Abstract
Background
Decreased glomerular filtration rate (GFR) leads to reduced production of 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3 (25(OH)D3). Effects of low GFR on vitamin D catabolism are less well understood. We tested associations of estimated GFR (eGFR) with the circulating concentration of 24,25-dihydroxyvitamin D3 (24,25(OH)2D3), the most abundant product of 25(OH)D3 catabolism, across populations with a wide range of GFR.
Study Design
Cross-sectional study.
Setting & Participants
9596 participants in 5 cohort studies and clinical trials: the Diabetes Control and Complications Trial (N=1193), Multi-Ethnic Study of Atherosclerosis (N=6470), Cardiovascular Health Study (N=932), Seattle Kidney Study (N=289), and Hemodialysis Study (N=712).
Predictor
eGFR.
Outcome
Circulating 24,25(OH)2D3 concentration.
Measurements
GFR was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation. Vitamin D metabolites were measured by mass spectrometry.
Results
Circulating 24,25(OH)2D3 concentration was correlated with circulating 25(OH)D3 concentration (Pearson r range, 0.64–0.88). This correlation was weaker with lower eGFR. Moreover, the increment in 24,25(OH)2D3 associated with higher 25(OH)D3 (“slope”) was lower with lower eGFR: 2.06 (95% CI, 2.01–2.10), 1.77 (95% CI, 1.74–1.81), 1.55 (95% CI, 1.48–1.62), 1.17 (95% CI, 1.05–1.29), 0.92 (95% CI, 0.74–1.10), 0.61 (95% CI, 0.22–1.00), and 0.37 (95% CI, 0.35–0.39) ng/mL 24,25(OH)2D3 per 10 ng/mL 25(OH)D3 for eGFR ≥90, 60–89, 45–59, 30–44, 15–29, and <15 mL/min/1.73 m2 and ESRD treated with hemodialysis, respectively. As a result, at a 25(OH)D3 concentration of 20 ng/mL, mean 24,25(OH)2D3 concentration was 2.92 (95% CI, 2.87–2.96), 2.68 (95% CI, 2.64–2.72), 2.35 (95% CI, 2.26–2.45), 1.92 (95% CI, 1.74–2.10), 1.69 (95% CI, 1.43–1.95), 1.14 (95% CI, 0.62–1.66), and 1.04 (95% CI,1.02–1.07) ng/mL for each category, respectively. This interaction was independent of other relevant clinical characteristics. Race, diabetes, urine albumin excretion, and the circulating concentrations of parathyroid hormone and fibroblast growth factor 23 more modestly modified the association of 24,25(OH)2D3 with 25(OH)D3.
Limitations
Lack of direct pharmacokinetic measurements of vitamin D catabolism.
Conclusions
Lower eGFR is strongly associated with reduced vitamin D catabolism as measured by circulating 24,25(OH)2D3 concentration.
INDEX WORDS: decreased renal function; low estimated glomerular filtration rate; vitamin D catabolism; 1,25-dihydroxyvitamin D3; 25-hydroxyvitamin D3; active vitamin D; chronic kidney disease (CKD); biomarker
Decreased glomerular filtration rate (GFR) leads to reduced production of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active vitamin D hormone, from 25-hydroxyvitamin D3 (25(OH)D3).1,2 Reduced 1,25(OH)2D3 production is due to reduced renal mass as well as downregulation of the renal 1-α hydroxylase enzyme (CYP27B1) by fibroblast growth factor 23 (FGF-23), phosphorous excess, and metabolic acidosis.3–5
Less is known regarding vitamin D catabolism. Steady-state concentrations of vitamin D metabolites have to represent a balance between production and catabolism.5 Vitamin D catabolism may therefore have important effects on vitamin D metabolite concentrations in blood and tissues. An improved understanding of vitamin D catabolism may help identify new diagnostic and therapeutic strategies to improve health in chronic kidney disease (CKD) because impaired vitamin D metabolism leads to secondary hyperparathyroidism and bone disease and may contribute to cardiovascular disease, progression to end stage renal disease, and premature death.3–11
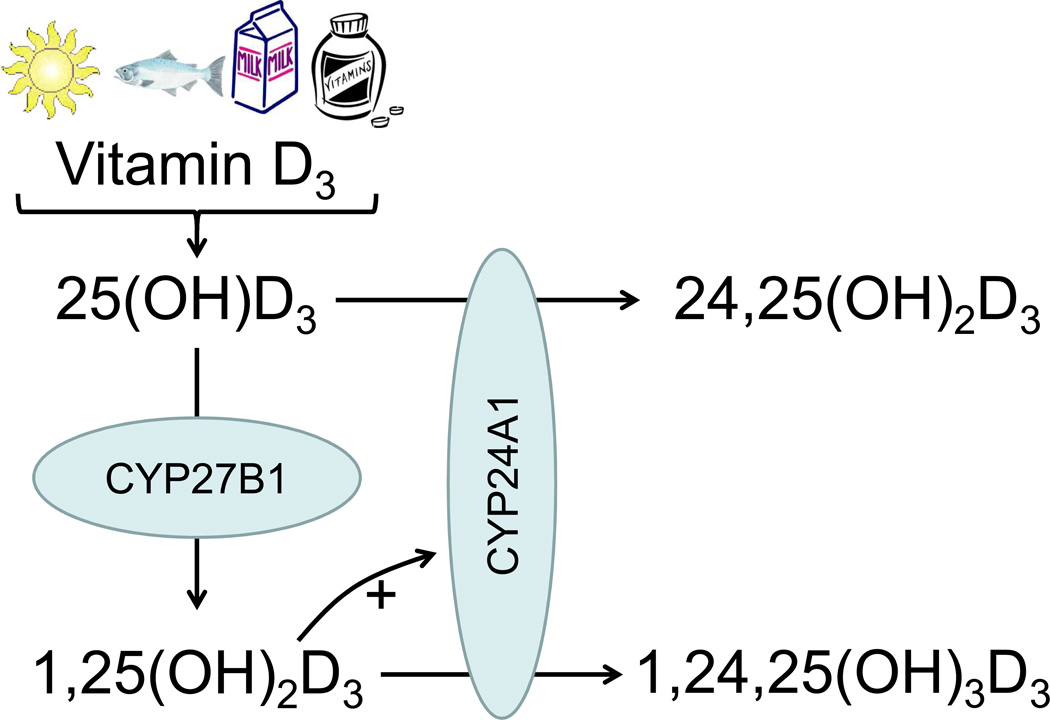
To better assess vitamin D catabolism in humans, we developed a novel high-throughput assay for circulating 24,25-dihydroxyvitamin D (24,25(OH)2D3).12 The most abundant product of vitamin D catabolism, 24,25(OH)2D3, is produced from 25(OH)D3 by CYP24A1, the 24α-hydroxylase enzyme.13 CYP24A1 also converts 1,25(OH)2D3 to 1,24,25-trihydroxyvitamin D3 (Figure 1). Hydroxylated products of CYP24A1 are further converted to more polar metabolites and excreted in urine or bile. In a cohort of patients referred to nephrology clinics, we demonstrated a strong, independent, direct correlation of estimated GFR (eGFR) with serum 24,25(OH)2D3 concentration.12 This observation suggests that CKD is characterized by reduced vitamin D catabolism, in addition to reduced 1,25(OH)2D3 production.
Figure 1. Vitamin D3 metabolism.
Vitamin D3 synthesized in the skin or consumed by mouth is metabolized to 25-hydroxyvitamin D3 (25(OH)D3), which can then be metabolized to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3, the active vitamin D hormone) by CYP27B1. Vitamin D3 catabolism is accomplished predominantly by CYP24A1, which metabolizes 25(OH)D3 to 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) and 1,25(OH)2D3 to 1,24,25-trihydroxyvitamin D3 (1,24,25(OH)3D3). CYP24A1 is induced by 1,25(OH)2D3.
In the current study, we tested associations of eGFR with circulating 24,25(OH)2D3 concentration across a wide range of eGFR using data from five cohort studies and clinical trials. Because 24,25(OH)2D3 production and circulating 24,25(OH)2D3 concentration are highly dependent on available substrate 25(OH)D3, we examined 24,25(OH)2D3 in the context of 25(OH)D3. We hypothesized that lower eGFR is associated with smaller increments in circulating 24,25(OH)2D3 concentration for a given increment in circulating 25(OH)D3 concentration. Such a finding would further support the theory that GFR loss leads to reduced vitamin D catabolism.
Methods
Study Populations
We measured serum or plasma 24,25(OH)2D3 concentrations in five cohort studies and clinical trials: the Diabetes Control and Complications Trial (DCCT), the Multi-Ethnic Study of Atherosclerosis (MESA), the Cardiovascular Health Study (CHS), the Seattle Kidney Study (SKS), and the Hemodialysis (HEMO) Study. We included all five studies in this cross-sectional analysis.
The DCCT was a randomized clinical trial that enrolled 1,441 participants with type 1 diabetes to test the effects of intensive diabetes therapy on the development of micro- and macro-vascular complications.14 We measured plasma vitamin D metabolite concentrations for all non-pregnant participants with available frozen samples collected at or near the end of the DCCT (N=1193).15
MESA is an observational cohort study of subclinical cardiovascular disease among people who were free of clinical cardiovascular disease at study entry.16 We measured serum vitamin D metabolite concentrations for all MESA participants with available frozen samples collected at baseline (N=6470 of 6814).17
CHS is an observational cohort study of cardiovascular disease among adults aged 65 or older.18 We measured serum vitamin D metabolites at the 1996–1997 CHS study visit (4–7 years after baseline) using a case-cohort design. In this study, we included the 932 participants from the randomly-selected cohort.
SKS is an observational cohort study of patients referred to nephrology clinics associated with the University of Washington (Seattle, WA).12 We measured baseline serum vitamin D metabolites using a case-cohort design.12 In this study, we included the 289 participants included in the randomly-selected cohort.
The HEMO Study was a randomized clinical trial that enrolled 1846 participants with ESRD treated with maintenance hemodialysis to test the effects of dialysis dose and membrane flux on mortality.19 In this study, we included the 712 participants for whom both 24,25(OH)2D3 and its interfering analyte(s) were measured at baseline, as described below.
Measurement of 24,25(OH)2D3
We measured 24,25(OH)2D3 using liquid chromatography–tandem mass spectrometry (LC-MS/MS) at the University of Washington Nutrition Obesity Research Center supervised by A.N.H‥ There were three variations of 24,25(OH)2D3 assay used across the populations. For the DCCT, MESA, and SKS, a liquid-liquid extraction was used to prepare samples prior to LC-MS/MS.12,15,17,20 After these analyses, it was discovered that the liquid chromatography method did not separate 24,25(OH)2D3 from another analyte or analytes, present at low concentrations, which we presumed based on elution time and fragmentation pattern to be 23S,25-dihydroxyvitamin D3 and/or 25,26-dihydroxyvitamin D3.21 In order to separate this interfering analyte(s) from 24,25(OH)2D3, methylamine was added to the mobile phase in the liquid chromatography method. This second assay was used to analyze samples from the CHS. For the HEMO cohort, immunoaffinity enrichment was added to the newer chromatographic method to measure 1,25(OH)2D3 and 1,25-dihydroxyvitaminD2 in addition to 24,25(OH)2D3, and the interfering analyte(s).21,22 All assays measured 25(OH)D3 and 25-hydroxyvitamin D2, calibrated to standards provided by the National Institute of Standards and Technology,23 concurrently with 24,25(OH)2D3.
For our primary analyses, in order to evaluate comparable 24,25(OH)2D3 values across all cohorts, we summed the concentrations of 24,25(OH)2D3 and the interfering analyte(s) for each participant in the CHS and the HEMO Study. In CHS, the mean concentration of the interfering dihydroxyvitamin D3 metabolite(s) was 0.73 ± 0.35 (standard deviation) ng/mL, while the mean concentration of specific 24,25(OH)2D3 was 2.77 ± 1.70 ng/mL. The correlation of the summed dihydroxyvitamin D3 metabolites with specific 24,25(OH)2D3 was 0.99 (Figure S1, available as online supplementary material). In the HEMO Study, the mean concentration of the interfering dihydroxyvitamin D3 metabolite(s) was 0.52 ± 0.31 ng/mL, the mean concentration of specific 24,25(OH)2D3 was 0.33 ± 0.23 ng/mL, and the correlation of the summed dihydroxyvitamin D3 metabolites with specific 24,25(OH)2D3 was 0.82 (Figure S2). We performed sensitivity analyses in the CHS to demonstrate that our primary associations of interest did not differ using the summed values versus the specific 24,25(OH)2D3 measurements. Specifically, we compared the association of eGFR with the summed value to the association of eGFR with specific 24,25(OH)2D3 concentration.
To verify that the 24,25(OH)2D3 assays yielded consistent results across the populations, we regularly measured a set of 20 quality control serum samples collected from healthy donors. Frozen serum was used to reflect real-world sample testing. Twenty samples (instead of two or three) were used to evaluate for drift specific to a subset of samples with increased sensitivity. The quality control samples were collected and divided into aliquots at the University of Vermont in 2008 and stored frozen at −80°C. The set of 20 samples was measured 9 times across the study populations and included measurements of 24,25(OH)2D3 using the liquid-liquid extraction method without methylamine in the chromatographic method and the method with methylamine. The average coefficient of variation (CV) observed for these 20 samples over the nine measurements (spanning 16 months) was 14.6%. Importantly, there was no significant trend detected for the mean of these 20 samples over the 16 months, and the CV of the 9 means was 7.9%, indicating that there was no drift in calibration of the assays across the populations. The measurements of 24,25(OH)2D3 by the liquid-liquid extraction method and the immunoaffinity method have previously been shown to agree well.21
Clinical Characteristics
In the DCCT, MESA, CHS, and SKS, serum creatinine concentrations were measured using methods traceable to isotope dilution mass spectrometry. The CKD-EPI (CKD Epidemiology Collaboration) equation was used to estimate GFR from serum creatinine and demographic variables.24 Urine albumin excretion was quantified as the mean of two 4-hour albumin excretion rates in the DCCT15 and as albumin-creatinine ratio from a single urine sample in MESA, CHS, and SKS. Diabetes was defined as the use of glucose-lowering medications, fasting glucose ≥126 mg/dL, or (in SKS only) random glucose ≥200 mg/dL. Hypertension was defined as the use of antihypertensive medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg. Intact parathyroid hormone (PTH) was measured using a second-generation immunoassay on the Beckman-Coulter DxI system. Intact fibroblast growth factor 23 (FGF-23) was measured by ELISA (Kainos) for the DCCT, MESA, and SKS. Carboxy-terminal FGF-23 was measured by ELISA (Immutopics) for CHS. 1,25(OH)2D3 was measured by mass spectrometry in the DCCT, SKS, and the HEMO Study.22
Statistical Analysis
All analyses used individual-level data from each of the five included cohorts. Bivariate relationships of 24,25(OH)2D3 concentration with 25(OH)D3 concentration were examined using scatterplots, locally-weighted scatterplot smoothing (LOWESS), Pearson correlation, and linear regression. These relationships were examined both by cohort and by eGFR, using data pooled across cohorts. LOWESS curves were calculated using all available data but truncated below the 2.5% percentile and above the 97.5% percentile of 25(OH)D3 concentration for presentation. Multivariable linear regression models were used to test associations of clinical characteristics (including eGFR) with 24,25(OH)2D3 concentration (the dependent variable), adjusting for potential confounding variables and cohort. We included 25(OH)D3 and interaction terms of each clinical characteristic with 25(OH)D3 to estimate how the clinical characteristics modified the relationship of 24,25(OH)2D3 with 25(OH)D3. For each category of a covariate, its interaction with 25(OH)D3 was reported as a slope (increment in 24,25(OH)2D3 per 10-ng/mL increment in 25(OH)D3) and an intercept was estimated for 25(OH)D3 = 20 ng/mL, a clinically relevant concentration. Mean values of other covariates were used to estimate values for the covariate of interest. The multivariable models pooled data from MESA, CHS, and SKS; the DCCT and HEMO Study were excluded because there was little or no overlap in eGFR values compared with other cohorts.
Results
Together, the DCCT, MESA, CHS, SKS, and HEMO Study covered a wide range of eGFR values (Table 1 and Figure 2). The distributions of age, sex, race/ethnicity, diabetes, hypertension, body mass index, urine albumin excretion, PTH, and FGF-23 also varied substantially across studies. Nonetheless, mean 25(OH)D3 concentrations were similar, and there was substantial overlap in the distributions of circulating 25(OH)D3 concentration.
Table 1.
Participant characteristics by cohort.
| DCCT | MESA | CHS | SKS | HEMO | |
|---|---|---|---|---|---|
| No. analyzed | 1193 | 6470 | 932 | 289 | 712 |
| Age (y) | 26.9 (7) | 62.1 (10.3) | 78.1 (4.8) | 60.7 (13) | 56.7 (14) |
| Female sex | 564 (47) | 3446 (53) | 372 (40) | 49 (17) | 402 (56) |
| Race/ethnicity | |||||
| White | 1151 (96.5) | 2509 (38.8) | 778 (83.5) | 197 (68.2) | 235 (33.2) |
| Black | 24 (2.0) | 1762 (27.2) | 148 (15.9) | 56 (19.4) | 449 (63.4) |
| Hispanic | 13 (1.1) | 1412 (21.8) | 2 (0.2) | 12 (4.2) | 21 (3.0) |
| Asian | 4 (0.3) | 787 (12.2) | 3 (0.3) | 8 (2.8) | 7 (1.0) |
| Other | 1 (0.1) | 0 (0) | 1 (0.1) | 16 (5.5) | 0 (0) |
| Diabetes | 1193 (100) | 803 (12) | 131 (14) | 161 (56) | 320 (45) |
| Hypertension | 104 (9) | 4266 (66) | 744 (80) | 274 (95) | 676 (95) |
| BMI category () | |||||
| <25 kg/m2 | 560 (46.9) | 1865 (28.8) | 323 (35.2) | 48 (16.6) | 356 (50.3) |
| 25-<30 kg/m2 | 487 (40.8) | 2552 (39.4) | 400 (43.6) | 89 (30.8) | 338 (47.7) |
| ≥30 kg/m2 | 146 (12.2) | 2053 (31.7) | 194 (21.2) | 152 (52.6) | 14 (2.0) |
| Mean eGFR (mL/min/1.73 m2) | 125 (12.5) | 78.2 (16.3) | 63.2 (16.5) | 45.6 (25.8) | - |
| eGFR category | |||||
| ≥ 90 mL/min/1.73 m2 | 1185 (99.3) | 1518 (23.5) | 47 (5) | 22 (7.6) | 0 |
| 60–89 mL/min/1.73 m2 | 7 (0.6) | 4125 (63.8) | 499 (53.5) | 46 (15.9) | 0 |
| 45–59 mL/min/1.73 m2 | 1 (0.1) | 699 (10.8) | 265 (28.4) | 52 (18) | 0 |
| 30–44 mL/min/1.73 m2 | 0 | 96 (1.5) | 94 (10.1) | 80 (27.7) | 0 |
| 15–29 mL/min/1.73 m2 | 0 | 21 (0.3) | 24 (2.6) | 71 (24.6) | 0 |
| < 15* mL/min/1.73 m2 | 0 | 5 (0.1) | 3 (0.3) | 18 (6.2) | 0 |
| Hemodialysis | 0 | 0 | 0 | 0 | 712 (100) |
| UAE (mg/d or mg/g**) | 10.0 [7.1–16.2] | 5.3 [3.3–11.0] | 9.8 [5.4–23.0] | 143.3 [20.6–704.3] | - |
| UAE category (mg/d or mg/g**) | |||||
| <30 | 1073 (89.9) | 5829 (90.5) | 676 (80) | 82 (28.6) | - |
| 30–299 | 105 (8.8) | 525 (8.1) | 137 (16.2) | 90 (31.4) | - |
| ≥300 | 15 (1.3) | 89 (1.4) | 32 (3.8) | 115 (40.1) | - |
| 24,25(OH)2D3(ng/mL) | 4.1 (2.1) | 3.7 (2.4) | 3.5 (1.9) | 2.8 (2.1) | 0.8 (0.5) |
| 25(OH)D3(ng/mL) | 23.9 (8.9) | 22.7 (10.6) | 25.3 (11.1) | 24 (14.1) | 14.6 (10.5) |
| Total 25(OH)D (ng/mL) | 25.4 (8.7) | 25.3 (10.9) | 28.1 (11.3) | 29.3 (15.3) | 18.6 (13.0) |
| Total 1,25(OH)2D (pg/mL) | 41.3 (11.8) | - | - | 33.6 (14.9) | 10.9 (11.9) |
| Calcium (mg/dL) | - | 9.6 (0.4) | 9.8 (0.6) | 9.0 (0.8) | 9.3 (0.9) |
| Phosphorus (mg/dL) | - | 3.7 (0.5) | 3.8 (0.6) | 3.8 (0.7) | 5.8 (1.9) |
| Parathyroid hormone (pg/mL) | 28.5 [21.6–37.2] | 40.6 [31.2–53.3] | 43.1 [33.0–57.0] | 72.8 [43.3–130.2] | 190 [83–426] |
| FGF-23 (pg/mL or RU/mL***) | 30.8 [24.9–35.9] | 37.7 [30.5–46.4] | 69.7 [53.3–96.7] | 59.8 [42.6–97.9] | 3118 [726–129278] |
Note: Unless otherwise indicated, values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range].
Limited data are missing for BMI (CHS), eGFR (MESA), and UAE (MESA, CHS, SKS). Conversion factors for units: calcium in mg/dL to mmol/L, ×0.2495; phosphorus in mg/dL to mmol/L, ×0.3229;
Not treated with dialysis.
UAE is based on albumin excretion rate (in mg/d) for DCCT, otherwise it reflects albumin-creatining ratio (mg per g of creatinine).
FGF-23 is in RU/mL for CHS, otherwise pg/mL.
Abbreviations and definitions: DCCT = Diabetes Control and Complications Trial; MESA = Multi-Ethnic Study of Atherosclerosis; CHS = Cardiovascular Health Study; SKS = Seattle Kidney Study; HEMO = Hemodialysis Study; BMI, body mass index; eGFR, estimated glomerular filtration rate; UAE, urinary albumin excretion; FGF, fibroblast growth factor; total 1,25(OH)2D, 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; total 25(OH)D, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3
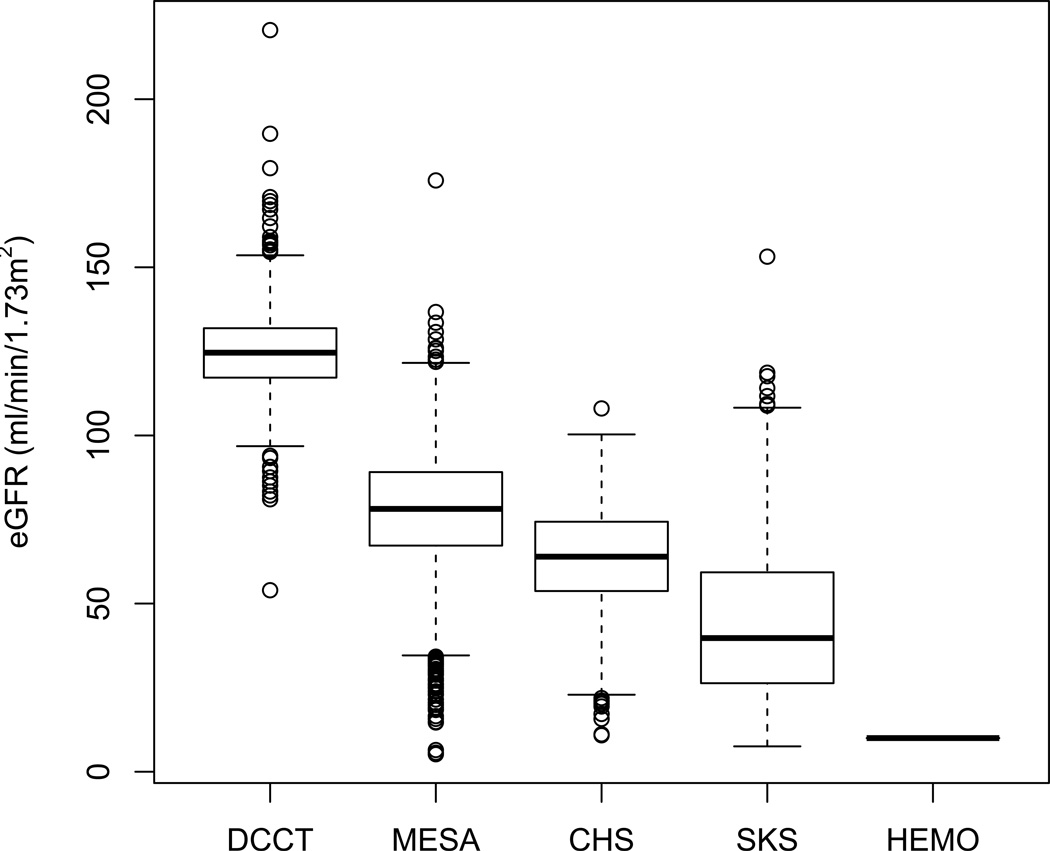
Figure 2. Distribution of estimated GFR among 9596 participants in the Diabetes Control and Complications Trial (DCCT), Multi-Ethnic Study of Atherosclerosis (MESA), Cardiovascular Health Study (CHS), Seattle Kidney Study (SKS), and Hemodialysis (HEMO) Study.
Box plots demonstrate the 5th, 25th, 50th, 75th, and 95th percentiles, with dots representing estimated GFR outside the 5th – 95th percentiles. All HEMO Study participants have end-stage renal disease treated with hemodialysis, represented here as an estimated GFR of 10 mL/min/1.73 m2.
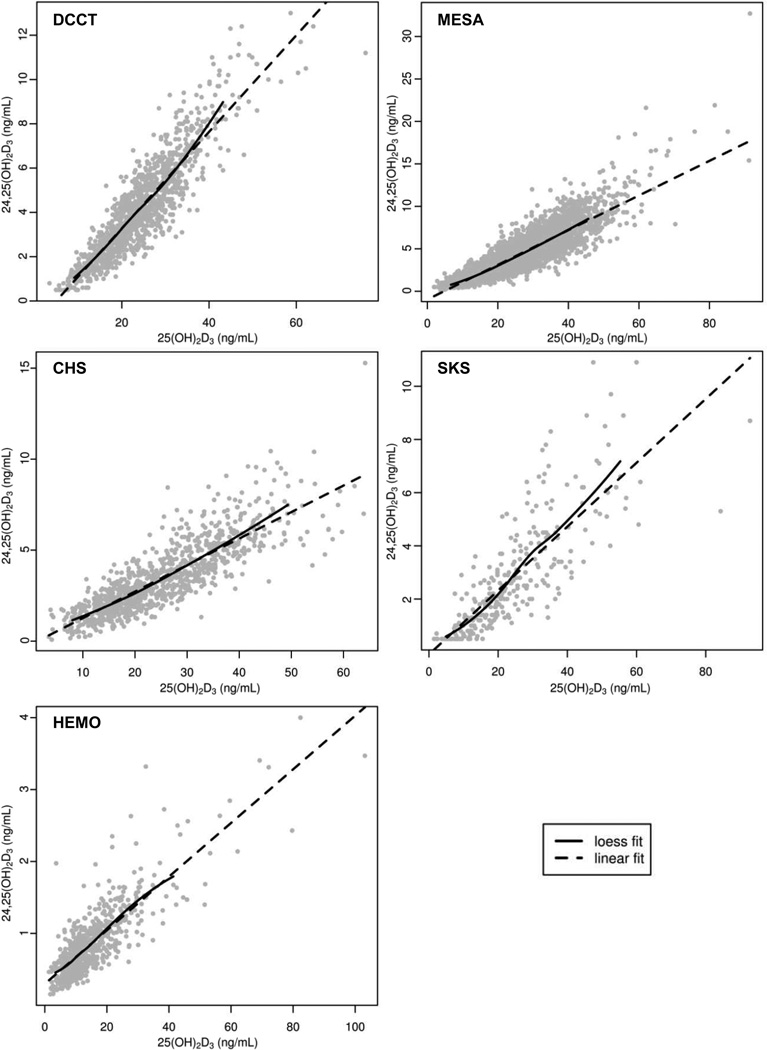
Within each cohort, there was a strong positive correlation of circulating 24,25(OH)2D3 concentration with circulating 25(OH)D3 concentration (Figure 3). These relationships appeared linear when LOWESS curves were compared to estimates generated using linear regression. The correlations of 24,25(OH)2D3 and 25(OH)D3 were strongest in DCCT and MESA (r=0.88 and r=0.84, respectively) and weaker in CHS and SKS (r=0.78 and r=0.64, respectively). Pooling data from the DCCT, MESA, CHS, and SKS, correlations of 24,25(OH)2D3 and 25(OH)D3 were 0.87, 0.83, 0.81, 0.76, 0.76, and 0.74 for eGFR ≥90, 60–89, 45–59, 30–44, 15–29, and <15 mL/min/1.73 m2, respectively.
Figure 3. Relationships of 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) with 25-hydroxyvitamin D3 (25(OH)D3) in five cohort studies and clinical trials.
The scatter plots display individual values for each participant. Solid lines represent mean 24,25(OH)2D3 concentrations estimated using locally-weighted scatterplot smoothing (LOWESS). Broken lines represent linear fit generated using unadjusted linear regression. LOWESS curves were calculated using all available data but truncated below the 2.5% percentile and above the 97.5% percentile of 25(OH)D3 concentration for presentation. Note that the ranges of the×and y axes vary in each panel. DCCT = Diabetes Control and Complications Trial; MESA = Multi-Ethnic Study of Atherosclerosis; CHS = Cardiovascular Health Study; SKS = Seattle Kidney Study; HEMO = Hemodialysis Study.
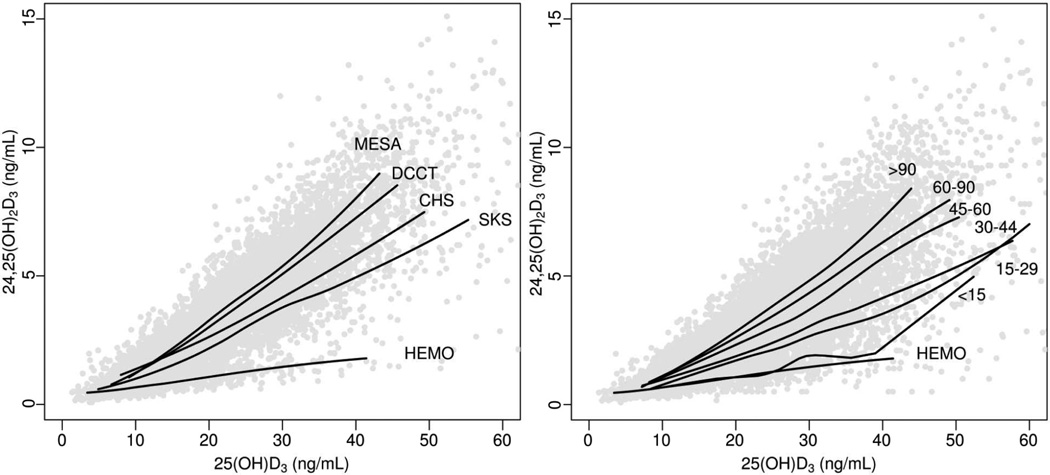
The increment in 24,25(OH)2D3 per unit higher 25(OH)D3 (slope) differed by cohort, being greatest in DCCT and MESA, followed by CHS, SKS, and the HEMO Study (Figure 4A). Pooling data across all five cohorts, the increment in 24,25(OH)2D3 per increment in 25(OH)D3 (slope) correlated directly with eGFR (Figure 4B). As a result, unadjusted mean 24,25(OH)2D3 concentration at a 25(OH)D3 concentration of 20 ng/mL was significantly lower with lower eGFR (Table 2).
Figure 4. Relationships of 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) with 25-hydroxyvitamin D3 (25(OH)D3) among 9596 participants in the Diabetes Control and Complications Trial (DCCT), Multi-Ethnic Study of Atherosclerosis (MESA), Cardiovascular Health Study (CHS), Seattle Kidney Study (SKS), and Hemodialysis (HEMO) Study.
The scatter plots display individual values for each participant. Solid lines represent mean 24,25(OH)2D3 concentrations estimated using locally-weighted scatterplot smoothing (LOWESS) among subgroups defined by cohort (Panel A) or category of estimated GFR (Panel B). LOWESS curves were calculated using all available data but truncated below the 2.5% percentile and above the 97.5% percentile of 25(OH)D3 concentration for presentation. The X axis was truncated at 60 ng/mL. Estimated GFR is in mL/min/1.73 m2.
Table 2.
Relationship of 24,25(OH)2D3 with 25(OH)D3 in all 5 studies.
| Mean 24,25(OH)2D3 at 25(OH)D3 = 20 ng/mL (ng/mL) |
Increment in 24,25(OH)2D3 per increment in 25(OH)D3 (slope, ng/mL per 10 ng/mL) |
|
|---|---|---|
| Cohort (Model A) | ||
| DCCT | 2.97 (2.90 to 3.03) | 2.16 (2.10 to 2.23) |
| MESA | 2.68 (2.65 to 2.72) | 1.84 (1.81 to 1.87) |
| CHS | 2.40 (2.30 to 2.49) | 1.33 (1.26 to 1.40) |
| SKS | 1.98 (1.77 to 2.20) | 0.88 (0.76 to 1.00) |
| HEMO | 1.04 (1.02 to 1.07) | 0.37 (0.35 to 0.39) |
| eGFR category (Model B) | ||
| ≥ 90 mL/min/1.73 m2 | 2.92 (2.87 to 2.96) | 2.06 (2.01 to 2.1) |
| 60–89 mL/min/1.73 m2 | 2.68 (2.64 to 2.72) | 1.77 (1.74 to 1.81) |
| 45–59 mL/min/1.73 m2 | 2.35 (2.26 to 2.45) | 1.55 (1.48 to 1.62) |
| 30–44 mL/min/1.73 m2 | 1.92 (1.74 to 2.10) | 1.17 (1.05 to 1.29) |
| 15–29 mL/min/1.73 m2 | 1.69 (1.43 to 1.95) | 0.92 (0.74 to 1.10) |
| < 15* mL/min/1.73 m2 | 1.14 (0.62 to 1.66) | 0.61 (0.22 to 1.00) |
| Hemodialysis | 1.04 (1.02 to 1.07) | 0.37 (0.35 to 0.39) |
Note: N=9596. Two parallel models are presented evaluating this relationship: (A) by cohort, or (B) by eGFR, pooling cohorts. The regression model evaluates 24,25(OH)2D3 as dependent variable and 25(OH)D3, cohort or eGFR, and the interaction of 25(OH)D3 with cohort or eGFR as independent variables. No additional covariates are included. intercepts (mean 24,25(OH)2D3 concentration at 25(OH)D3 = 20 ng/mL) and Slopes (increment in 24,25(OH)2D3 per 10-ng/mL increment in 25(OH)D3) are derived from linear regression‥ 95% confidence intervals are given in parentheses.
Abbreviations: eGFR, estimated glomerular filtration rate; DCCT = Diabetes Control and Complications Trial; MESA = Multi-Ethnic Study of Atherosclerosis; CHS = Cardiovascular Health Study; SKS = Seattle Kidney Study; HEMO = Hemodialysis Study. 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3
Patients treated with hemodialysis are not included in this category.
Pooling data from MESA, CHS, and SKS in a multivariable model, lower eGFR was associated with a significantly reduced increment in 24,25(OH)2D3 concentration per given increment in 25(OH)D3 concentration (slope) and a significantly reduced mean 24,25(OH)2D3 concentration at a 25(OH)D3 of 20 ng/mL, adjusting for relevant potential confounding clinical characteristics (Table 3). Other clinical characteristics were also independently associated with 24,25(OH)2D3 concentration, though not as strongly as eGFR. Diabetes was associated with reduced slope and reduced mean 24,25(OH)2D3 concentration at a 25(OH)D3 of 20 ng/mL. Black or Asian race and higher urine ACR were associated with reduced slope, but no significant difference in mean 24,25(OH)2D3 concentration at a 25(OH)D3 of 20 ng/mL. Age, sex, and body mass index were not independently associated with 24,25(OH)2D3. In CHS, results were similar in terms of the comparison of specific 24,25(OH)2D3 to the sum of specific 24,25(OH)2D3 and its interfering analyte(s) as the outcome (Table S1).
Table 3.
Adjusted associations of eGFR and other clinical characteristics with serum 24,25(OH)2D3 concentration in MESA, CHS, and SKS.
| Independent variable | Mean 24,25(OH)2D3 at 25(OH)D3 = 20 ng/mL (ng/mL) |
Increment in 24,25(OH)2D3 per increment in 25(OH)D3 (slope, ng/mL per 10 ng/mL) |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Age Category | 0.02 | <0.001 | ||
| <55 y | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| 55–64 y | 2.91 (2.78 to 3.04) | 2.16 (2.05 to 2.26) | ||
| 65–74 y | 2.83 (2.70 to 2.97) | 2.16 (2.05 to 2.28) | ||
| ≥75 y | 2.78 (2.63 to 2.93) | 1.94 (1.82 to 2.06) | ||
| Sex | <0.001 | 0.5 | ||
| Male | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| Female | 2.82 (2.71 to 2.94) | 2.14 (2.05 to 2.23) | ||
| Race/ethnicity | 0.07 | <0.001 | ||
| White | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| Black | 2.99 (2.87 to 3.11) | 2.03 (1.92 to 2.13) | ||
| Hispanic | 3.07 (2.94 to 3.19) | 2.15 (2.05 to 2.26) | ||
| Asian | 2.92 (2.78 to 3.05) | 1.85 (1.72 to 1.97) | ||
| Other | 2.62 (1.91 to 3.32) | 2.76 (2.28 to 3.24) | ||
| Diabetes | <0.001 | <0.001 | ||
| No | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| Yes | 2.74 (2.59 to 2.89) | 1.88 (1.76 to 2.01) | ||
| BMI category | 0.3 | <0.001 | ||
| <25 kg/m2 | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| 25-<30 kg/m2 | 2.98 (2.87 to 3.10) | 2.06 (1.97 to 2.15) | ||
| ≥30 kg/m2 | 2.93 (2.81 to 3.05) | 1.96 (1.87 to 2.06) | ||
| eGFR category | <0.001 | <0.001 | ||
| >90 mL/min/1.73 m2 | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| 60–89 mL/min/1.73 m2 | 2.81 (2.70 to 2.93) | 1.92 (1.84 to 1.99) | ||
| 45–59 mL/min/1.73 m2 | 2.58 (2.42 to 2.73) | 1.74 (1.63 to 1.85) | ||
| 30–44 mL/min/1.73 m2 | 2.29 (2.07 to 2.52) | 1.25 (1.11 to 1.39) | ||
| 15–29 mL/min/1.73 m2 | 2.07 (1.76 to 2.38) | 0.94 (0.74 to 1.15) | ||
| <15 mL/min/1.73 m2 | 1.45 (0.86 to 2.04) | 1.10 (0.68 to 1.52) | ||
| Urine ACR * | 0.9 | 0.001 | ||
| <30 mg/g | 2.94 (2.83 to 3.06) | 2.12 (2.03 to 2.21) | ||
| 30–299 mg/g | 2.92 (2.76 to 3.07) | 1.98 (1.86 to 2.11) | ||
| ≥300 mg/g | 2.96 (2.72 to 3.19) | 1.90 (1.72 to 2.08) | ||
Note: N=7691. A single combined regression model evaluates 24,25(OH)2D3 as dependent variable and 25(OH)D3, clinical characteristics (including eGFR), and interactions of 25(OH)D3 with each clinical characteristic as independent variables. All variables included in the model are shown. intercepts (mean 24,25(OH)2D3 concentration at 25(OH)D3 = 20 ng/mL) and Slopes (increment in 24,25(OH)2D3 per 10-ng/mL increment in 25(OH)D3) are derived from linear regression. 95% confidence intervals are listed in parentheses.
Reported are the results of a single multivariable regression model, with circulating 24,25(OH)2D3 concentration as dependent variable. In addition to circulating 25(OH)D3 concentration, the model includes age, sex, race/ethnicity, diabetes, body mass index, estimated GFR, urine ACR, and interactions of each of these variables with circulating 25(OH)D3 concentration as independent variables. Estimates are reported at mean values of the other independent variables. P-values test the null hypothesis that the slope (or intercept) does not differ by level of the independent variable (p-value for interaction).
ACR, albumin-creatinine ratio; BMI, body mass index; eGFR, estimated glomerular filtration rate; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3
We evaluated associations of circulating PTH and FGF-23 concentrations with circulating 24,25(OH)2D3 concentration separately within MESA, CHS, and SKS because the ranges of PTH and FGF-23 varied substantially by cohort and different methods were used to assay FGF-23 across cohorts. Adjusting for potential confounders, including eGFR, higher FGF-23 was associated with a significantly higher slope and a significantly higher mean 24,25(OH)2D3 concentration at a 25(OH)D3 of 20 ng/mL in MESA (Table 4). Similar trends were not observed in CHS and SKS. In the same models, higher PTH was associated with a significantly reduced increment in 24,25(OH)2D3 per given increment in 25(OH)D3 (slope) in MESA, with similar trends in CHS and SKS that were not statistically significant. Higher PTH was associated with a significantly lower mean 24,25(OH)2D3 concentration at a 25(OH)D3 of 20 ng/mL in SKS, but not MESA or CHS.
Table 4.
Adjusted associations of circulating PTH and FGF-23 concentrations with serum 24,25(OH)2D3 concentration, evaluated in 3 individual cohorts.
| Independent variable |
Mean 24,25(OH)2D3 at 25(OH)D3 = 20 ng/mL (ng/mL) |
Increment in 24,25(OH)2D3 per increment in 25(OH)D3 (slope, ng/mL per 10 ng/mL) |
||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| MESA | ||||
| PTH () | 0.2 | <0.001 | ||
| ≤ 31.2 | 3.21 (2.96 to 3.46) | 2.54 (2.35 to 2.73) | ||
| 31.3–40.6 | 3.22 (2.97 to 3.47) | 2.34 (2.14 to 2.54) | ||
| 40.7–53.3 | 3.23 (2.98 to 3.48) | 2.18 (1.98 to 2.38) | ||
| ≥ 53.4 | 3.14 (2.88 to 3.40) | 2.21 (2.00 to 2.41) | ||
| FGF-23 | <0.001 | <0.001 | ||
| ≤ 30.5 | 3.16 (2.92 to 3.41) | 2.41 (2.21 to 2.6) | ||
| 30.6–37.7 | 3.26 (3.01 to 3.51) | 2.5 (2.30 to 2.69) | ||
| 37.8–46.4 | 3.31 (3.05 to 3.56) | 2.65 (2.45 to 2.85) | ||
| ≥ 46.5 | 3.39 (3.14 to 3.64) | 2.51 (2.31 to 2.70) | ||
| CHS | ||||
| PTH | 0.6 | 0.08 | ||
| ≤ 33.2 | 2.78 (0.94 to 4.62) | 1.89 (0.50 to 3.28) | ||
| 33.3–42.6 | 2.91 (1.05 to 4.77) | 1.64 (0.24 to 3.04) | ||
| 42.7–56.8 | 2.84 (0.96 to 4.73) | 1.71 (0.30 to 3.12) | ||
| ≥ 56.9 | 2.73 (0.84 to 4.62) | 1.61 (0.18 to 3.04) | ||
| FGF-23 | 0.7 | 0.6 | ||
| ≤ 53.0 | 2.59 (0.74 to 4.44) | 2.12 (0.73 to 3.51) | ||
| 53.1–69.0 | 2.71 (0.88 to 4.54) | 2.02 (0.65 to 3.39) | ||
| 69.1–93.8 | 2.66 (0.82 to 4.49) | 2.12 (0.72 to 3.51) | ||
| ≥ 93.9 | 2.78 (0.92 to 4.63) | 2.15 (0.74 to 3.56) | ||
| SKS | ||||
| PTH | 0.05 | 0.4 | ||
| ≤ 43.4 | 3.15 (1.60 to 4.7) | 1.3 (0.38 to 2.21) | ||
| 43.5–72.8 | 3.02 (1.40 to 4.63) | 1.12 (0.13 to 2.10) | ||
| 72.9–130.0 | 2.60 (0.96 to 4.23) | 1.25 (0.25 to 2.24) | ||
| ≥ 130.1 | 2.33 (0.7 to 3.95) | 0.95 (-0.08 to 1.98) | ||
| FGF-23 | 0.5 | 0.8 | ||
| ≤ 42.6 | 3.00 (1.41 to 4.59) | 1.55 (0.59 to 2.51) | ||
| 42.7–59.8 | 3.42 (1.76 to 5.09) | 1.40 (0.45 to 2.36) | ||
| 59.9–97.9 | 3.48 (1.78 to 5.18) | 1.42 (0.37 to 2.46) | ||
| ≥ 98.0 | 3.47 (1.70 to 5.24) | 1.35 (0.26 to 2.44) | ||
Note: PTH and FGF-23 expressed in pg/mL, except that FGF-23 expressed in RU/mL for CHS. Reported are the results of three multivariable regression models (one for each cohort), with circulating 24,25(OH)2D3 concentration as dependent variable. In addition to circulating 25(OH)D3 concentration, each model includes PTH, FGF-23, age, sex, race/ethnicity, diabetes, body mass index, estimated glomerular filtration rate, urine albumin-creatinine ratio, and interactions of each of these variables with circulating 25(OH)D3 concentration as independent variables. Estimates are reported at mean values of the other independent variables. P-values test the null hypothesis that the slope or intercept does not differ by level of PTH or FGF-23.
Abbreviations: MESA = Multi-Ethnic Study of Atherosclerosis; CHS = Cardiovascular Health Study; SKS = Seattle Kidney Study. FGF, fibroblast growth factor; PTH, parathyroid hormone; 24,25(OH)2D3, 24,25-dihydroxyvitamin D3; 25(OH)D3, 25-hydroxyvitamin D3
Discussion
Across five cohort studies and clinical trials with a wide range of eGFRs, the relationship of circulating 24,25(OH)2D3 concentration with circulating 25(OH)D3 concentration was significantly modified by eGFR. Specifically, with lower eGFR, the correlation of 24,25(OH)2D3 with 25(OH)D3 concentration was weaker, the increment in 24,25(OH)2D3 per unit higher 25(OH)D3 (slope) was reduced, and mean 24,25(OH)2D3 concentration at a given 25(OH)D3 concentration was lower. These observations were consistent when 24,25(OH)2D3 concentration was evaluated on the study level, on the participant level, and using multivariable regression. Other clinical characteristics also modified the relationship of 24,25(OH)2D3 with 25(OH)D3, but none as strongly as eGFR.
Our results are consistent with and extend those of smaller studies examining targeted populations. Very low or undetectable circulating concentrations of 24,25(OH)2D3 were observed in hemodialysis patients.25–30 In addition, 24,25(OH)2D3 concentration was found to correlate directly with eGFR in three CKD cohorts, one of which was the SKS, included in this analysis.12,31,32 Compared to these studies, we evaluated a much broader population, including people with type 1 diabetes and normal or high eGFR (DCCT), participants in two diverse community-based cohorts (MESA and CHS), a cohort with moderate-severe CKD not requiring renal replacement therapy (SKS), and prevalent hemodialysis patients (HEMO Study).
Accounting for available 25(OH)D3, 24,25(OH)2D3 production is a measure of CYP24A1-mediated 25(OH)D3 clearance. Metabolism by CYP24A1 is thought to be the major route of 25(OH)D3 catabolism.13 Therefore, assuming that circulating 24,25(OH)2D3 concentration is proportional to 24,25(OH)2D3 production, our results suggest that lower GFR, across its full range, is associated with reduced vitamin D catabolism. The weaker correlation of 24,25(OH)2D3 with 25(OH)D3 further suggests that inter-individual differences in vitamin D catabolism are accentuated with lower GFR. Our results suggest that CKD is a state in which vitamin D metabolism is stagnant and vitamin D catabolism and 1,25(OH)2D3 production are reduced.
A number of potential mechanisms may explain reduced CYP24A1-mediated 25(OH)D3 clearance in CKD. Renal tubules are a known site of CYP24A1 activity.33 Reduced circulating 24,25(OH)2D3 concentration may therefore be a sign of reduced net tubular CYP24A1 function. This could be due to decreased tubular delivery of 25(OH)D3 secondary to reduced glomerular filtration, impaired luminal 25(OH)D3 uptake by megalin and cubilin,34 reduced CYP24A1 protein content, or impaired CYP24A1 function. Renal CYP24A1 expression is known to be stimulated by FGF-23 and inhibited by PTH.3–5 Our observed positive and negative correlations of FGF-23 and PTH with 24,25(OH)2D3 concentration suggest that these regulatory effects explain inter-individual differences in 24,25(OH)2D3, to some extent. However, associations of eGFR with 24,25(OH)2D3 were more consistent and much stronger than associations of PTH or FGF-23 with 24,25(OH)2D3, suggesting that regulation of CYP24A1 transcription is not the predominant mechanism of reduced vitamin D catabolism in CKD.
Low circulating 24,25(OH)2D3 concentration could reflect reduced non-renal CYP24A1-mediated 25(OH)D3 clearance, instead of or in addition to reduced renal CYP24A1-mediated 25(OH)D3 clearance.29 Most cells of the body express the vitamin D receptor, and all of these are thought to express CYP24A1.13 1,25(OH)2D3 is known to potently induce CYP24A1, probably to prevent 1,25(OH)2D3 toxicity. Therefore, in CKD, low circulating 24,25(OH)2D3 concentration could reflect in part systemic tissue-level 1,25(OH)2D3 deficiency.
In addition to informing pathophysiology, better understanding of the effects of low 24,25(OH)2D3 concentration may eventually help guide vitamin D–related interventions in CKD. Current clinical care focuses on measuring 25-hydroxyvitamin D and PTH concentrations to direct the initiation and titration of vitamin D supplements and vitamin D receptor agonists. However, these biomarkers may not adequately capture tissue 1,25(OH)2D3 function: 25(OH)D3 is relatively inactive and requires regulated conversion to 1,25(OH)2D3 for full hormonal activity, while PTH reflects functional 1,25(OH)2D3 deficiency at only one of many relevant biological sites and is influenced by factors other than 1,25(OH)2D3, such as calcium. Low 24,25(OH)2D3 relative to 25(OH)D3 may better reflect impaired vitamin D metabolic function and therefore provide a stronger indication for treatment. Ultimately, to determine whether 24,25(OH)2D3 is a clinically useful biomarker, additional studies are needed to determine whether low 24,25(OH)2D3 is associated with clinical outcomes, is modifiable, and identifies patients who respond favorably to vitamin D–related interventions.
Furthermore, 25(OH)D3 is also metabolized by pathways that do not produce 24,25(OH)2D3. For example, metabolism of 25(OH)D3 by cytochrome P450 3A4 (CYP3A4) in the liver and intestine produces 4β,25-dihydroxyvitamin D3.35 Neither the effects of CKD on alternate catabolic pathways nor the net effects of CKD on overall vitamin D catabolism are known. One pharmacokinetic study documented a nearly 50% reduction in the clearance of radiolabeled 25(OH)D3 in hemodialysis patients, compared with healthy control participants.25 This is consistent with our findings utilizing circulating 24,25(OH)2D3 and strongly suggests that net vitamin D catabolism is reduced in hemodialysis patients. Two similar pharmacokinetic studies in CKD yielded conflicting results.36,37 Additional pharmacokinetic studies and studies assessing multiple catabolic pathways across a wide range of GFR would be useful to better define this pathophysiology.
Limitations of this study include the lack of direct pharmacokinetic measurements of vitamin D catabolism; the cross-sectional design, which is subject to confounding and precludes assessment of changes in 24,25(OH)2D3 within individuals over time; the focus on a single biomarker of vitamin D catabolism; and the inclusion of an interfering analyte(s) in the measurement of 24,25(OH)2D3. Regarding the interfering analyte(s), its concentration was low compared with specific 24,25(OH)2D3 in CHS, the summed value utilized in primary analyses correlated strongly with specific 24,25(OH)2D3 (r=0.99 in CHS), and sensitivity analyses in the CHS verified that associations of eGFR with specific 24,25(OH)2D3 were similar to those of the summed value used in primary analyses.
This study also has important strengths, including the use of five diverse cohorts that together cover the full range of GFR and enhance external validity, the large number of novel 24,25(OH)2D3 measurements utilized to evaluate vitamin D catabolism on an unprecedented scale, and the ability to assess the impact of important regulatory hormones. As such, it represents an important initial step in understanding vitamin D catabolism in CKD.
In conclusion, lower eGFR is strongly associated with reduced vitamin D catabolism as measured by circulating 24,25(OH)2D3 concentration. Further studies are needed to more fully understand vitamin D catabolism and its changes in CKD, to determine whether assessment of vitamin D catabolism augments clinical care, and to develop new therapeutic approaches to treat impaired vitamin D metabolism in CKD.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of DCCT, MESA, CHS, SKS, and the HEMO Study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org. A full list of principal CHS investigators and institutions can be found at www.chs-nhlbi.org.
Support: This research was supported by grants R01HL096875, R01HL102214, R01HL080295, and R01HL096851 as well as contracts N01HC95159 through N01HC95169 and contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079 through N01HC85083, and N01HC85086 from the National Heart, Lung and Blood Institute; grants R01DK087726, R01DK088762, R01DK081473, and RC4DK090766 from the National Institute of Diabetes and Digestive and Kidney Diseases; grant AG023629 from the National Institute on Aging; grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources; and grant 0575021N from the American Heart Association. These sponsors had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Supplementary Material
Table S1: Sensitivity analysis comparing association of eGFR with specific serum 24,25(OH)2D3 concentration to association of eGFR with summed concentration of dihydroxyvitamin D3 metabolites in CHS.
Figure S1: Correlation of serum concentrations of summed dihydroxyvitamin D3 metabolites with specific 24,25(OH)2D3 in CHS.
Figure S2: Correlation of serum concentrations of summed dihydroxyvitamin D3 metabolites with specific 24,25(OH)2D3 in HEMO. Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Contributor Information
Ian H. de Boer, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
Michael C. Sachs, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Michel Chonchol, Division of Nephrology, Department of Medicine, University of Colorado, Denver, CO.
Jonathan Himmelfarb, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Andrew N. Hoofnagle, Department of Laboratory Medicine, University of Washington, Seattle, WA; Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Joachim H. Ix, Division of Nephrology, Department of Medicine, University of California, San Diego, San Diego, CA.
Robin A. Kremsdorf, Division of Nephrology, Department of Pediatrics, Seattle Children’s Hospital, Seattle, WA; Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Yvonne S. Lin, Department of Pharmaceutics, University of Washington, Seattle, WA.
Rajnish Mehrotra, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Cassianne Robinson-Cohen, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
David S. Siscovick, Cardiovascular Health Research Unit, Department of Medicine, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
Michael W. Steffes, Department of Laboratory Medicine, University of Minnesota, Minneapolis, MN.
Kenneth E. Thummel, Department of Pharmaceutics, University of Washington, Seattle, WA.
Russell P. Tracy, Department of Laboratory Medicine, University of Vermont, Burlington, VT.
Zhican Wang, Department of Pharmaceutics, University of Washington, Seattle, WA.
Bryan Kestenbaum, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
References
- 1.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 2.Dusso A, Lopez-Hilker S, Lewis-Finch J, et al. Metabolic clearance rate and production rate of calcitriol in uremia. Kidney Int. 1989 Mar;35(3):860–864. doi: 10.1038/ki.1989.64. [DOI] [PubMed] [Google Scholar]
- 3.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney international. 2011 Apr;79(7):715–729. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 4.Nigwekar SU, Bhan I, Thadhani R. Ergocalciferol and cholecalciferol in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Jul;60(1):139–156. doi: 10.1053/j.ajkd.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Seminars in nephrology. 2013 Mar;33(2):158–168. doi: 10.1016/j.semnephrol.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. The Journal of clinical investigation. 1984 Dec;74(6):2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989 Aug 3;321(5):274–279. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 8.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007 Oct;72(8):1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 9.Ravani P, Malberti F, Tripepi G, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009 Jan;75(1):88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009 Aug;20(8):1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendrick J, Cheung AK, Kaufman JS, et al. Associations of plasma 25-hydroxyvitamin D and 1,25- dihydroxyvitamin D concentrations with death and progression to maintenance dialysis in patients with advanced kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012 Oct;60(4):567–575. doi: 10.1053/j.ajkd.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosworth CR, Levin G, Robinson-Cohen C, et al. The serum 24,25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney international. 2012 Sep;82(6):693–700. doi: 10.1038/ki.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012 Jul 1;523(1):9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 14.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Oct 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 15.de Boer IH, Sachs MC, Cleary PA, et al. Circulating vitamin D metabolites and kidney disease in type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2012 Dec;97(12):4780–4788. doi: 10.1210/jc.2012-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002 Nov 1;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. The American journal of clinical nutrition. 2013 Jun;97(6):1243–1251. doi: 10.3945/ajcn.112.054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991 Feb;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. The New England journal of medicine. 2002 Dec 19;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 20.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2010 Jun 1;878(19):1639–1642. doi: 10.1016/j.jchromb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem. 2012 Dec;58(12):1711–1716. doi: 10.1373/clinchem.2012.185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clinical Chemistry. 2011 Sep;57(9):1279–1285. doi: 10.1373/clinchem.2010.161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008 Aug;88(2):511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012 Jul 5;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray RW, Weber HP, Dominguez JH, Lemann J., Jr The metabolism of vitamin D3 and 25-hydroxyvitamin D3 in normal and anephric humans. The Journal of clinical endocrinology and metabolism. 1974 Dec;39(6):1045–1056. doi: 10.1210/jcem-39-6-1045. [DOI] [PubMed] [Google Scholar]
- 26.Haddad JG, Jr, Min C, Mendelsohn M, Slatopolsky E, Hahn TJ. Competitive protein-binding radioassay of 24,25-dihydroxyvitamin D in sera from normal and anephric subjects. Arch Biochem Biophys. 1977 Aug;182(2):390–395. doi: 10.1016/0003-9861(77)90519-7. [DOI] [PubMed] [Google Scholar]
- 27.Weisman Y, Eisenberg Z, Leib L, Harell A, Shasha SM, Edelstein S. Serum concentrations of 24,25- dihydroxy vitamin D in different degrees of chronic renal failure. Br Med J. 1980 Sep 13;281(6242):712–713. doi: 10.1136/bmj.281.6242.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horst RL, Littledike ET, Gray RW, Napoli JL. Impaired 24,25-dihydroxyvitamin D production in anephric human and pig. The Journal of clinical investigation. 1981 Jan;67(1):274–280. doi: 10.1172/JCI110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zerwekh JE, McPhaul JJ, Jr, Parker TF, Pak CY. Extra-renal production of 24,25-dihydroxyvitamin D in chronic renal failure during 25 hydroxyvitamin D3 therapy. Kidney international. 1983 Feb;23(2):401–406. doi: 10.1038/ki.1983.33. [DOI] [PubMed] [Google Scholar]
- 30.Koenig KG, Lindberg JS, Zerwekh JE, Padalino PK, Cushner HM, Copley JB. Free and total 1,25- dihydroxyvitamin D levels in subjects with renal disease. Kidney Int. 1992 Jan;41(1):161–165. doi: 10.1038/ki.1992.22. [DOI] [PubMed] [Google Scholar]
- 31.Ishimura E, Nishizawa Y, Inaba M, et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999 Mar;55(3):1019–1027. doi: 10.1046/j.1523-1755.1999.0550031019.x. [DOI] [PubMed] [Google Scholar]
- 32.Dai B, David V, Alshayeb HM, et al. Assessment of 24,25(OH)2D levels does not support FGF23- mediated catabolism of vitamin D metabolites. Kidney international. 2012 Nov;82(10):1061–1070. doi: 10.1038/ki.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helvig CF, Cuerrier D, Hosfield CM, et al. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney international. 2010 Sep;78(5):463–472. doi: 10.1038/ki.2010.168. [DOI] [PubMed] [Google Scholar]
- 34.Nykjaer A, Dragun D, Walther D, et al. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999 Feb 19;96(4):507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Lin YS, Zheng XE, et al. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012 Apr;81(4):498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dusso A, Lopez-Hilker S, Lewis-Finch J, et al. Metabolic clearance rate and production rate of calcitriol in uremia. Kidney international. 1989 Mar;35(3):860–864. doi: 10.1038/ki.1989.64. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CH, Patel S, Buchsbaum BL. Calcitriol metabolism in patients with chronic renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1991 Feb;17(2):185–190. doi: 10.1016/s0272-6386(12)81127-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.