Abstract
The mitochondrion plays a crucial role in the immune system particularly in regulating the responses of monocytes and macrophages to tissue injury, pathogens, and inflammation. In systemic diseases such as atherosclerosis and chronic kidney disease (CKD) it has been established that disruption to monocyte and macrophage function can lead to chronic inflammation. Polarization of macrophages into the pro-inflammatory (M1) and anti-inflammatory (M2) phenotypes results in distinct metabolic reprograming which corresponds to the progression and resolution of inflammation. In this review, we will discuss the role of the mitochondrion in monocyte and macrophage function and how these cells specifically influence the pathophysiology of atherosclerosis and CKD. We propose that assessing monocyte bioenergetics in different disease states could (1) enhance our understanding of the energetic perturbations occurring in systemic inflammatory conditions and (2) aid in identifying therapeutic interventions to mitigate these disorders in patients.
Keywords: monocyte, macrophage, atherosclerosis, chronic kidney disease, oxidative stress, metabolic shift, biomarker
Introduction
In the innate immune system, monocytes and macrophages are derived from myeloid progenitor cells and are vital in the resolution of inflammation caused by tissue injury or infection. The early inflammatory signaling-mediated phenotypic changes induce extravasation and differentiation of circulating monocytes to tissue macrophages, the immune cells that are responsible for phagocytosis and tissue repair. (Luscinskas et al., 1996, Pardali and Waltenberger, 2012, Davies et al., 2013). These diverse functions are carried out by two distinct classes, the M1 (classically activated) or M2 (alternatively activated) macrophages. M1 macrophages mediate the pro-inflammatory response through TNF-α, IL-1β, reactive oxygen species (ROS) and inducible nitric oxide synthase (iNOS) derived nitric oxide. (Mills, 2012, Martinez et al., 2008). M2 macrophages are anti-inflammatory in nature and secrete cytokines such as IL-10, IL-4 and TGF-β to aid in wound repair and healing (Martinez et al., 2008, Novak and Koh, 2013). Metabolism, particularly bioenergetics, plays a central role in regulating the physiological roles of the M1 and M2 phenotypes.
In contrast to their physiological function, the monocyte/macrophage system is perturbed in a number of pathologies associated with chronic inflammation such as atherosclerosis and chronic kidney disease (CKD). The role of monocytes and macrophages in the process of atherosclerotic lesion formation has been widely studied and characterized along with dysfunction of the M1 - M2 transition (Ghattas et al., 2013, Gui et al., 2012). Vascular complications associated with atherosclerosis such as hypertension, tissue ischemia and diabetes can lead to renal injury and result in CKD (Kokubo, 2013, Khatami, 2013, Yamagishi and Imaizumi, 2005). In addition, microvascular complications in patients with CKD can develop cardiovascular complications.
In this review, we will discuss the role of mitochondria in physiological monocyte and macrophage function and this is altered in atherosclerosis and CKD. We will also describe the potential benefits of evaluating monocyte bioenergetics as a translational approach to monitor systemic disease progression and/or identify therapeutic strategies to mitigate disease.
Function of monocytes and macrophages in physiology
Monocytes can be divided into three subtypes based on surface receptor expression. There are 3 major populations of circulating monocytes which are classified by the expression of cluster-determinant (CD) antigens. The “so called” classical monocytes are CD14++CD16− and produce the highest levels of IL-10, a cytokine that mediates tissue repair, and reflects the majority of circulating monocytes in healthy individuals (Wong et al., 2011). The non-classical monocytes are CD14+CD16++ and have an important role in patrolling the vascular endothelium and produce the highest levels of inflammatory cytokines, TNF-α and IL-1β, in response to pathogens and are thought to be involved in phagocytosis (Wong et al., 2011). The intermediate monocytes are CD14++CD16+ and produce the lowest levels of cytokines and chemokines. It is postulated that the classical monocytes mature over time to intermediate then non-classical monocytes (Wong et al., 2011).
Monocytes circulate in the bloodstream and patrol the endothelium for signs of inflammatory distress. In atherosclerosis, inflammation, associated with accumulation of oxidized LDL (oxLDL) in the arterial sub-intimal space, causes monocytes to infiltrate into the tissue where they mature into macrophages (Imhof and Aurrand-Lions, 2004). The macrophages also can be divided into different sub-types depending on their exposure to the prevailing inflammatory microenvironment. Factors such as the cytokine milieu and pathogens dictate the polarization of the macrophages into either the M1 or M2 phenotype (Fig. 1). Exposure to cytokines such as TNF-α IFN-γ leads to production of M1 macrophages, whereas TGF-β and IL-10 produce M2 macrophages (Martinez et al., 2008). Pathogen associated molecular patterns (PAMPs) such as lipopolysaccharide, flagellin from bacteria and double stranded RNA from viruses can activate the toll like receptor (TLR) pathway to engage the NF-κB system to produce inflammatory cytokines that modulate the M1 macrophage phenotype(Zhang and Wang, 2014). Macrophages can switch their metabolism during inflammation from being dependent on glycolysis for ATP synthesis in the M1 state, to relying on oxidative phosphorylation in the M2 state (Rodriguez-Prados et al., 2010, Vats et al., 2006). Interestingly, M1 and M2 macrophages are dynamic and can convert from one form to another, hence the oxidative phosphorylation remains intact in M1 macrophages and glycolytic machinery remains functional in M2 macrophages, allowing for the pathways to be upregulated based on macrophage polarization(Davis et al., 2013, Lumeng et al., 2007).
Figure 1. Differentiation and metabolism of monocytes and tissue macrophages.
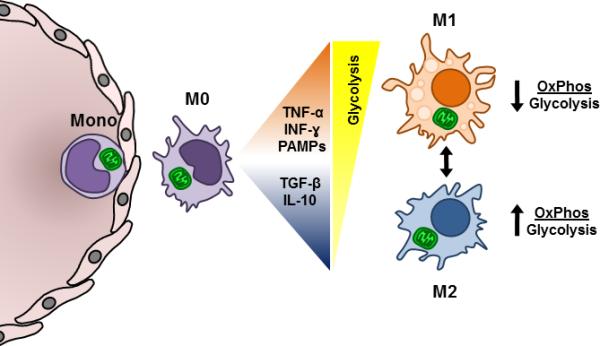
The circulating monocyte is shown exiting the vasculature and proceeding into the tissue and differentiating into a tissue macrophage (M0). These cells utilize oxidative phosphorylation to meet their energetic demand. Upon differentiation, the cytokine and pathogen environment directs their fate to either the M1 or M2 phenotype in the presence of TNFα, PAMPS, INFγ, or TGFβ and IL-10, respectively. M1 macrophages rely on glycolysis for energy production and as such have a lower ratio of oxidative phosphorylation to glycolysis. On the other hand, M2 macrophages preferentially utilize oxidative phosphorylation, and so have a higher ratio of oxidative phosphorylation to glycolysis.
Tissue oxygen tension is also a critical modulator of phenotype switching and metabolic alterations in tissue macrophages. HIF1α-mediated differentiation of macrophage to the M1 phenotype and corresponding upregulation of anerobic glycolytic genes in inflamed tissues suggest critical roles for cellular metabolism and tissue oxygen levels in modulating cell function (Nizet and Johnson, 2009, Shapiro et al., 2011). It is important to note that M1 macrophages can transform into M2 macrophages during the resolution phase of inflammation (Fig.1). As oxygen levels increase, peroxisome proliferator activated receptor-γ (PPAR-γ) is induced, which stimulates mitochondrial biogenesis and a shift from anaerobic glycolysis to oxidative phosphorylation, particularly through fatty acid oxidation (Huang et al., 1999, Vats et al., 2006). It has been shown that pharmacological inhibition of the mitochondrial oxidative phosphorylation, inhibits the expression of markers of the M2 phenotype. (Vats et al., 2006). Bioenergetic analysis of murine macrophages demonstrated that M1 mediators, but not M2, increased glycolysis, and decreased oxidative phosphorylation (Haschemi et al., 2012).
The focus of this review is on the modulation of the metabolism of monocyte/macrophages during their normal biological function in innate immunity. Interestingly, it is also clear that lymphocytes or T cells which are part of the adaptive immune system also undergo a metabolic switch during inflammation (Pearce, 2010). T cells are major regulators of the inflammatory environment in atherosclerosis as they can produce INF-γ and other inflammatory cytokines as well as anti-inflammatory cytokines depending on their effector or suppressor status(Pastrana et al., 2012). Similar to macrophages, T-cell activation and the resultant cytokine production requires a metabolic switch from a primarily oxidative phosphorylation phenotype to one of aerobic glycolysis (Pearce, 2010).
Function of monocytes and macrophages in pathology
Atherosclerosis
Monocytes are precursors to atherosclerotic lesion macrophages, which are both instrumental in the initiation and progression of the disease. Higher levels of circulating blood monocytes are significantly associated with obesity and increased risk of peripheral arterial disease (Nasir et al., 2005, Kullo et al., 2002). A recent study found that high levels of CD14++CD16+ intermediate monocytes predicted a high incidence of cardiovascular events such as myocardial infarction (Rogacev et al., 2012). Monocyte mitochondrial DNA damage and decreased complex I and IV activity along with increased cytokine release has been identified in mouse models of atherosclerosis (Yu et al., 2013).
CD36 receptor on macrophages mediates phagocytosis of oxLDL, leading to formation of foam cells. Uptake of oxLDL stimulates the switch to the M2 phenotype (Rios et al., 2013). Clearance of foam cells is the next step in this inflammatory cascade, and this is accomplished by other macrophages that engulf and clear the foam cells, a process termed efferocytosis (Fig.2A). M2 macrophages promote efferocytosis, therefore the recruitment and the M2 phenotype is important in foam cell homeostasis (Tabas, 2010). Efferocytosis has been shown to induce production of IL-10 and TGF-β, anti-inflammatory mediators that can help in tissue repair and resolution of inflammation (Henson et al., 2001). During physiology, the uptake of oxLDL and the clearance of damaged cells by efferocytosis are critical in prevention of plaque formation.
Figure 2. Physiological and pathological fates of macrophages.
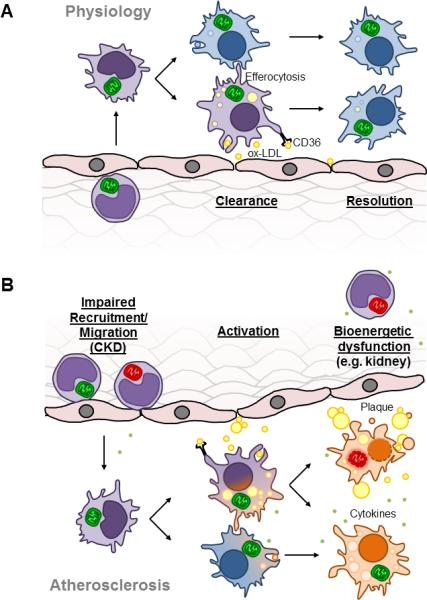
A. Scheme depicting physiological monocyte extravasation into the subintimal space of a vessel and differentiation into a macrophage to clear oxidized LDL (oxLDL) through the CD36 scavenger receptor. These macrophages can then differentiate into M2 macrophages to resolve the inflammation as well as clear damaged cells by efferocytosis. B. In atherosclerosis, impaired recruitment and migration of monocytes facilitates increased macrophage differentiation in the subintimal space. High levels of ox-LDL leads to oxidative stress and bioenergetic dysfunction in macrophages which may proceed to apoptosis or the M1 macrophage phenotype.
Increased levels of circulating monocytes has a direct effect on increasing the number of macrophages that populate the intimal region, and studies have shown that there is increased macrophage density in ruptured human atherosclerotic plaques (Lendon et al., 1991). Macrophages produce ROS upon exposure to oxLDL, and it has been shown that a substantial portion of the ROS is mitochondrially derived (Wang et al., 2014, Bae et al., 2009, Park et al., 2009). ROS can damage various mitochondrial components such as TCA cycle enzymes, electron transport chain proteins and mitochondrial DNA (Zeevalk et al., 2005, Ide et al., 2001, Graziewicz et al., 2002). This may prevent the metabolic shift to oxidative phosphorylation decreasing the numbers of M2 macrophages available to take part in the clearance of lipid laden foam cells. Indeed, as illustrated in Figure 2B the increased uptake of ox-LDL and oxidative damage may facilitate the M2 to M1 macrophage transition. M2 macrophages have a lower propensity to become foam cells, but high phagocytic activity demonstrating the protective role of M2 macrophages in atherosclerosis (Chinetti-Gbaguidi et al., 2011). Further, mitochondrial damage can lead to cytochrome c release and apoptosis in the foam cells (Fig. 2B), and disrupts the ability of neighboring macrophages to ingest these apoptotic bodies (Eguchi et al., 1997). This causes enlargement of the lesion, and an uncontrolled secondary necrotic cell death, plaque instability and rupture (Seimon and Tabas, 2009).
Monocyte polarization plays a vital role in prognosis of atherosclerosis, yet their mitochondrial regulation and dynamics has not been fully elucidated. Understanding the metabolic regulation of the bioenergetic monocyte populations presents a novel therapeutic target for atherosclerosis. There is also evidence that an intact mitochondrial system is important for M2 macrophages that are involved in foam cell clearance, thereby indicating modulation of macrophage metabolism as a therapeutic intervention.
Chronic Kidney Disease
Diabetes is a systemic disease associated with severe cellular bioenergetic dysfunction in a broad range of tissues (Rains and Jain, 2011, Jagielski and Piesiewicz, 2011, Giacco and Brownlee, 2010, Locatelli et al., 2003, Ritov et al., 2005, Aneja et al., 2008). A common secondary complication of diabetes is chronic kidney disease (CKD), where progressive decline in renal function over time necessitates dialysis or transplantation. In addition, both the innate and adaptive immune system show dysfunction in CKD patients which has been linked to the increased risk of morbidity and mortality (Middleton and Pun, 2010). As shown in Figure 2, monocytes from CKD patients have been shown to have impaired adhesion and migratory capabilities and this is thought to contribute to the development of atherosclerotic complications (Al-Chaqmaqchi et al., 2013). The intermediate monocytes (CD14++CD16+) are the most prominent monocytes in the circulation of CKD patients and have been used as selective predictors of adverse outcomes such as cardiovascular disease and mortality (Heine et al., 2012).
As CKD progresses there is a chronic state of systemic inflammation that can further induce oxidative stress and cellular bioenergetic dysfunction. Several reports have shown that pro-inflammatory cytokines such as IL-6, IL-10 and TNFα are elevated in the circulation of CKD patients (Himmelfarb et al., 2004, Sardenberg et al., 2004, Dounousi et al., 2012) which can negatively affect immune cell mitochondrial function. In particular, mononuclear cells from Type 2 diabetics have lower mitochondrial mass, higher mitochondrial membrane potential and increased superoxide generation (Widlansky et al., 2010). It has also been reported that mitochondrial respiratory complex IV (COX), subunits I and IV are upregulated in PBMC from CKD patients; however, complex IV activity is significantly decreased (Granata et al., 2009). The findings from these reports support the concept that the inflammatory conditions during CKD can directly affect mitochondrial complexes within peripheral blood cells. Notably, both the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and nuclear respiratory factor-1 (NRF-1) genes, involved in mitochondrial biogenesis and function, respectively, are down regulated in PBMC in CKD patients on peritoneal dialysis (Zaza et al., 2013). CKD patients on dialysis also have an increased risk of developing sepsis (Sardenberg et al., 2004) and this is thought to be influenced by alterations in monocyte mitochondrial function. Indeed, a reduction in F1Fo adenosine-5’-triphosphate synthase activity was linked to dysfunctional mitochondrial bioenergetics in immune cells from patients with septic shock (Japiassu et al., 2011).
This disruption in mitochondrial function can elicit further oxidative stress. It has been reported that intracellular ROS and DNA oxidative damage is induced in PBMCs during CKD (Granata et al., 2009). Consequently, these events can negatively affect other organs in the body since monocytes accumulate both in the peripheral circulation and in sites of interstitial inflammation (Wallquist et al., 2013). This is important because both elevated oxidative stress and mitochondrial dysfunction can lead to increased apoptosis in CKD monocytes (Dounousi et al., 2012) and tissues. Interestingly, the oxidative burst, which is necessary for innate immunity, is suppressed in diabetics suggesting that decreased oxidative burst activity could be related to the severity of renal dysfunction (Dickerson et al., 2012), a suppressed immune response, accumulation of damaged monocytes, and disease pathology. It is clear that mitochondrial homeostasis and gene expression is perturbed in immune cells during diabetes and is likely a critical factor in the development of atherosclerosis in these patients.
Future Outlook
The emerging role of bioenergetic function in inflammation and monocyte activation in CKD and atherosclerosis and its complications suggests that therapeutic interventions at the level of the mitochondrion could be beneficial and that bioenergetic biomarkers maybe a new approach to monitoring disease progression. The concept that circulating blood cells can serve as a surrogate for the severity of systemic diseases such as diabetes and sepsis has been recognized previously (Zharikov and Shiva, 2013, Avila et al., 2012, Guo et al., 2009, Widlansky et al., 2010, Satoh et al., 2010). In turn this has led us to propose the development of an integrated value of cellular mitochondrial function we have termed the Bioenergetic health index (BHI) (Chacko et al, 2014). It is clear that has the field progresses mitochondria in leukocytes and platelets are emerging as both biomarkers of metabolic stress and mediators of the complex pathologies associated with diseases such as CKD and atherosclerosis (Chacko et al., 2013, Kramer et al., 2014, Mitchell et al., 2013, Chacko et al., 2011).
Organelle Facts.
Mitochondrial oxidative phosphorylation and glycolysis support monocyte/macrophage function.
Macrophages undergo a metabolic switch from glycolysis to oxidative phosphorylation during inflammation.
Mitochondrial ROS are produced by macrophages following oxidized LDL exposure
Monocyte mitochondrial DNA and electron transport chain activity are damaged in mouse models of atherosclerosis.
Mitochondria in monocytes from patients with chronic kidney disease are impaired.
Genes involved in mitochondrial biogenesis (PGC-1α and NRF-1) are down-regulated in PBMC's of CKD patients undergoing peritoneal dialysis.
Acknowledgements
The authors appreciate support from the American Heart Association (SR), NIH T32HL007457 (TM), NIH T32HL07918 (PAK), P30DK056336 (BKC), NIDDK Diabetic Complications Consortium (DiaComp, www.diacomp.org), grant DK076169 (sub-award VDU) the O'Brien Center P30 DK079337.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Chaqmaqchi HA, Moshfegh A, Dadfar E, Paulsson J, Hassan M, Jacobson SH, Lundahl J. Activation of Wnt/β-catenin pathway in monocytes derived from chronic kidney disease patients. PLoS One. 2013;8:e68937. doi: 10.1371/journal.pone.0068937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, Sack MN. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2012;120:248–251. doi: 10.1055/s-0031-1285833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res. 2009;104:210–218. doi: 10.1161/CIRCRESAHA.108.181040. 221p following 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP, Ferrick D, Singal AK, Ballinger SW, Bailey SM, Hardy RW, Jianhua Zhang J, Zhi D, Darley-Usmar VM. The Bioenergetic Health Index: A New Concept in Mitochondrial Translational Research. Clinical Science. 2014 doi: 10.1042/CS20140101. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93:690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti-Gbaguidi G, Baron M, Bouhlel MA, Vanhoutte J, Copin C, Sebti Y, Derudas B, Mayi T, Bories G, Tailleux A, Haulon S, Zawadzki C, Jude B, Staels B. Human atherosclerotic plaque alternative macrophages display low cholesterol handling but high phagocytosis because of distinct activities of the PPARgamma and LXRalpha pathways. Circ Res. 2011;108:985–995. doi: 10.1161/CIRCRESAHA.110.233775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4:e00264–00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R, Deshpande B, Gnyawali U, Lynch D, Gordillo GM, Schuster D, Osei K, Roy S. Correction of aberrant NADPH oxidase activity in blood-derived mononuclear cells from type II diabetes mellitus patients by a naturally fermented papaya preparation. Antioxid Redox Signal. 2012;17:485–491. doi: 10.1089/ars.2011.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dounousi E, Koliousi E, Papagianni A, Ioannou K, Zikou X, Katopodis K, Kelesidis A, Tsakiris D, Siamopoulos KC. Mononuclear leukocyte apoptosis and inflammatory markers in patients with chronic kidney disease. Am J Nephrol. 2012;36:531–536. doi: 10.1159/000345352. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541–1551. doi: 10.1016/j.jacc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, Carella M, Schena FP, Grandaliano G, Pertosa G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics. 2009;10:388. doi: 10.1186/1471-2164-10-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziewicz MA, Day BJ, Copeland WC. The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Res. 2002;30:2817–2824. doi: 10.1093/nar/gkf392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y. Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediators Inflamm. 2012;2012:693083. doi: 10.1155/2012/693083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wu J, Du J, Ran J, Xu J. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets. 2009;20:588–593. doi: 10.3109/09537100903288422. [DOI] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, Amir S, Lubec G, Park J, Esterbauer H, Bilban M, Brizuela L, Pospisilik JA, Otterbein LE, Wagner O. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine GH, Ortiz A, Massy ZA, Lindholm B, Wiecek A, Martínez-Castelao A, Covic A, Goldsmith D, Süleymanlar G, London GM, Parati G, Sicari R, Zoccali C, Fliser D, (ERA-EDTA), E. R. a. C. M. E. m. w. g. o. t. E. R. A.-E. D. a. T. A. Monocyte subpopulations and cardiovascular risk in chronic kidney disease. Nat Rev Nephrol. 2012;8:362–369. doi: 10.1038/nrneph.2012.41. [DOI] [PubMed] [Google Scholar]
- Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Le P, Klenzak J, Freedman S, McMenamin ME, Ikizler TA, Group P. Impaired monocyte cytokine production in critically ill patients with acute renal failure. Kidney Int. 2004;66:2354–2360. doi: 10.1111/j.1523-1755.2004.66023.x. [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- Jagielski AK, Piesiewicz A. [Diabetes as the challenge to 21 century medicine--insights from clinical and biochemical investigations]. Postepy biochemii. 2011;57:191–199. [PubMed] [Google Scholar]
- Japiassu AM, Santiago AP, d'Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC, Bozza FA, Oliveira MF. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit Care Med. 2011;39:1056–1063. doi: 10.1097/CCM.0b013e31820eda5c. [DOI] [PubMed] [Google Scholar]
- Khatami MR. Ischemic nephropathy: more than a simple renal artery narrowing. Iran J Kidney Dis. 2013;7:82–100. [PubMed] [Google Scholar]
- Kokubo Y. Carotid atherosclerosis in kidney disease. Contrib Nephrol. 2013;179:35–41. doi: 10.1159/000346720. [DOI] [PubMed] [Google Scholar]
- Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30). Am J Cardiol. 2002;89:1441–1443. doi: 10.1016/s0002-9149(02)02366-4. [DOI] [PubMed] [Google Scholar]
- Lendon CL, Davies MJ, Born GV, Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. doi: 10.1016/0021-9150(91)90235-u. [DOI] [PubMed] [Google Scholar]
- Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:1272–1280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas FW, Ding H, Tan P, Cumming D, Tedder TF, Gerritsen ME. L-and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Middleton JP, Pun PH. Hypertension, chronic kidney disease, and the development of cardiovascular risk: a joint primacy. Kidney Int. 2010;77:753–755. doi: 10.1038/ki.2010.19. [DOI] [PubMed] [Google Scholar]
- Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- Mitchell T, Johnson MS, Ouyang X, Chacko BK, Mitra K, Lei X, Gai Y, Moore DR, Barnes S, Zhang J, Koizumi A, Ramanadham S, Darley-Usmar VM. Dysfunctional mitochondrial bioenergetics and oxidative stress in Akita(+/Ins2)-derived beta-cells. Am J Physiol Endocrinol Metab. 2013;305:E585–599. doi: 10.1152/ajpendo.00093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999-2002. Arterioscler Thromb Vasc Biol. 2005;25:1966–1971. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali E, Waltenberger J. Monocyte function and trafficking in cardiovascular disease. Thromb Haemost. 2012;108:804–811. doi: 10.1160/TH12-04-0276. [DOI] [PubMed] [Google Scholar]
- Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana JL, Sha X, Virtue A, Mai J, Cueto R, Lee IA, Wang H, Yang XF. Regulatory T cells and Atherosclerosis. J Clin Exp Cardiolog. 2012;2012:2. doi: 10.4172/2155-9880.S12-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL. Metabolism in T cell activation and differentiation. Current opinion in immunology. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios FJ, Koga MM, Pecenin M, Ferracini M, Gidlund M, Jancar S. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm. 2013;2013:198193. doi: 10.1155/2013/198193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, Grosse-Dunker G, Heisel I, Hornof F, Jeken J, Rebling NM, Ulrich C, Scheller B, Bohm M, Fliser D, Heine GH. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Sardenberg C, Suassuna P, Watanabe R, Cruz Andreoli MC, Aparecida Dalboni M, Faria Seabra V, Draibe SA, Cendoroglo Neto M, Jaber B. Balance between cytokine production by peripheral blood mononuclear cells and reactive oxygen species production by monocytes in patients with chronic kidney disease. Ren Fail. 2004;26:673–681. doi: 10.1081/jdi-200037122. [DOI] [PubMed] [Google Scholar]
- Satoh N, Shimatsu A, Himeno A, Sasaki Y, Yamakage H, Yamada K, Suganami T, Ogawa Y. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33:e7. doi: 10.2337/dc09-1315. [DOI] [PubMed] [Google Scholar]
- Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50(Suppl):S382–387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. ScientificWorldJournal. 2011;11:2509–2529. doi: 10.1100/2011/397971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallquist C, Paulson JM, Hylander B, Lundahl J, Jacobson SH. Increased accumulation of CD16+ monocytes at local sites of inflammation in patients with chronic kidney disease. Scand J Immunol. 2013;78:538–544. doi: 10.1111/sji.12115. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage Mitochondrial Oxidative Stress Promotes Atherosclerosis and Nuclear Factor-kappaB-Mediated Inflammation in Macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Translational research : the journal of laboratory and clinical medicine. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–2299. doi: 10.2174/1381612054367300. [DOI] [PubMed] [Google Scholar]
- Yu E, Calvert PA, Mercer JR, Harrison J, Baker L, Figg NL, Kumar S, Wang JC, Hurst LA, Obaid DR, Logan A, West NE, Clarke MC, Vidal-Puig A, Murphy MP, Bennett MR. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation. 2013;128:702–712. doi: 10.1161/CIRCULATIONAHA.113.002271. [DOI] [PubMed] [Google Scholar]
- Zaza G, Granata S, Masola V, Rugiu C, Fantin F, Gesualdo L, Schena FP, Lupo A. Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS One. 2013;8:e77847. doi: 10.1371/journal.pone.0077847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevalk GD, Bernard LP, Song C, Gluck M, Ehrhart J. Mitochondrial inhibition and oxidative stress: reciprocating players in neurodegeneration. Antioxid Redox Signal. 2005;7:1117–1139. doi: 10.1089/ars.2005.7.1117. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang CC. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int. 2014;13:138–152. doi: 10.1016/s1499-3872(14)60024-2. [DOI] [PubMed] [Google Scholar]
- Zharikov S, Shiva S. Platelet mitochondrial function: from regulation of thrombosis to biomarker of disease. Biochem Soc Trans. 2013;41:118–123. doi: 10.1042/BST20120327. [DOI] [PubMed] [Google Scholar]


