; 95% confidence interval [CI], 0.61–0.97). There was a graded association between higher Southern pattern scores and higher risk of all-cause mortality, such that in the fully-adjusted model, participants in the highest quartile had 1.5-fold higher risk of death as compared to participants in the lowest quartile (HR, 1.51; 95% CI, 1.19–1.92). Similar results were observed when modeling dietary pattern scores as continuous variables. These associations were not modified by age, gender, race or diabetes status (P for interaction > 0.1 for all).
Table 3.
Incidence rates and HRs of mortality as a function of dietary pattern scores.
| Q1 | Q2 | Q3 | Q4 | Per 1-U increase in pattern score | |
|---|---|---|---|---|---|
| Convenience | |||||
| No. of events | 234 | 199 | 198 | 185 | |
| IR | 42.89 (37.74, 48.76) | 37.43 (32.58, 43.01) | 36.45 (31.71, 41.90) | 32.80 (28.40, 37.89) | |
| HR Model 1 | reference | 0.94 (0.78, 1.14) | 0.89 (0.73, 1.08) | 0.92 (0.74, 1.14) | 0.94 (0.86, 1.03) |
| HR Model 2 | reference | 1.00 (0.82, 1.22) | 0.95 (0.78, 1.16) | 0.97 (0.77, 1.21) | 0.96 (0.88, 1.05) |
| Plant Based | |||||
| No. of events | 216 | 213 | 209 | 178 | |
| IR | 40.32 (35.28, 46.07) | 39.24 (34.31, 44.88) | 38.44 (33.57, 44.03) | 31.67 (27.35, 36.69) | |
| HR Model 1 | 1.00 (reference) | 0.84 (0.69, 1.02) | 0.87 (0.71, 1.05) | 0.68 (0.55, 0.85) | 0.86 (0.79, 0.94) |
| HR Model 2 | 1.00 (reference) | 0.93 (0.76, 1.13) | 0.96 (0.78, 1.17) | 0.77 (0.61, 0.97) | 0.96 (0.83, 0.99) |
| Sweets/Fats | |||||
| No. of events | 180 | 208 | 204 | 224 | |
| IR | 32.93 (28.46, 38.11) | 38.16 (33.31, 43.71) | 37.04 (32.39, 42.49) | 41.24, 36.27, 47.13) | |
| HR Model 1 | 1.00 (reference) | 1.03 (0.84, 1.26) | 0.98 (0.79, 1.20) | 1.13 (0.89, 1.43) | 1.01 (0.92, 1.11) |
| HR Model 2 | 1.00 (reference) | 1.07 (0.87, 1.31) | 0.97 (0.78, 1.21) | 1.09 (0.86, 1.40) | 0.98 (0.89, 1.09) |
| Southern | |||||
| No. of events | 164 | 189 | 229 | 234 | |
| IR | 28.33 (24.31, 33.02) | 34.42 (29.86, 39.71) | 42.39 (37.24, 48.25) | 45.33 (39.87, 51.52) | |
| HR Model 1 | 1.00 (reference) | 1.23 (0.99, 1.52) | 1.44 (1.17, 1.77) | 1.78 (1.42, 2.24) | 1.28 (1.18, 1.39) |
| HR Model 2 | 1.00 (reference) | 1.19 (0.96, 1.48) | 1.26 (1.02, 1.56) | 1.51 (1.19, 1.92) | 1.21 (1.11, 1.33) |
| Alcohol/Salads | |||||
| No. of events | 225 | 228 | 189 | 174 | |
| IR | 41.21 (36.16, 46.96) | 43.72 (38.40, 49.78) | 34.02 (29.05, 39.24) | 31.00 (26.72, 35.96) | |
| HR Model 1 | 1.00 (reference) | 1.09 (0.91, 1.32) | 0.86 (0.70, 1.04) | 0.81 (0.66, 0.99) | 0.91 (0.85, 0.98) |
| HR Model 2 | 1.00 (reference) | 1.09 (0.91, 1.33) | 0.91 (0.75, 1.12) | 0.83 (0.67, 1.03) | 0.94 (0.87, 1.01) |
Note: Unless otherwise indicated, values given as IR of mortality per 1,000 person-years of follow-up (95% CI) and HR of mortality (95% CI). For each pattern, Q1 represents least consistency with pattern whereas Q4 represents most consistency with pattern. Model 1 is adjusted for age, gender, race, geographic region of residence, and energy intake. Model 2 is adjusted for variables in Model 1 plus lifestyle factors (self-reported frequency of exercise per week, current smoking), comorbidities (history of heart disease and hypertension), educational achievement (< vs. ≥ high school diploma), annual family income (< vs. ≥ $20,000/y), and natural log-transformed urinary albumin-creatinine ratio, and estimated glomerular filtration rate.
Abbreviations: Q, quartile; IR, incidence rate; HR, hazard ratio.
Dietary Pattern Scores and Incident ESRD
Over a mean 6.4 years of follow-up, a total of 141 individuals initiated dialysis. There were no statistically significant associations of Convenience, Plant-Based, Sweets/Fats or Alcohol/Salads patterns with risk of ESRD in models adjusted for age, race, sex, geographic region of residence, and caloric intake, or in models further adjusted for socioeconomic and lifestyle factors, co-morbidities and baseline kidney function (Table 4). In models adjusted for age, race, sex, geographic region of residence, and caloric intake, higher Southern scores were associated with greater risk of incident ESRD when modeled as a continuous variable (HR per 1-unit increase in Southern pattern score, 1.27; 95%CI, 1.04–1.56); however, this association was no longer present in the fully-adjusted model (HR, 0.94; 95%CI, 0.76–1.17). Adjustment for eGFR and ACR were primarily responsible for this attenuation. In sensitivity analyses modeling all-cause death as a competing risk, the results did not change (Table S2).
Table 4.
Incidence rates and HRs of ESRD as a function of dietary pattern scores.
| Q1 | Q2 | Q3 | Q4 | Per 1-U increase in pattern score | |
|---|---|---|---|---|---|
| Convenience | |||||
| No. of events | 44 | 38 | 39 | 20 | |
| IR | 6.81 (5.07, 9.15) | 6.17 (4.48, 8.46) | 6.25 (4.56, 8.56) | 3.09 (1.99, 4.79) | |
| HR Model 1 | 1.00 (reference) | 0.95 (0.61, 1.47) | 1.07 (0.69, 1.67) | 0.60 (0.34, 1.08) | 0.91 (0.72, 1.14) |
| HR Model 2 | 1.00 (reference) | 1.37 (0.87, 2.16) | 1.31 (0.83, 2.06) | 0.73 (0.41, 1.31) | 1.01 (0.81, 1.26) |
| Plant Based | |||||
| No. of events | 44 | 34 | 31 | 32 | |
| IR | 6.97 (5.19, 9.36) | 5.35 (3.82, 7.50) | 4.88 (3.43, 6.94) | 5.05 (3.57, 7.14) | |
| HR Model 1 | 1.00 (reference) | 0.76 (0.48, 1.19) | 0.74 (0.46, 1.19) | 0.86 (0.52, 1.41) | 1.09 (0.89, 1.32) |
| HR Model 2 | 1.00 (reference) | 1.08 (0.67, 1.75) | 0.74 (0.45, 1.23) | 1.18 (0.71, 1.98) | 1.19 (0.97, 1.48) |
| Sweets/Fats | |||||
| No. of events | 45 | 32 | 40 | 24 | |
| IR | 7.17 (5.35, 9.61) | 5.07 (3.58, 7.16) | 6.32 (4.63, 8.62) | 3.73 (2.50, 5.57) | |
| HR Model 1 | 1.00 (reference) | 0.88 (0.56, 1.39) | 1.36 (0.86, 2.15) | 0.99 (0.54, 1.82) | 1.09 (0.87, 1.37) |
| HR Model 2 | 1.00 (reference) | 0.89 (0.55, 1.45) | 1.29 (0.79, 2.09) | 0.99 (0.52, 1.93) | 1.15 (0.87, 1.51) |
| Southern | |||||
| No. of eents | 20 | 36 | 43 | 42 | |
| IR | 3.12 (2.01, 4.83) | 5.81 (4.19, 8.05) | 6.67 (4.94, 8.98) | 6.69 (4.95, 9.06) | |
| HR Model 1 | 1.00 (reference) | 1.35 (0.78, 2.37) | 1.30 (0.74, 2.28) | 1.25 (0.68, 2.30) | 1.27 (1.04, 1.56) |
| HR Model 2 | 1.00 (reference) | 1.40 (0.79, 2.47) | 1.02 (0.58, 1.79) | 0.73 (0.38, 1.42) | 0.94 (0.76, 1.17) |
| Alcohol/Salads | |||||
| No. of events | 42 | 44 | 33 | 22 | |
| IR | 6.56 (4.85, 8.88) | 7.12 (5.30, 9.57) | 5.17 (3.67, 7.27) | 3.44 (2.27, 5.23) | |
| HR Model 1 | 1.00 (reference) | 1.14 (0.74, 1.75) | 0.91 (0.57, 1.45) | 0.73 (0.42, 1.26) | 0.87 (0.72, 1.07) |
| HR Model 2 | 1.00 (reference) | 1.04 (0.67, 1.63) | 1.19 (0.74, 1.94) | 0.88 (0.50, 1.53) | 0.95 (0.76, 1.19) |
Note: Unless otherwise indicated, values given as IR of ESRD per 1,000 person-years of follow-up (95% CI) and HR of ESRD (95% CI). For each pattern, Q1 represents least consistency with pattern whereas Q4 represents most consistency with pattern. Model 1 is adjusted for age, gender, race, geographic region of residence, and energy intake. Model 2 is adjusted for variables in Model 1 plus lifestyle factors (self-reported frequency of exercise per week, current smoking), comorbidities (history of heart disease and hypertension), educational achievement (< vs. ≥ high school diploma), annual family income (< vs. ≥ $20,000/y), and natural log-transformed urinary albumin-creatinine ratio, and estimated glomerular filtration rate.
Abbreviations: ESRD, end-stage renal disease; Q, quartile; IR, incidence rate; HR, hazard ratio.
DISCUSSION
In participants with CKD from this large national cohort, higher scores for a dietary pattern characterized by fried foods, organ meats and sweetened beverages—foods commonly found in Southern cuisines—were independently associated with higher risk of mortality. In contrast, higher consumption of a diet rich in fish, fruits and vegetables was associated with lower mortality risk over time.
Prior studies have reported associations of dietary patterns with indices of kidney health. A cross-sectional analysis of 5,042 participants of the Multi-Ethnic Study of Atherosclerosis (MESA) showed that greater consistency with a diet pattern characterized by high intake of whole grains, fruit and vegetables was associated with lower prevalence of microalbuminuria independently of known risk factors.17 Consistent with these results, prior data from REGARDS showed higher estimated sodium and saturated fat intake to be associated with higher albuminuria.18, 19 In addition, a prospective study of 3,121 participants from the Nurses’ Health Study showed that a Dietary Approaches to Stop Hypertension (DASH)–style diet was independently associated with lower odds of kidney function decline.20 However, to our knowledge, no prior study has specifically examined associations of dietary patterns with death and incident ESRD in persons with CKD. Our study helps to address this gap by showing that a Southern pattern characterized by higher intake of fried foods, processed meats and sugar-sweetened beverages was associated with increased mortality risk, whereas a Plant-Based diet was associated with lower mortality risk in individuals with CKD.
In addition to demonstrating that excess consumption of processed and fried foods impairs survival while high intake of fruits and vegetables appears to be protective in CKD patients, these findings have important potential clinical implications. Current CKD practice guidelines for nutrition generally focus on limiting the consumption of specific macronutrients (saturated fat, protein) or micronutrients (sodium, phosphorus) in patients with CKD.21 However, individuals do not typically consume just these macro- or micronutrients, making it challenging for the average patient to comply with dietary restrictions such as reducing protein intake, especially when adjustments to the intake of multiple other nutrients are required at the same time. Based upon the data in this study, an alternative or complementary approach may be to focus on modifying general patterns of eating, which may be easier for patients to conceptualize, and therefore actualize, with potential benefits for cardiovascular health and survival in CKD patients.
Although we found independent associations of Southern and Plant-Based pattern scores with mortality risk, we observed no statistically significant associations of any pattern scores with risk of ESRD. There may be several reasons for this. First, given the relatively modest number of ESRD events, it is possible that we were underpowered to detect associations of these patterns with incident ESRD. Second, it is possible that indices of kidney disease severity were in the causal pathway between certain patterns and development of ESRD. Adjustment for baseline eGFR and urinary ACR was primarily responsible for attenuating the association of Southern pattern score with incident ESRD, suggesting that kidney injury may be an intermediate between consumption of a Southern dietary pattern and risk of ESRD. Future studies with repeated measures of eGFR and ACR and ESRD follow-up are needed to tease out these possibilities.
Participants with high Southern dietary pattern scores predominantly lived in the stroke belt and stroke buckle, areas of the Southeastern United States with disproportionately high rates of not only stroke but a number of other chronic disease conditions including cognitive impairment and diabetes.22–24 Higher Southern dietary pattern scores were also marked by disparities in demographic, socioeconomic and clinical characteristics, such that, as compared to individuals in the lowest quartile of scores, individuals in the highest quartile were far more likely to be younger, black, male, have low socioeconomic status, and have obesity and diabetes. These results provide novel insights into regional and socio-demographic factors that may impact diet intake and, as a consequence, mortality risk in persons with CKD and perhaps the general US population as well.
Our study has several limitations. As is the case for any study relying on FFQ data, dietary reporting errors may have reduced the accuracy of individual dietary intake measurements and resulted in misclassification.25 However, this would generally bias results towards the null, potentially reducing the magnitude of the observed associations between dietary patterns and mortality. Next, roughly one third of the cohort did not return the FFQ. Those who did not return the FFQ were more likely to have annual incomes less than $20,000 and less than a high school education.15 However, we still had approximately 900 people of lower socioeconomic status and did not find evidence that those returning the FFQ in this group were different in terms of race, age, gender or history of cardiovascular disease from those who returned the FFQ, even when restricted to individuals who died or developed ESRD during follow-up, so we do not believe this biased the results substantially. Next, although we adjusted for available lifestyle factors such as cigarette smoking and physical activity, we cannot exclude residual confounding from other unmeasured, non-dietary lifestyle, cultural, and regional factors that may be linked with dietary habits and may partly influence these associations. Further, we also could not account for potential genetic factors that may influence these relationships. Beyond their known anti-inflammatory and antioxidant effects, high intake of alkali-inducing fruits and vegetables may have specific benefits in individuals with CKD by attenuating the deleterious effect of acid retention on endothelin and aldosterone secretion.26, 27 Since we did not have any measures of acid-base status, we could not evaluate this possibility in this study. Finally, while the use of empirically derived diet patterns has the advantage of identifying patterns of eating behavior specific to the population being studied, it is possible that this may have also reduced the generalizability of patterns to other populations, particularly those living outside the United States.
In conclusion, a Southern dietary pattern characterized by high intake of fried foods, organ meats, and sweetened beverages, and by disproportionately high residence in the Southeastern United States, was independently associated with increased risk of mortality in individuals with CKD. In contrast, a Plant-Based dietary pattern high in fruits, vegetables and fish was associated with lower risk of mortality. These data underscore the vital role of nutrition in optimizing CKD outcomes and provide novel insights into dietary factors that may contribute to geographic and racial disparities in kidney disease risk in the United States.
Supplementary Material
Table S1: Food group factor loadings for 5 empirically derived dietary patterns.
Table S2: HRs of ESRD as function of dietary pattern scores accounting for competing risk of death.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Support: This study was supported by a cooperative agreement (grant U01 NS041588) from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH). OMG was supported by NIH grants K23DK081673 (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]), R03DK095005 (NIDDK), and R01NS080850 (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS, the NIDDK, or the NIH. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation of the manuscript. The manuscript was sent to Amgen Corporation for review prior to submission for publication.
Footnotes
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Descriptive Text for Online Delivery of Supplementary Material
Food group factor loadings for 5 empirically derived dietary patterns.
HRs of ESRD as function of dietary pattern scores accounting for competing risk of death.
Financial Disclosure: DGW is a member of the Amgen National Nephrology Advisory Board. OMG, PM, WMM, and DGW have received research support from the Amgen Corporation. The other authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50(2):343–357. doi: 10.1038/ki.1996.323. [DOI] [PubMed] [Google Scholar]
- 2.Ma JZ, Ebben J, Xia H, Collins AJ. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10(3):610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 3.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63(5):1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24(4):1232–1237. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navaneethan SD, Schold JD, Arrigain S, et al. Serum bicarbonate and mortality in stage 3 and stage 4 chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(10):2395–2402. doi: 10.2215/CJN.03730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon V, Kopple JD, Wang X, et al. Effect of a very low-protein diet on outcomes: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2009;53(2):208–217. doi: 10.1053/j.ajkd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Lynch KE, Lynch R, Curhan GC, Brunelli SM. Prescribed dietary phosphate restriction and survival among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(3):620–629. doi: 10.2215/CJN.04620510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mc Causland FR, Waikar SS, Brunelli SM. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney Int. 2012 doi: 10.1038/ki.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Cin Nutr. 2003;78(3 Suppl):508S–513S. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62(5):177–203. doi: 10.1301/nr.2004.may.177-203. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 15.Newby PK, Noel SE, Grant R, Judd S, Shikany JM, Ard J. Race and region are associated with nutrient intakes among black and white men in the United States. J Nutr. 2011;141(2):296–303. doi: 10.3945/jn.110.130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR., Jr Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;87(6):1825–1836. doi: 10.1093/ajcn/87.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaron KJ, Campbell RC, Judd SE, Sanders PW, Muntner P. Association of dietary sodium and potassium intakes with albuminuria in normal-weight, overweight, and obese participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Clin Nutr. 2011;94(4):1071–1078. doi: 10.3945/ajcn.111.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Judd S, Le A, et al. Associations of dietary fat with albuminuria and kidney dysfunction. Am J Clin Nutr. 2010;92(4):897–904. doi: 10.3945/ajcn.2010.29479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: a subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis. 2011;57(2):245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2000;35 (suppl 2):S1–140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 22.Barker LE, Kirtland KA, Gregg EW, Geiss LS, Thompson TJ. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40(4):434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Howard G, Labarthe DR, Hu J, Yoon S, Howard VJ. Regional differences in African Americans’ high risk for stroke: the remarkable burden of stroke for Southern African Americans. Ann Epidemiol. 2007;17(9):689–696. doi: 10.1016/j.annepidem.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadley VG, Unverzagt FW, McGuire LC, et al. Incident cognitive impairment is elevated in the stroke belt: the REGARDS study. Ann Neurol. 2011;70(2):229–236. doi: 10.1002/ana.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett W. Nutritional epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 26.Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77(7):617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 27.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010;78(11):1128–1135. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Food group factor loadings for 5 empirically derived dietary patterns.
Table S2: HRs of ESRD as function of dietary pattern scores accounting for competing risk of death.