1. Introduction
Direct damage reversal (DDR) mechanisms correct DNA lesions without cutting the DNA backbone. Instead, the damaging chemical group is directly removed from the modified DNA base itself, making DDR the least invasive DNA repair approach possible. DDR pathways are also unique in that they involve only one type of protein, meaning that one protein has to be able to fulfill all the tasks, it has to find the damage, verify it, and remove it. DDR pathways are only available for very few select types of DNA lesions; in humans, direct repair mechanisms are limited to a subset of base-alkylation products [1–3]. DNA alkylation is highly mutagenic and cytotoxic and ubiquitously occurs, for instance, through natural endogeneous alkylating agents [4, 5]. The focus of the work described here, the O6-alkylguanine DNA alkyltransferase (AGT), is a highly-conserved protein responsible for direct repair of alkylated guanine and to a lesser degree thymine bases [3, 5–10]. In addition to protecting the genome under normal conditions, AGT has also recently become a highly interesting target for inhibitor development because it interferes with the deliberate application of DNA alkylation during chemotherapy. Inhibitors of AGT are currently in clinical trial, with the aim of mitigating this consequence of AGT action [1, 11–16].
Human AGT is a small, monomeric protein (Mr ~ 21.5 kDa) [17]. Crystal structures of AGT are available for the apo- [18, 19], alkylated [18, 20], and DNA-bound forms of the protein [2, 21] (Figure 1). These structures show that it binds to the minor groove of DNA via a helix–loop–helix motif and flips the damaged base into an active site cleft (Figure 1A), where nucleophilic attack from a cysteine residue results in transfer of the alkyl group to the protein [22]. No mechanism for dealkylation of the active site cysteine has been discovered; thus AGT appears to be a “single cycle” or “suicide” enzyme. A comparison of crystal structures of AGT in its active form prior to DNA repair, and its methylated form following repair shows that a short helix near the active site becomes unstructured upon alkylation (Figure 1B). It has been suggested that the modest destabilization of the native fold by 0.5–1.2 kcal/mole accelerates ubiquitinylation and the subsequent degradation of alkylated AGT [17, 18, 20, 23–25]. Thus each AGT molecule can catalyze only a single alkyltransfer reaction and the DNA-repair capacity of a cell depends on its concentration of unreacted AGT molecules.
Figure 1. AGT structures.
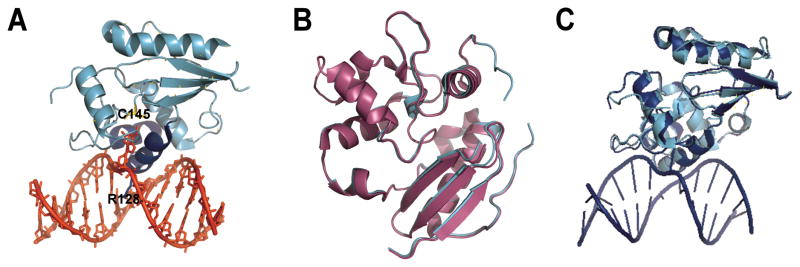
(A) AGT (cyan and blue) binds to the minor groove of the DNA double helix (shown in red) via a helix-loop-helix motif (blue), leading to flipping of the alkylated base into the active site cleft of the protein where it is transferred onto a cysteine (C145). An arginine finger (R128) stabilizes the void left by the flipped base. (Coordinates from 1T38.pdb) (B) Overlay of the alkylated (magenta; 1EH6.pdb) and the apo (cyan; 1EH7.pdb) forms of AGT. Alkylation destabilizes a short alpha helix near the active site. (C) Overlay of the AGT monomer bound to a DNA lesion (blue; 1T38.pdb) with the unbound protein (cyan, 1EH6.pdb). DNA binding does not introduce significant structural changes in AGT (RMSD 0.6 Å, determined with DaliLite, EMBL-EBI).
The available crystal structures show monomeric AGT bound at isolated DNA sites, occupying 8 bp on one face of the DNA cylinder [2, 21, 22]. However, solution studies indicate that AGT binds cooperatively, with statistical binding site size of 4 bp/monomer [17, 26, 27]. These disparate binding site sizes can be reconciled by models in which adjacent proteins occupy partially-overlapping sites [26, 27]. The resulting DNA segments coated with AGT molecules [17, 26–30] are likely to contribute to lesion-search processes. Mutational studies suggest that cooperative DNA binding plays a role in the physiological activities of AGT. Mutations located in the proposed protein-protein interface of AGT and far from the DNA contact surface were found to have strong effects on binding cooperativity in vitro and resistance to alkylating agents in vivo [30].
Cooperative interactions are frequently encountered in protein-DNA complexes [31–40]. The mechanisms of these cooperative interactions are as diverse as their target functions, which include modulation of DNA-affinity [34–36], stabilization of DNA superstructures [37, 38], and regulation of enzyme activity [33]. Atomic force microscopy (AFM) imaging has been applied to study a number of cooperative protein-DNA systems ranging from dimeric protein complexes to long multimeric nucleofilaments [31, 38–40]. AFM provides direct access to structural features of cooperative protein-DNA complexes at the level of the individual molecules and can provide data essential for an understanding of the underlying mechanisms. Importantly, the concentration-dependent length of the protein complexes on the DNA, which is directly related to the intrinsic DNA affinity and the cooperativity of the interactions can be derived from AFM images. These characteristics will be discussed in the context of the AGT-DNA system, below.
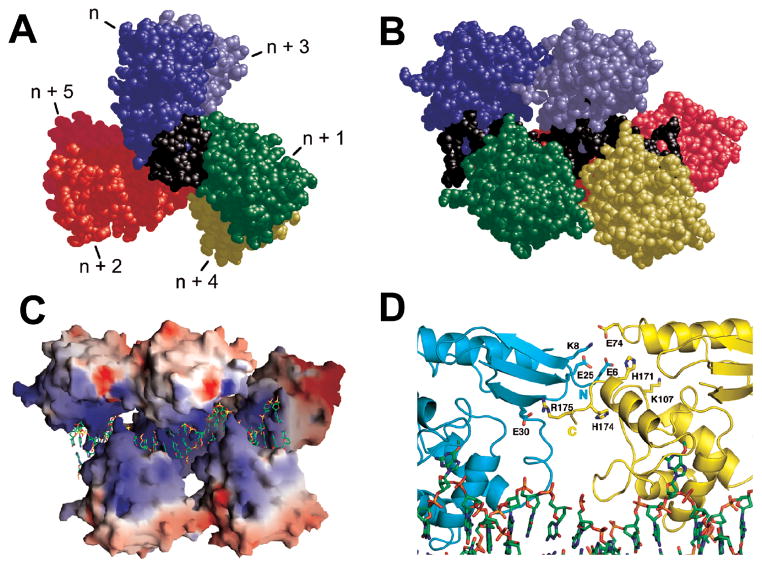
Models of cooperative AGT assemblies have been built, based on the crystal structures of DNA-bound AGT monomers [2, 21] and taking into account observed binding densities and similar affinities for single-stranded and duplex substrates [26, 27]. In these models (for example Figure 2), each protein monomer in the cooperative unit is rotated with respect to its neighbors by 138° (corresponding to an apparent binding site size of 4 bp). A surface-charge representation of the cooperative complex model reveals a positively-charged channel of AGT monomers that is occupied by the negatively charged DNA. In this model, each nth and (n+3)rd monomer in the complex interact directly, but contacts between adjacent monomers are weak or non-existent. This model of non-specific cooperative AGT-DNA lesion search complexes can be tested by single molecule AFM imaging.
Figure 2. Model of a cooperative AGT-DNA complex.
View down (A) and perpendicular to the DNA axis (B). The repeating unit of this model is one molecule of AGT (colors) plus 4 bp of DNA (black). Repeating units were based on a crystal structure [2] and juxtaposed so as to preserve B-form DNA parameters, producing a three-start helical array with contacts between proteins n and n+3. (C) Electrostatic surface potentials of the helical protein array (red −8kT, blue +8kT). (D) Interface between protein units n (cyan) and n+3 (yellow). The charged side chains provide interaction points. C-termini are pointed to the left in (B, C and D). Reprinted with permission from [27], copyright Elsevier, 2009.
2. Model description of non-specific cooperative protein-DNA interactions
The McGhee-von Hippel binding model is often used to characterize non-specific cooperative protein-DNA interactions [41, 42]. While this model simplifies some of the complex biological processes, it provides useful guidelines for an overall description and comparison of cooperatively interacting systems. Simplifying assumptions used in the model include a single binding mode for all protein molecules within the cooperative unit, characterized by a unique binding site size s, equilibrium constant K, and cooperativity parameter ω. Fixing these values implies that binding affinity and cooperativity are not DNA sequence-, base composition-, or structure-dependent and that the cooperativity remains constant regardless of the position of a protein within a cooperative assembly and the number of interactions involved in its integration in the cooperative unit. Estimates for s, K, and ω can be obtained experimentally by sedimentation equilibrium centrifugation or gel mobility shift techniques, and evaluated using a variant of the Scatchard equation (Equation 1) [26, 27].
| (eq. 1) |
Here ν is the protein binding density on the DNA and [P] is the free protein concentration supporting the equilibrium. When binding is defined according to the McGhee-von Hippel model, the mean cooperative cluster size C̄ is predicted to be [42]:
| (eq. 2) |
For AGT binding to long DNA substrates of 1,000 and ~2,700 bp length, values of s = (6.81 ± 0.14) and (6.32 ± 0.12) bp, K = (7960 ± 916) and (9667 ± 1499), and ω = (44.2 ± 3.8) and (35.9 ± 6.8) were obtained, respectively, from sedimentation equilibrium analytical ultracentrifugation data [7]. The observed limiting binding site sizes were larger than the value of ~4 bp/protein obtained with shorter DNA oligonucleotides [26]. This likely reflects packing inhomogeneities that occur when the substrate is large and which are largely absent near binding saturation, when short substrates are used. The dependence of cluster size C̄ on AGT concentration predicted by McGhee-von Hippel theory using these values of s, K, and ω is shown in Figure 3 [7].
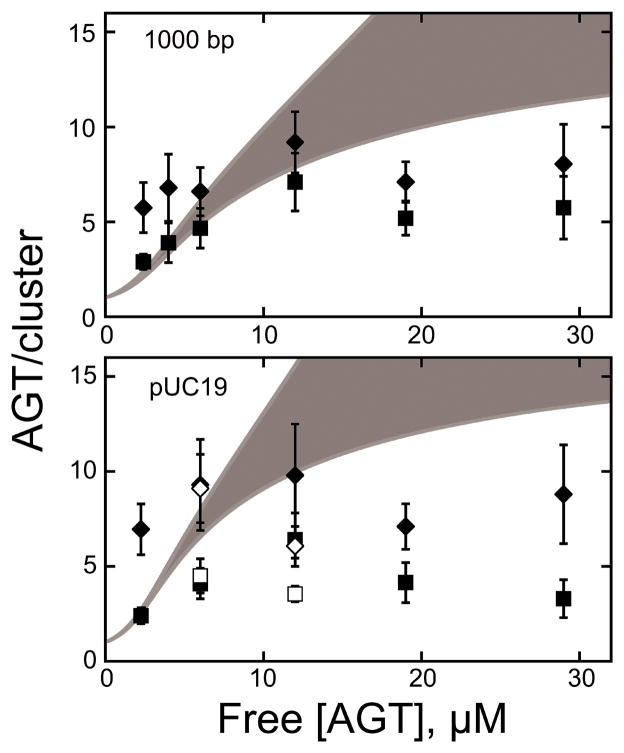
Figure 3. Measurement of binding parameters and prediction of cooperative cluster sizes.
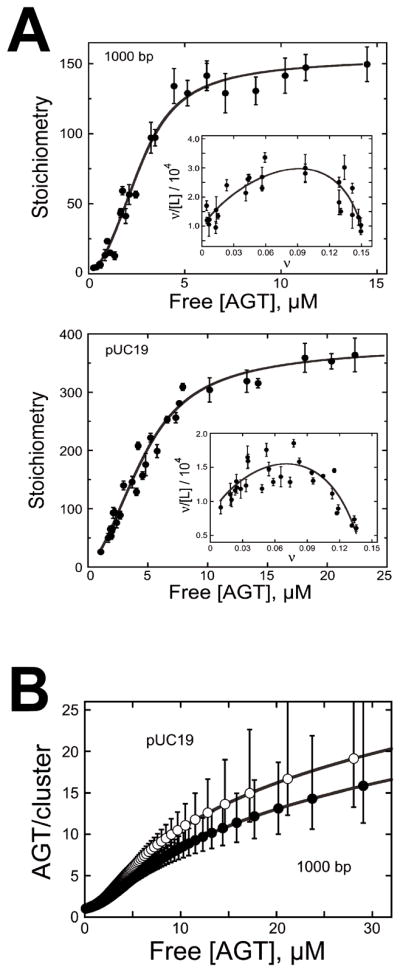
[7] (A) Dependence of binding stoichiometry on free AGT concentration for the 1,000 bp fragment (upper panel) and linear pUC19 DNA (lower panel). Stoichiometries were inferred from weight-average molecular weights measured at sedimentation equilibrium. Error bars are 95% confidence limits for the individual parameters. The smooth curve is an isotherm calculated with Eq. 1 using parameters determined from the Scatchard plots (insets). Insets: The solid curves are fits of Eq. 1, returning K = 9667 ± 1499, ω = 35.9 ± 6.8 and s = 6.32 ± 0.12 for binding the 1,000 bp fragment and K = 7960 ± 916, ω = 44.2 ± 3.8 and s = 6.81 ± 0.14 for binding linear pUC19 DNA. (B) Ranges for C̄ (AGT/cluster) were calculated with Eq. 2 using values of ν, ω, and s and corresponding error ranges determined from Scatchard plot fits (shown in (A)).
3. Characterization of non-specifically DNA bound AGT clusters by AFM
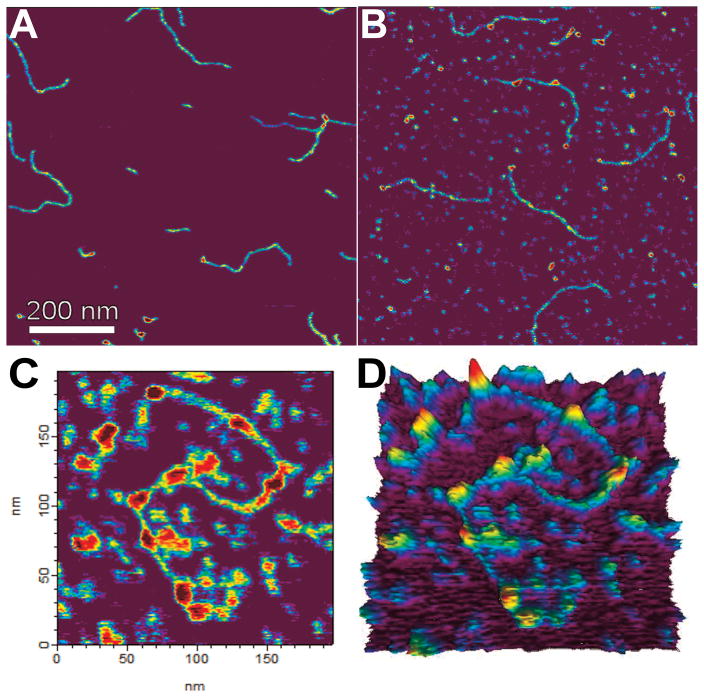
AFM imaging allowed the direct characterization of AGT complexes with long DNA substrates (1,000 and ~2,700 bp length, same as above; Figure 4) [7]. Since these DNA substrates did not contain any introduced O6-methylguanine sites, binding should be dominated by non-specific interactions. Thus, we believe the observable complexes are representative of AGT assemblies in the process of damage search, rather than specific lesion-bound structures.
Figure 4. AFM images of AGT-DNA complexes.
AFM images of the 1,000 bp DNA fragment in the absence of protein (A) and after incubation with AGT at 6 μM (B) or 12 μM AGT (C). Note the increasing numbers of AGT clusters on the DNA with increasing protein concentration. A 3-dimensional representation of the data from (C) is shown in (D).
DNA bending in cooperative clusters
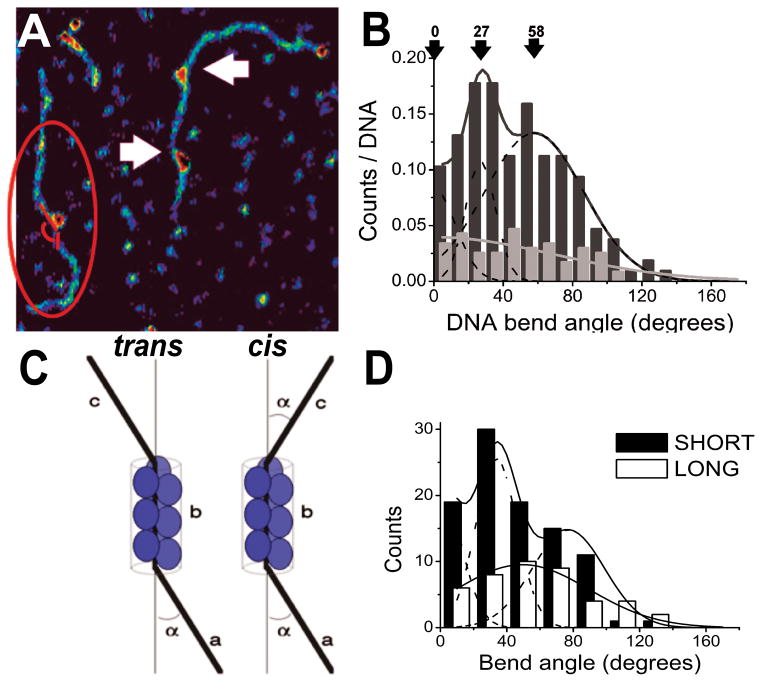
AFM images of AGT-DNA complexes show DNA bending that is localized to the sites flanking AGT clusters (arrows Figure 5A) [7]. Analysis of DNA bend angles gave a triphasic distribution (Figure 5B) consistent with models in which AGT arrays adopt a helical arrangement (Figure 2) with each protein monomer bending the DNA by ~27°. Surface-deposition of these structures results in different possible relative orientations of cis or trans of the entering and leaving DNA segments in two dimensions (Figure 5C). The relative orientations of flanking DNA segments depend on the number of monomers in the protein cluster, since each monomer is rotated by 138° with respect to its nearest neighbors and bends the DNA in the plane of the interaction. Consistent with this prediction, AGT clusters grouped according to their lengths showed distinct differences in their bend angle distributions (Figure 5D).
Figure 5. DNA bending introduced by AGT binding.
(A) DNA bend angles were measured along the DNA contour flanking the protein clusters (red lines). Arrows indicate DNA bending at cluster ends. (B) Bend angle distributions in the presence of AGT (black, n = 139) display three peaks, centered around 0 ± 13 degrees, 27 ± 9 degrees and 58 ± 29 degrees (R2 > 0.97, Gaussian decomposition: dashed lines). In the absence of AGT (grey, n = 75), bend angles measured at peaks on the DNA show a broad distribution centered at zero degrees. (C) Model of 2-dimensional arrangement of DNA strands entering (a) and exiting (c) the protein cluster (with length b). In the trans orientation, the overall angle between (a) and (c) is 0 degrees, in cis the two angles add up to a total of 2α. (D) Separate Gaussian fits (lines) to short (≤ 5 proteins, black bars) and long (> 6 proteins; white bars) cluster populations suggest for the short clusters mean bend angles of 0 ± 10 degrees, 26 ± 13 degrees, and 60 ± 27 degrees (dashed lines showing the three Gaussian components; R2 > 0.99, n = 81), for the longer complexes a broad distribution centered at a mean bend angle of (50 ± 35) degrees (R2 > 0.97, n = 33).
AGT binding has little or no sequence specificity
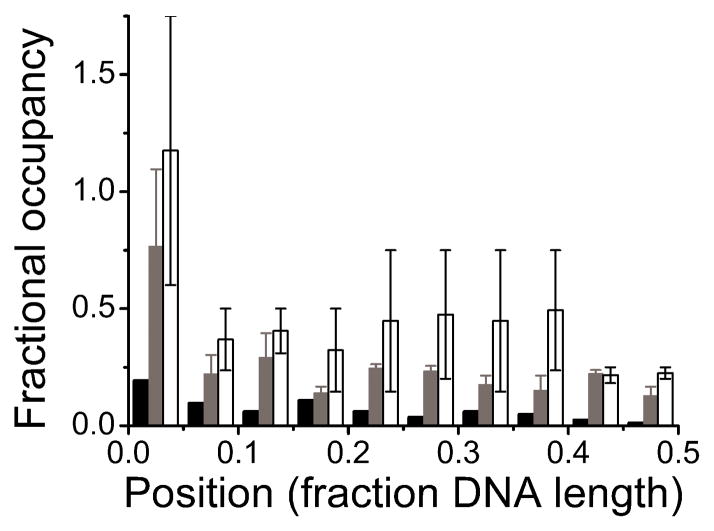
The distribution of complexes among internal sites on the two substrates was nearly uniform, with little evidence of sequence or base composition preferences (Figure 6). The absence of sequence specificity accords well with results obtained with short oligonucleotides, and with the expectation that AGT should function well in any sequence context [29, 43]. However, the AFM data also indicated a preferential binding at DNA fragment ends that could not be resolved by other techniques. AGT is known to accomplish its repair function by “flipping” a target base out of the duplex stack [2, 18, 21, 44] and the enhanced affinity for DNA ends may be a consequence of the reduced stability of the DNA double helix at molecular ends (“breathing”). Consistent with this notion, base-flipping activity by AGT was indeed found to be enhanced at DNA fragment ends [7]. The observation that AGT binding is sequence independent, justifies use of the McGhee-von Hippel binding model for analysis of AGT-DNA interactions.
Figure 6. Distributions of AGT clusters on 1,000 bp DNA.
Elevated binding densities at ends reflect preferential interactions. Away from the ends, uniform binding densities are incompatible with DNA sequence or base-composition preference. Because the unmodified DNA ends used in these experiments cannot be distinguished, locations are reported in units of DNA length from 0% (at a DNA end) to 50% (at the DNA center). Low and high [AGT] correspond to incubations at 2 μM (grey, 2.6 +/− 0.3 peaks per DNA, n = 220), and 12 μM AGT (white, 4.6 +/− 2.2 peaks per DNA, n = 159), respectively. For comparison, DNA in the absence of protein is shown (black, 0.7 peaks per DNA, n = 54), demonstrating a low background, likely due to salt contaminations on the DNA.
Cooperative cluster size of AGT
The dimensions of the DNA bound clusters were larger than those of non-DNA-bound AGT monomers in the images, indicating that the assemblies on DNA contained several closely-juxtaposed AGT molecules (Figure 7). In principle, AFM imaging allows measurement of the average dimensions of protein-DNA complexes, including the length of cooperative clusters. However, AFM images represent convolutions of the sample topography and the AFM probe geometry. The contribution from the AFM tip (~2 – 20 nm) to image dimensions becomes significant for samples with particle radii similar to those of the AFM tip (Figure 8A, B). Since typical protein and nucleic acid molecules fall into this size range, a determination of the tip radius is required, in order to deconvolute particle and tip dimensions. The diameters of the DNA fragments in the images provide an internal calibration standard that is appropriate for this purpose (Figure 8C) [45, 46]. In the simplest geometrical model (Figure 8D), the AFM tip is approximated by a spherical section and the DNA cross-section by a rectangular box with height hDNA:
| (eq. 3) |
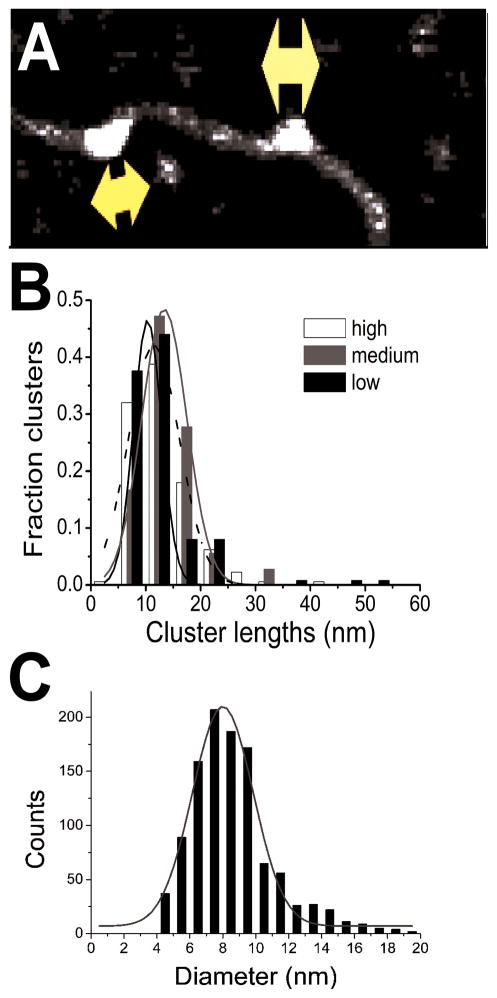
Figure 7. Length distributions of AGT clusters on DNA and diameters of free AGT molecules.
(A) Cluster lengths measured along the DNA axes. (B) Cluster lengths on 1,000 bp DNA fragments for [AGT] = 2 μM (black, n = 125), 6 μM (grey, n = 36) and 12 μM (white, n = 178). Data are shown as fractions of the total number of complexes. Gaussian fits (lines) to the distributions give comparable cluster lengths of 10 nm, 13 nm, and 11 nm for 2 μM, 6 μM, and 12 μM AGT, respectively (R2 ≥ 0.97). (C) Diameter distribution for free AGT molecules. The mean (~8 nm) is smaller than those found for AGT-DNA complexes.
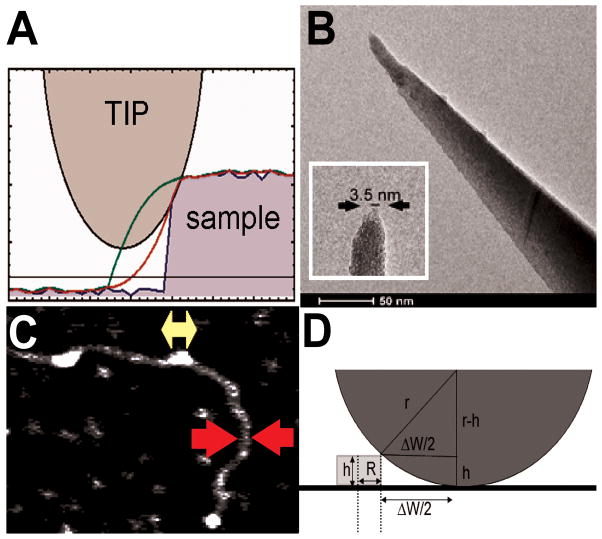
Figure 8. Correcting for the contribution of AFM tip dimensions to apparent cluster lengths.
(A) Mathematica simulations show convolution of sample features (blue line) in the images with finite AFM tip dimensions (green line), which can be corrected for with knowledge of the tip size to obtain a better estimate of true particle dimensions (red line). (B) Transmission electron micrograph of an AFM tip. The zoom in the inset shows a tip apex with approximately 3.5 nm final diameter, similar to typical protein molecular dimensions. (C) The width of duplex DNA in AFM images (red arrows) can be used as an innate calibration standard to correct measured protein cluster lengths (yellow arrow) for tip contributions. (D) Image features (radius W/2) can be represented as the sum of AFM tip radius (r) and sample radius (R) contributions. In the simplest model, the sample particle section is approximated by a box shape with height h and length 2R, and the tip induced broadening of image features is: ΔW = 2 × sqrt(2hr-h2).
Here, the radius of duplex DNA RDNA = 1 nm [47, 48] and the radius of curvature of the AFM tip rT is found from the discrepancy between the apparent DNA width W in the images and the theoretical DNA diameter 2RDNA [45]. The tip radii used in these studies of AGT cluster length spanned the range 1.3 nm ≤ rT ≤ 21.6 nm. Direct evaluation of AFM tips by electron microscopy confirmed that the tip sizes obtained by this method are in the correct range [45].
Tip-size-corrected cluster lengths (Lcorrected) were obtained using Equation 4, approximating the AGT sample clusters by a box with length L and height hS as described above for the DNA sections [7, 45]:
| (eq. 4) |
Figure 9 shows uncorrected, measured AGT cluster lengths (diamond shapes) as maximum limits as well as AFM tip effect corrected cluster lengths (Lcorrected, square shapes) as minimum limits, as functions of AGT concentration [7]. In contrast to the gradual increase in cluster size predicted by McGhee-von Hippel theory (Figure 3 and grey areas in Figure 9), the AFM data indicate a distinct limitation in cluster length to approximately 7 monomers for high protein concentration (> 6 μM).
Figure 9. AGT cluster length.
[7] Comparison of cluster sizes from AFM, expressed as protein molecules/cluster (symbols), with cluster size predictions from the McGhee-von Hippel model (grey zones) for the two DNA substrates (1,000 bp and pUC19 ~2,700 bp). For AFM data, rtip-corrected values (■) are shown as lower-limit estimates of the number of AGT monomers per cluster (Eq. 4), and uncorrected values as measured from the images (◆) as upper-limit estimates. Open symbols (□,◇) denote measurements made after glutaraldehyde crosslinking (see Fig. 10).
To exclude the possibility that cluster size limitation might be due to protein dissociation or isomerization of complexes during deposition on the mica substrate, representative samples were covalently crosslinked with glutaraldehyde prior to deposition [7]. As can be seen from Figures 9 and 10, crosslinking did not significantly change the contour lengths of AGT clusters, consistent with the notion that the deposition process did not affect the distribution of proteins on the DNA.
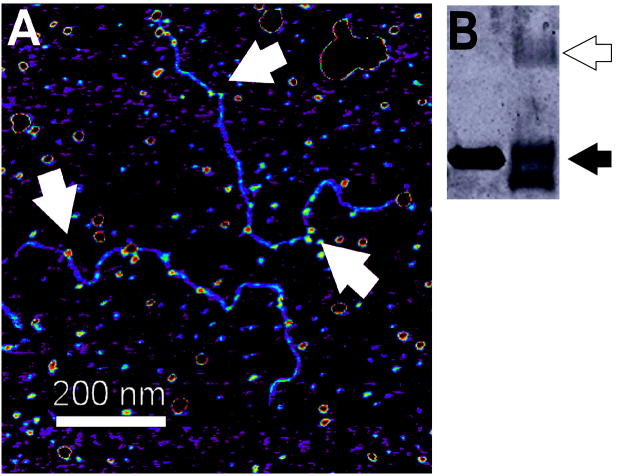
Figure 10. Crosslinked AGT-DNA complexes.
(A) Samples containing linear pUC19 (60 nM) and AGT (12μM) were crosslinked with 0.1% glutaraldehyde (10 min, 37°C) before application to the mica substrate. Arrows indicate AGT clusters on DNA. (B) SDS-PAGE analysis showed that >50% of AGT molecules were crosslinked to a neighbor by this treatment (black arrow monomer, white arrow dimer).
4. Discussion
A model accounting for length constraint in cooperative AGT clusters
With exception of the DNA ends, the uniform distributions of AGT density seen in the AFM images (Figure 6) are compatible with the sequence-independent binding assumed in the McGhee-von Hippel model. However, the deviation of measured cluster lengths from those predicted by theory (Figure 9) suggests that a mechanism limiting the size of cooperative clusters must operate in the AGT-DNA system. Cooperativity in protein-DNA interactions can be affected by conformational changes within the protein molecules [5, 31, 32, 34–36, 51], within the DNA [52, 53], or both [37, 38]. The structural basis for cooperativity in AGT-DNA interactions is unlikely to be mediated by rearrangements within the protein, since crystal structures of AGT in its apo-form and bound to DNA reveal no significant changes in the protein upon DNA binding [2] (Figure 1C). On the other hand, AGT slightly untwists the DNA double helix (Δtwist = −7.1 ± 0.3 degrees/protein monomer [27]). This change in structure may modulate DNA affinity or cooperativity. Since protein-protein contacts constrain the conformation of the cooperative unit, torsional stress accumulates as cooperative clusters form. Clusters are expected to increase in size until ΔG(DNA twist) = −ΔG(cooperative), where ΔG(DNA twist) is the energy cost due to DNA deformation and ΔG(cooperative) is the free energy difference associated with cooperative interactions. This is an example of a mechanism that would cause binding cooperativity to change with binding density, in contrast to the uniform cooperativity specified by the McGhee-von Hippel model. The dependence of ΔG(DNA twist) on the twist angle φ (in radians) is given by Equation 5, in which H is the DNA torsional Hooke’s constant (~3 × 10−19 erg/cm [54]) and L is the DNA length over which the twist acts [55]. ΔG(cooperative) is given in Equation 6, where ω is the cooperativity parameter (i.e., the equilibrium constant for cooperative interactions, R the gas constant and T the temperature (Kelvin).
| (eq. 5) |
| (eq. 6) |
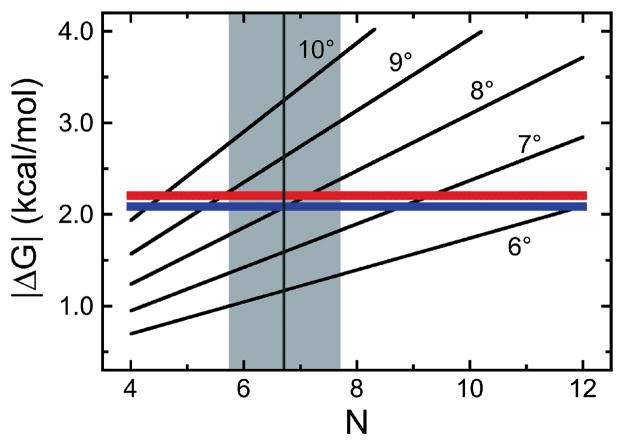
The dependence of ΔG(DNA twist) and ΔG(cooperative) on the number of protein molecules in a cooperative cluster (N) and the twist angle per protein monomer is shown in Figure 11. For a cluster size of ~7 (typical of the high [AGT] limits), ΔG(DNA twist) = −ΔG(cooperative) at a net unwinding of ~8.5 degrees/protein, only slightly greater than the value measured by topoisomerase assay (7.1 ± 0.3 degrees/protein [27]). While this coincidence does not prove that torsional stress limits the size of AGT clusters, it shows that such a mechanism is capable of limiting AGT cluster growth to the observed lengths, shorter than those predicted by the McGhee-von Hippel model. Biological systems may use such fine-tuning of energetic balances as a regulatory mechanism to control the lengths of cooperative protein complexes on DNA.
Figure 11. Limiting energetics in AGT cluster formation.
[7] The quadratic model (Eq. 5) was used to calculate the cumulative ΔG(DNA twist) as functions of proteins/cluster (N) for DNA twist angles of 6–10 degrees/protein. The red and blue lines give |ΔG(cooperative)| (Eq. 6) for the addition of a single protein molecule to complexes formed on linear pUC19 DNA and the 1,000 bp fragment, respectively. The intersections of these functions indicate where ΔG(DNA twist) = −ΔG(cooperative). A vertical grey line (grey zone between minimum and maximum estimates of cluster length) is plotted for a cluster length of 6.7 proteins, the mean of all length estimates for [AGT] ≥ 6μM from AFM experiments with the 1,000 bp DNA.
Functional implications of short cooperative AGT clusters
The small average cluster size of non-specific cooperative AGT complexes (N ~ 7 under conditions tested here) may allow cooperative units to function wherever short stretches of free DNA are available. With a spacing of 4bp/protein monomer, 7 proteins cover ~28 bp, a length that fits easily into inter-nucleosome spacer regions [56]. The supported bend angles indicate that AGT can occupy bent or looped DNA segments and the preference for DNA ends and lack of preference for strand internal positions further suggest structural specificity of AGT in its lesion search. Finally, the correlation of binding cooperativity with torsional free energy predicts that the largest clusters (and hence greatest binding densities) will be found on torsionally-relaxed, B-form DNA. Since the exposure of such DNA depends on the activities of chromatin remodeling and topoisomerase enzymes, and since these activities are required for DNA replication and transcription, the structures of AGT clusters predict that its activities will be concentrated near replication forks and regions of actively transcribed chromatin. The potential of AGT to interact with replication- and transcription-complexes, and the structures of the resulting assemblies, are attractive targets for future study.
Cooperativity in AGT lesion search and repair
The low binding preference of AGT to lesion sites compared to non-specific DNA (< 10-fold) [57] suggests that interactions with other proteins and/or non-specifically bound complexes translocating on the DNA to the site of a lesion may play a role in the recruitment of AGT to its target sites. Kinetic measurements have indicated that formation of the specific complex is preceded by transfer from adjacent non-specific sites [57]. Intriguingly, recent biochemical studies also suggest one-step cooperative, specific binding of AGT to alkyl-lesions in DNA with 2:1 stoichiometry [57], consistent with footprinting data showing protection of ~18 nt on the lesion-containing strand [58] and in contrast to the monomeric structures seen in crystallographic data with shorter DNA substrates [2, 21]. To conclude, there is still much to learn about when and how conversion between different cooperative AGT-DNA cluster states may occur and how the structural features of these complexes relate to function.
Acknowledgments
These studies were supported by the Deutsche Forschungsgemeinschaft (DFG, Forschungszentrum FZ82 to IT) and National Institutes of Health (NIH, GM-070662 to MGF).
Footnotes
It is worth noting that the resulting values likely present slight overestimations of AFM tip diameters, because DNA widths in AFM images obtained in air on dried samples are typically somewhat broadened from their theoretical value by a fine layer of salt that amounts at the DNA-mica interface [49, 50]. Using the theoretical DNA radius of 1 nm therefore suggests a slightly larger contribution to DNA broadening by the AFM tip than is in truth the case, with a fraction of the broadening instead attributable to the assembled salt layer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nature Reviews Cancer. 2012;12:104–120. doi: 10.1038/nrc3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nature Structural & Molecular Biology. 2004;11:714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 3.Mishina Y, Duguid EM, He C. Direct reversal of DNA alkylation damage. Chemical Reviews. 2006;106:215–232. doi: 10.1021/cr0404702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrivastav N, Li D, Essigmann JM. Chemical biology of mutagenesis and DNA repair: cellular responses to DNA alkylation. Carcinogenesis. 2010;31:59–70. doi: 10.1093/carcin/bgp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drablos F, Feyzi E, Aas PA, Vaagbo CB, Kavli B, Bratlie MS, Pena-Diaz J, Otterlei M, Slupphaug G, Krokan HE. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair (Amst) 2004;3:1389–1407. doi: 10.1016/j.dnarep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Fang Q, Kanugula S, Tubbs JL, Tainer JA, Pegg AE. Repair of O4-alkylthymine by O6-alkylguanine-DNA alkyltransferases. The Journal of Biological Chemistry. 2010;285:8185–8195. doi: 10.1074/jbc.M109.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tessmer I, Melikishvili M, Fried MG. Cooperative cluster formation, DNA bending and base-flipping by O6-alkylguanine-DNA alkyltransferase. Nucleic Acids Research. 2012;40:8296–8308. doi: 10.1093/nar/gks574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Research. 1990;50:6119–6129. [PubMed] [Google Scholar]
- 9.Goodtzova K, Kanugula S, Edara S, Pauly GT, Moschel RC, Pegg AE. Repair of O6-benzylguanine by the Escherichia coli Ada and Ogt and the human O6-alkylguanine-DNA alkyltransferases. The Journal of Biological Chemistry. 1997;272:8332–8339. doi: 10.1074/jbc.272.13.8332. [DOI] [PubMed] [Google Scholar]
- 10.Kooistra R, Zonneveld JBM, Watson AJ, Margison GP, Lohman PHM, Pastink A. Identification and characterisation of the Drosophila melanogaster O-6-alkylguanine-DNA alkyltransferase cDNA. Nucleic Acids Research. 1999;27:1795–1801. doi: 10.1093/nar/27.8.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabik CA, Njoku MC, Dolan ME. Inactivation of O6-alkylguanine DNA alkyltransferase as a means to enhance chemotherapy. Cancer Treatment Reviews. 2006;32:261–276. doi: 10.1016/j.ctrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 13.Pegg AE. Multifaceted roles of alkyltransferase and related proteins in DNA repair, DNA damage, resistance to chemotherapy, and research tools. Chemical Research in Toxicology. 2011;24:618–639. doi: 10.1021/tx200031q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2002;20:2388–2399. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 15.Kaina B, Margison GP, Christmann M. Targeting O(6)-methylguanine-DNA methyltransferase with specific inhibitors as a strategy in cancer therapy. Cellular and Molecular Life Sciences: CMLS. 2010;67:3663–3681. doi: 10.1007/s00018-010-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson AJ, Sabharwal A, Thorncroft M, McGown G, Kerr R, Bojanic S, Soonawalla Z, King A, Miller A, Waller S, Leung H, Margison GP, Middleton MR. Tumor O(6)-methylguanine-DNA methyltransferase inactivation by oral lomeguatrib. Clinical Cancer Research: an official journal of the American Association for Cancer Research. 2010;16:743–749. doi: 10.1158/1078-0432.CCR-09-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasimas JJ, Pegg AE, Fried MG. DNA-binding mechanism of O6-alkylguanine-DNA alkyltransferase. Effects of protein and DNA alkylation on complex stability. The Journal of Biological Chemistry. 2003;278:7973–7980. doi: 10.1074/jbc.M211854200. [DOI] [PubMed] [Google Scholar]
- 18.Daniels DS, Mol CD, Arvai AS, Kanugula S, Pegg AE, Tainer JA. Active and alkylated human AGT structures: a novel zinc site, inhibitor and extrahelical base binding. The EMBO Journal. 2000;19:1719–1730. doi: 10.1093/emboj/19.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wibley JE, Pegg AE, Moody PC. Crystal structure of the human O(6)-alkylguanine-DNA alkyltransferase. Nucleic Acids Research. 2000;28:393–401. doi: 10.1093/nar/28.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels DS, Tainer JA. Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O(6)-alkylguanine-DNA alkyltransferase. Mutation Research. 2000;460:151–163. doi: 10.1016/s0921-8777(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 21.Duguid EM, Rice PA, He C. The structure of the human AGT protein bound to DNA and its implications for damage detection. Journal of Molecular Biology. 2005;350:657–666. doi: 10.1016/j.jmb.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 22.Tubbs JL, Pegg AE, Tainer JA. DNA binding, nucleotide flipping, and the helix-turn-helix motif in base repair by O-6-alkylguanine-DNA alkyltransferase and its implications for cancer chemotherapy. DNA Repair. 2007;6:1100–1115. doi: 10.1016/j.dnarep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanugula S, Goodtzova K, Pegg AE. Probing of conformational changes in human O6-alkylguanine-DNA alkyl transferase protein in its alkylated and DNA-bound states by limited proteolysis. The Biochemical Journal. 1998;329(Pt 3):545–550. doi: 10.1042/bj3290545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pegg AE, Wiest L, Mummert C, Stine L, Moschel RC, Dolan ME. Use of antibodies to human O6-alkylguanine-DNA alkyltransferase to study the content of this protein in cells treated with O6-benzylguanine or N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis. 1991;12:1679–1683. doi: 10.1093/carcin/12.9.1679. [DOI] [PubMed] [Google Scholar]
- 25.Brunk E, Mollwitz B, Rothlisberger U. Mechanism to trigger unfolding in O(6)-alkylguanine-DNA alkyltransferase. Chembiochem. 2013;14:703–710. doi: 10.1002/cbic.201200566. [DOI] [PubMed] [Google Scholar]
- 26.Melikishvili M, Rasimas JJ, Pegg AE, Fried MG. Interactions of human O(6)-alkylguanine-DNA alkyltransferase (AGT) with short double-stranded DNAs. Biochemistry. 2008;47:13754–13763. doi: 10.1021/bi801666c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams CA, Melikishvili M, Rodgers DW, Rasimas JJ, Pegg AE, Fried MG. Topologies of complexes containing O6-alkylguanine-DNA alkyltransferase and DNA. Journal of Molecular Biology. 2009;389:248–263. doi: 10.1016/j.jmb.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasimas JJ, Kar SR, Pegg AE, Fried MG. Interactions of human O6-alkylguanine-DNA alkyltransferase (AGT) with short single-stranded DNAs. The Journal of Biological Chemistry. 2007;282:3357–3366. doi: 10.1074/jbc.M608876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried MG, Kanugula S, Bromberg JL, Pegg AE. DNA binding mechanism of O6-alkylguanine-DNA alkyltransferase: stoichiometry and effects of DNA base composition and secondary structure on complex stability. Biochemistry. 1996;35:15295–15301. doi: 10.1021/bi960971k. [DOI] [PubMed] [Google Scholar]
- 30.Adams CA, Fried MG. Mutations that probe the cooperative assembly of O(6)-alkylguanine-DNA alkyltransferase complexes. Biochemistry. 2011;50:1590–1598. doi: 10.1021/bi101970d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurkowska RZ, Rajavelu A, Anspach N, Urbanke C, Jankevicius G, Ragozin S, Nellen W, Jeltsch A. Oligomerization and Binding of the Dnmt3a DNA Methyltransferase to Parallel DNA Molecules HETEROCHROMATIC LOCALIZATION AND ROLE OF Dnmt3L. Journal of Biological Chemistry. 2011;286:24200–24207. doi: 10.1074/jbc.M111.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajavelu A, Jurkowska RZ, Fritz J, Jeltsch A. Function and disruption of DNA Methyltransferase 3a cooperative DNA binding and nucleoprotein filament formation. Nucleic Acids Research. 2012;40:569–580. doi: 10.1093/nar/gkr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li N, Henry E, Guiot E, Rigolet P, Brochon JC, Xi XG, Deprez E. Multiple Escherichia coli RecQ helicase monomers cooperate to unwind long DNA substrates: a fluorescence cross-correlation spectroscopy study. The Journal of Biological Chemistry. 2010;285:6922–6936. doi: 10.1074/jbc.M109.069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Liu Y, Yang Z, Utzat C, Wang G, Basu AK, Zou Y. Cooperative interaction of human XPA stabilizes and enhances specific binding of XPA to DNA damage. Biochemistry. 2005;44:7361–7368. doi: 10.1021/bi047598y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu D, Fillet S, Meng C, Alguel Y, Kloppsteck P, Bergeron J, Krell T, Gallegos MT, Ramos J, Zhang X. Crystal structure of TtgV in complex with its DNA operator reveals a general model for cooperative DNA binding of tetrameric gene regulators. Genes & Development. 2010;24:2556–2565. doi: 10.1101/gad.603510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fickert R, Muller-Hill B. How Lac repressor finds lac operator in vitro. Journal of Molecular Biology. 1992;226:59–68. doi: 10.1016/0022-2836(92)90124-3. [DOI] [PubMed] [Google Scholar]
- 37.Pugh BF, Cox MM. Stable binding of recA protein to duplex DNA. Unraveling a paradox. The Journal of Biological Chemistry. 1987;262:1326–1336. [PubMed] [Google Scholar]
- 38.Umemura K, Okada T, Kuroda R. Cooperativity and intermediate structures of single-stranded DNA binding-assisted RecA-single-stranded DNA complex formation studied by atomic force microscopy. Scanning. 2005;27:35–43. doi: 10.1002/sca.4950270107. [DOI] [PubMed] [Google Scholar]
- 39.Tessmer I, Moore T, Lloyd RG, Wilson A, Erie DA, Allen S, Tendler SJ. AFM studies on the role of the protein RdgC in bacterial DNA recombination. Journal of Molecular Biology. 2005;350:254–262. doi: 10.1016/j.jmb.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 40.Hamon L, Pastre D, Dupaigne P, Le Breton C, Le Cam E, Pietrement O. High- resolution AFM imaging of single-stranded DNA-binding (SSB) protein--DNA complexes. Nucleic Acids Research. 2007;35:e58. doi: 10.1093/nar/gkm147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGhee JD, von Hippel PH. Theoretical aspects of DNA-protein interactions: co- operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. Journal of Molecular Biology. 1974;86:469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- 42.Kowalczykowski SC, Paul LS, Lonberg N, Newport JW, McSwiggen JA, von Hippel PH. Cooperative and noncooperative binding of protein ligands to nucleic acid lattices: experimental approaches to the determination of thermodynamic parameters. Biochemistry. 1986;25:1226–1240. doi: 10.1021/bi00354a006. [DOI] [PubMed] [Google Scholar]
- 43.Bender K, Federwisch M, Loggen U, Nehls P, Rajewsky MF. Binding and repair of O6-ethylguanine in double-stranded oligodeoxynucleotides by recombinant human O6-alkylguanine-DNA alkyltransferase do not exhibit significant dependence on sequence context. Nucleic Acids Research. 1996;24:2087–2094. doi: 10.1093/nar/24.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zang H, Fang Q, Pegg AE, Guengerich FP. Kinetic analysis of steps in the repair of damaged DNA by human O6-alkylguanine-DNA alkyltransferase. The Journal of Biological Chemistry. 2005;280:30873–30881. doi: 10.1074/jbc.M505283200. [DOI] [PubMed] [Google Scholar]
- 45.Winzer AT, Kraft C, Bhushan S, Stepanenko V, Tessmer I. Correcting for AFM tip induced topography convolutions in protein-DNA samples. Ultramicroscopy. 2012;121:8–15. doi: 10.1016/j.ultramic.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Sander B, Tria G, Shkumatov AV, Kim E-Y, Grossmann JG, Tessmer I, Svergun DI, Schindelin H. Structural characterization of gephyrin by AFM and SAXS reveals a mixture of compact and extended states. Acta Cryst D. 2013 doi: 10.1107/S0907444913018714. in press. [DOI] [PubMed] [Google Scholar]
- 47.Watson JD, Crick FH. The structure of DNA. Cold Spring Harbor Symposia on Quantitative Biology. 1953;18:123–131. doi: 10.1101/sqb.1953.018.01.020. [DOI] [PubMed] [Google Scholar]
- 48.Mandelkern M, Elias JG, Eden D, Crothers DM. The dimensions of DNA in solution. Journal of Molecular Biology. 1981;152:153–161. doi: 10.1016/0022-2836(81)90099-1. [DOI] [PubMed] [Google Scholar]
- 49.Vesenka J, Manne S, Yang G, Bustamante CJ, Henderson E. Humidity effects on atomic force microscopy of gold-labeled DNA on mica. Scanning Microscopy. 1993;7:781–788. [PubMed] [Google Scholar]
- 50.Tang J, Li JW, Wang C, Bai CL. Enhancement of resolution of DNA on silylated mica using atomic force microscopy. Journal of Vacuum Science & Technology B. 2000;18:1858–1860. [Google Scholar]
- 51.Jia D, Jurkowska RZ, Zhang X, Jeltsch A, Cheng XD. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 2007;449:248–251. doi: 10.1038/nature06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benoff B, Yang H, Lawson CL, Parkinson G, Liu J, Blatter E, Ebright YW, Berman HM, Ebright RH. Structural basis of transcription activation: the CAP-alpha CTD-DNA complex. Science. 2002;297:1562–1566. doi: 10.1126/science.1076376. [DOI] [PubMed] [Google Scholar]
- 53.Diamant H, Andelman D. Binding of molecules to DNA and other semiflexible polymers. Physical Review E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics. 2000;61:6740–6749. doi: 10.1103/physreve.61.6740. [DOI] [PubMed] [Google Scholar]
- 54.Crothers DM, Drak J, Kahn J, Levene SD. DNA bending, flexibility and helical repeat by cyclization kinetics. Methods Enzymol. 1992;212:3–29. doi: 10.1016/0076-6879(92)12003-9. [DOI] [PubMed] [Google Scholar]
- 55.Bloomfield VA, Crothers DM, Tinoco I., Jr . Nucleic Acids: Structures, Properties, and Functions. University Science Books; Sausalito: 2000. pp. 176–181. [Google Scholar]
- 56.Arya G, Maitra A, Grigoryev SA. A structural perspective on the where, how, why, and what of nucleosome positioning. Journal of Biomolecular Structure & Dynamics. 2010;27:803–820. doi: 10.1080/07391102.2010.10508585. [DOI] [PubMed] [Google Scholar]
- 57.Melikishvili M, Fried MG. Lesion-specific DNA-binding and repair activities of human O(6)-alkylguanine DNA alkyltransferase. Nucleic Acids Research. 2012;40:9060–9072. doi: 10.1093/nar/gks674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hazra TK, Roy R, Biswas T, Grabowski DT, Pegg AE, Mitra S. Specific recognition of O6-methylguanine in DNA by active site mutants of human O6-methylguanine-DNA methyltransferase. Biochemistry. 1997;36:5769–5776. doi: 10.1021/bi963085i. [DOI] [PubMed] [Google Scholar]