Abstract
Inorganic arsenic (InAs) is metabolized through a series of methylation reactions catalyzed by arsenic(III)-methyltransferase (AS3MT), resulting in the generation of monomethylarsonic (MMAs) and dimethylarsinic acids (DMAs). AS3MT activity requires the presence of the methyl donor S-adenosylmethionine (SAM), a product of folate-dependent one-carbon metabolism, and a reductant. Although glutathione (GSH), the primary endogenous antioxidant, is not required for As methylation, GSH stimulates As methylation rates in vitro. However, the relationship between GSH redox and As methylation capacity in humans is unknown. We wished to test the hypothesis that a more oxidized plasma GSH redox status is associated with decreased As methylation capacity, and examine whether these associations are modified by folate nutritional status. Concentrations of plasma GSH and GSSG, plasma folate, total blood As (bAs), total urinary As (uAs), and uAs metabolites were assessed in a cross-sectional study of n = 376 Bangladeshi adults who were chronically exposed to As in drinking water. We observed that a decreased plasma GSH/GSSG ratio (reflecting a more oxidized redox state) was significantly associated with increased urinary %MMA, decreased urinary %DMA, and increased total bAs in folate-deficient individuals (plasma folate ≤ 9.0 nmol/L). Concentrations of plasma GSH and GSSG were independently associated with increased and decreased As methylation capacity, respectively. No significant associations were observed in folate-sufficient individuals, and interactions by folate status were statistically significant. Our findings suggest that GSH/GSSG redox regulation might contribute to the large interindividual variation in As methylation capacity observed in human populations.
Keywords: Arsenic, arsenic methylation, Bangladesh, folate, glutathione, glutathione disulfide, oxidative stress, redox
INTRODUCTION
Over 140 million people throughout Bangladesh, India, Vietnam, Nepal, and Cambodia are chronically exposed to arsenic (As) in drinking water at concentrations over 10 μg/L, the World Health Organization guideline [1]. As a Class I human carcinogen [2], As is associated with increased risk for cancers of the skin, lung, bladder, liver, and kidney [3], although the carcinogenic mechanisms of As are incompletely understood [4].
Inorganic arsenic (InAs) is metabolized, primarily in the liver, through a series of methylation reactions catalyzed by arsenic(III)-methyltransferase (AS3MT), resulting in the formation of monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA) [5]. The methyl group for the reaction is donated from S-adenosylmethionine (SAM) [5], a product of folate-dependent one-carbon metabolism [6]. Since methylation increases As excretion and reduces As body burden [7], it is believed to be a detoxification process [8]. However, in vitro and in vivo studies have identified MMAIII as the most toxic As form [9, 10], suggesting that As methylation also involves bioactivation [11]. Epidemiologic studies consistently find that a reduced capacity to fully methylate InAs, as indicated by higher %MMA(III+V) and lower %DMA(III+V) in urine, is associated with increased risk for As-induced skin lesions and cancers of the skin, lung, and bladder [12]. As such, the identification of factors that facilitate complete methylation of InAs to DMA might provide insight into interventions to reduce risk for As-related diseases [13].
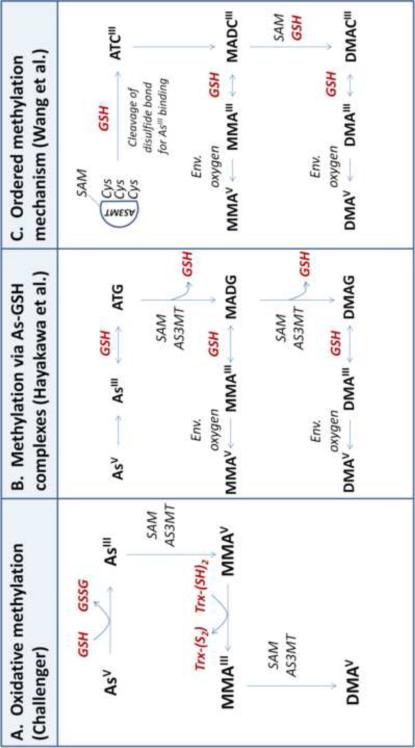
Although the proposed As methylation pathways presented in Figure 1 are distinct, they all require the presence of a reductant, such as glutathione (GSH) [14]. As the primary endogenous antioxidant, GSH readily donates an electron for reduction reactions, forming its oxidized form, glutathione disulfide (GSSG), in the process [15]. A lower ratio of GSH to GSSG reflects a more oxidized intracellular redox state [15]. While GSH is not required for As methylation to proceed, GSH can serve as the reducing agent necessary for AS3MT activity [16-19]. In addition, GSH can stimulate AS3MT-catalyzed methylation rates in experimental systems already containing another reductant [20].
Figure 1. Involvment of glutathione in arsenic methylation mechanisms.
Inorganic arsenic (InAs) is metabolized in the liver via a series of methylation reactions, resulting in the formation of monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). Glutathione (GSH), while not absolutely required for As methylation, can serve as the reductant necessary for AS3MT activity. Three distinct AS3MT-catalyzed methylation pathways have been proposed in the literature (A-C):
A. Oxidative methylation. In this pathway first proposed by Challenger [21, 22], AS3MT catalyzes the oxidative methylation of arsenite (AsIII) and methylarsonous acid (MMAIII) to methylarsonic acid (MMAV) and dimethylarsinic acid (DMAV), respectively. A reductant (such as GSH) is required for the reduction of the pentavalent arsenicals (AsV and MMAV) to their trivalent counterparts (AsIII and MMAIII).
B. Successive methylation via As-GSH complexes. Hayakawa et al. [23] proposes a pathway in which As-GSH complexes are substrates for AS3MT. Here, AsIII nonenzymatically complexes with GSH to generate arsenic triglutathione (ATG), which is methylated by AS3MT to form monomethylarsonic diglutathione (MADG). MADG can be further methylated to dimethylarsinic glutathione (DMAG). As-GSH complexes (ATG, MADG, and DMAG) are in equilibrium with trivalent arsenicals (AsIII, MMAIII, and DMAIII) based on the concentration of GSH. The trivalent methylated arsenicals (MMAIII and DMAIII) can be oxidized to pentavalent arsenicals (MMAV and DMAV) by environmental oxygen.
C. Ordered methylation mechanism. Wang et al. [24] propose a model in which a reductant (thiol or nonthiol) changes the conformation of AS3MT to influence enzyme activity. In this scheme, SAM binds to AS3MT; then, a reductant (such as GSH) cleaves a disulfide bond in the enzyme, which exposes active-site cysteine (Cys) residues for AsIII binding. AsIII binds the Cys residues, forming arsenic tricysteine (ATCIII), and the methyl group from SAM is transferred to ATCIII on the AS3MT enzyme, resulting in monomethylarsonic dicysteine (MADCIII). MADCIII can remain bound to AS3MT and be further methylated to dimethylarsinic cysteine (DAMCIII). MADCIII and DAMCIII can dissociate from AS3MT, forming MMAIII and DMAIII, respectively. Trivalent species are then oxidized to pentavalent arsenicals by environmental oxygen.
GSH might facilitate As methylation by donating electrons for the reduction of pentavalent arsenate (AsV) to trivalent arsenite (AsIII), shown in the oxidative methylation pathway first proposed by Challenger [21, 22] (Figure 1A), or by forming As-GSH complexes that are substrates for AS3MT, as proposed by Hayakawa et al. [23] (Figure 1B). Recent work by Wang et al. found that a reductant (such as GSH) is needed to cleave a disulfide bond within AS3MT, which allows AsIII to bind the enzyme [24] (Figure 1C). Additionally, active-site cysteine (Cys) residues that are potentially redox-sensitive have been identified in AS3MT [25]. Redox-sensitive Cys residues are prone to oxidative modifications such as S-glutathionylation— the reversible formation of a mixed disulfide between a Cys residue and GSH or GSSG—which might also regulate AS3MT activity [26].
We wished to test the hypothesis that a more reduced GSH redox state is associated with increased As methylation capacity, as indicated by a decrease in urinary %MMA, increase in urinary %DMA, and increase in the urinary secondary methylation index (SMI, defined as the ratio of DMA to MMA). We measured the GSH/GSSG ratio in plasma and in blood; the plasma ratio is believed to better reflect GSH/GSSG status in liver [27], the primary site of As methylation, and was therefore chosen for our focus in the present analyses. We also examined whether the associations were modified by folate status. We used data from the Folate and Oxidative Stress (FOX) study [28], which was originally designed to assess the dose-response relationship between total As exposure and markers of oxidative stress.
SUBJECTS AND METHODS
Eligibility criteria and study design
In the FOX study, we recruited 378 men and women between the ages of 30 and 65 y between April 2007 and April 2008 in Araihazar, Bangladesh. Study participants were selected on well water As (wAs) exposure to ensure that the study sample reflected the full range of wAs exposures in the region, as previously described [28]. Participants were excluded if they were pregnant, had taken nutritional supplements within the past 3 months, or had known diabetes, cardiovascular disease, or renal disease.
Analytic techniques
Sample collection and handling
Blood samples were drawn and processed immediately at our field clinic in Araihazar. Aliquots of blood and plasma were stored at –80°C. They were then transported to Dhaka on dry ice and stored at –80°C. Samples were then shipped, frozen on dry ice, to Columbia University for analysis.
Water As
Field sample collection and laboratory procedures are described previously in detail [29, 30]. Briefly, at the time of the recruitment visit of the FOX study, new well water samples were collected in 20-mL polyethylene scintillation vials and acidified to 1% with high-purity Optima HCl (Fisher Scientific, Pittsburg, PA, USA) at least 48 hr before analysis [31]. Water samples were analyzed by high-resolution inductively coupled plasma mass spectrometry after 1:10 dilution and addition of a Ge spike to correct fluctuations in instrument sensitivity. Standards were run multiple times in each batch. The intra- and inter-assay coefficients of variation (CVs) were 3.8% and 6.0%, respectively.
Urinary As metabolites and urinary creatinine
Urinary As (uAs) metabolite species were measured using HPLC separation of arsenobetaine (AsB), arsenocholine (AsC), AsV, AsIII, MMAs, and DMAs, followed by detection with ICP-MS [32]. Total uAs concentrations were determined by summing the concentrations of AsV, AsIII, MMAs, and DMAs, excluding AsC and AsB from the sum. The limit of detection for each uAs metabolite was 0.1 μg/L. Urinary creatinine (uCr) was analyzed using a colorimetric assay based on the Jaffe reaction [33], and urinary As concentrations were calculated with and without adjustment for uCr. The intra-assay CVs were 4.5% for AsV , 3.8% for AsIII, 1.5% for MMA, and 0.6% for DMA; the inter-assay CVs were 10.6% for AsV , 9.6% for AsIII, 3.5% for MMA, and 2.8% for DMA.
Total blood As
Total blood As (bAs) concentrations were determined by Perkin-Elmer Elan DRC II ICPMS equipped with an AS 93+ autosampler, as previously described [34]. The limit of detection for bAs was 0.1 μg/L. The intra- and inter-assay CVs were 3.2% and 5.7%, respectively.
Plasma folate and cobalamin
Plasma folate and cobalamin were measured using a radioproteinbinding assay (SimulTRAC-S, MP Biomedicals, Orangeburg, NY). For determination of folate concentration, folic acid as pteroylglutamic acid was used for calibration, and its 125I-labeled analog was used as the tracer; for cobalamin, cyanocobalamin was used for calibration, and its 57Co-labeled analog was used as the tracer. The intra- and inter-assay CVs for folate were 6% and 14%, respectively, and the intra- and inter-assay CVs for cobalamin were 4% and 8%, respectively.
Blood and plasma glutathione and glutathione disulfide
Whole blood and plasma GSH and GSSG were assayed based on the protocol by Jones et al [35], as previously described [28]. Briefly, blood was collected in the field laboratory in Bangladesh and immediately transferred into Eppendorf tubes containing 5% perchloric acid (PCA), 0.1 M boric acid, and γ-glutamyl glutamate (internal standard). Samples were derivatized in Bangladesh, and the derivatized samples were stored at –80°C until delivered to Columbia University on dry ice for HPLC analysis. Metabolites were detected using a Waters 474 scanning fluorescence detector, with 335 nm excitation and 515 nm emission (Waters Corp., Milford, MA). Intra-assay CVs were all between 5 and 10%, and inter-assay CVs were between 11 and 18%.
Calculation of the reduction potential
The reduction potential of the thiol/disulfide plasma GSH/GSSG redox pair (Eh) was calculated using the Nernst equation (Eh=Eo + RT/nF ln [disulfide]/[thiol]2 where Eo=standard potential for the redox couple, R=gas constant, T=absolute temperature, n=2 for the number of electrons transferred, and F=Faraday's constant) [36]. A more positive Eh value reflects a more oxidized redox state.
Statistical methods
Descriptive statistics were calculated for the overall sample. Chi-square test and the Wilcoxon rank-sum test were used to detect group differences in categorical and continuous variables, respectively. Spearman correlations were used to examine bivariate associations between quantitative variables, including associations between covariates with urinary As metabolite percentages and plasma GSH and GSSG concentrations. Certain covariates (gender, age, ever cigarette smoking, and water As) were selected based on biologic plausibility and previous studies in the literature. Other biologically-plausible confounders (BMI, plasma cobalamin, ever betelnut use, television ownership, and uCr) were considered by examining their bivariate associations with plasma GSH variables and As exposure variables in this dataset; covariates were included in the regression models if they were associated with both exposure and outcome at significance level of 0.2. To reduce extraneous variation in plasma GSH and GSSG, we additionally adjusted these variables for plasma GSH laboratory batch as a categorical variable using the residual method; batch-adjusted plasma GSH and GSSG variables were used in all analyses.
Linear regression models were constructed with plasma GSH variables as predictors and urinary As metabolite variables as the outcomes. Variables with skewed distributions were log-transformed to normalize the distributions of the variables and/or improve the linearity of the relationships between the predictors and outcomes; transformed variables included bAs, wAs, urinary SMI, plasma GSH, plasma GSSG, and plasma GSH/GSSG. Relationships were also examined by using linear models to calculate gender- and wAs-adjusted mean urinary %MMA and %DMA within categories of plasma GSH/GSSG (categorized at tertiles in the overall study sample), stratified by plasma folate status. Differences in the associations between predictor (GSH, GSSG, GSH/GSSG ratio) and outcomes of methylation variables between plasma folate strata were examined and detected by Wald tests. Of the total n = 378 participants in the study sample, two participants were excluded for missing data (n = 1 participant with missing plasma folate, n = 1 participant with missing plasma GSH), leaving n = 376 participants in the analysis. All statistical analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC).
RESULTS
[notdef]Demographic and clinical characteristics of the study participants, overall and by plasma folate status, are shown in Table 1. In the overall sample, the average age was 43 y, and there were roughly equal numbers of males and females. Folate-deficient individuals (plasma folate concentrations < 9.0 nmol/L) comprised approximately 29% of the sample. The folate-deficient group had a higher proportion of males, ever cigarette smokers, and betel nut users; higher mean age; and lower mean BMI. The folate-deficient group also had higher %InAs and %MMA and lower %DMA in urine.
Table 1.
Descriptive characteristics for study sample by plasma folate status.
| Baseline variables | Total sample N=376 | Folate deficient (<9.0 nmol/L) N=110 | Folate sufficient (≥9.0 nmol/L) N=266 | Group difference P |
|---|---|---|---|---|
| Age (yrs) | 43.1±8.03a (30 to 63) | 44.4±8.0 (30 to 62) | 42.6±8.3 (31 to 63) | 0.02 |
| Male | 182 (48.4)b | 70 (64.6) | 112 (42.1) | <0.0001 |
| BMI (kg/m2) | 20.4±3.5 (13.8 to 35.3)c | 19.7±3.0 (13.8 to 31.7) | 20.7±3.6 (14.5 to 35.3)e | 0.02 |
| Ever cigarette smoking | 136 (36.2) | 59 (53.6) | 77 (29.0) | <0.0001 |
| Ever betel nut use | 160 (42.6) | 59 (53.6) | 101 (38.0) | 0.003 |
| Television ownership | 219 (58.2) | 57 (51.8) | 162 (60.9) | 0.07 |
| Plasma folate (nmol/L) | 12.9±7.2 (2.4 to 60.6) | 7.1±1.4 (2.4 to 8.9) | 15.3±7.2 (9.1 to 60.6) | <0.0001 |
| Plasma cobalamin (μM) | 204±113 (44 to 1183)d | 191±93 (59 to 557) | 209±119 (44 to 1183)f | 0.20 |
| Plasma GSH (μmol/L) | 2.62±0.71 (1.00 to 5.52) | 2.61±0.72 (1.22–4.55) | 2.62±0.71 (1.00 to 5.52) | 0.94 |
| Plasma GSSG (μmol/L) | 2.14±0.60 (0.81 to 4.66) | 2.07±0.65 (0.92–4.66) | 2.16±0.58 (0.81 to 4.04) | 0.06 |
| Plasma GSH/GSSG | 1.29±0.44 (0.39 to 3.02) | 1.34±0.44 (0.59 to 2.67) | 1.28±0.45 (0.39 to 3.02) | 0.21 |
| Plasma GSH Eh (mV) | –98.7±7.3 (–118.0 to −73.4) | –98.6±6.8 (–112.6 to –83.6) | –98.7±7.5 (−118.0 to −73.4) | 0.92 |
| Water As (μg/L) | 138±124 (0.4 to 700) | 164±143 (0.4 to 700) | 128±114 (0.4 to 447) | 0.04 |
| Blood As, total (μg/L) | 13.4±9.8 (1.2 to 57.0) | 15.4±10.9 (1.3 to 51.3) | 12.5±9.1 (1.2 to 57.0) | 0.03 |
| Urinary As, total (μg/L) | 202±226 (3 to 1990) | 209±207 (7 to 992) | 119±223 (3 to 1990) | 0.43 |
| Urinary Cr (mg/dL) | 53±44 (4 to 224) | 53±42 (4 to 212) | 55±44 (6 to 224) | 0.83 |
| Urinary As/Cr (μg/g Cr) | 417±329 (16 to 1832) | 464±361 (16 to 1743) | 397±312 (18 to 1832) | 0.14 |
| Urinary %InAs | 17.7±5.5 (6.7 to 51.8) | 18.6±5.9 (8.3 to 42.9) | 17.4±5.3 (6.7 to 51.8) | 0.05 |
| Urinary %MMA | 13.9±5.0 (3.6 to 30.0) | 15.3±5.5 (3.6 to 30.0) | 13.4±4.7 (4.2 to 28.5) | 0.002 |
| Urinary %DMA | 68.3±7.9 (38.3 to 88.0) | 66.1±8.5 (39.5 to 88.0) | 69.3±7.4 (38.3 to 85.7) | 0.004 |
Mean±SD (range) (all such values)
N(%) (all such values)
N=375
N=370
N=265
N=260.
Males had higher mean urinary %MMA (16.0% vs. 12.0%, P < 0.0001) and lower mean urinary %DMA (66.6% vs. 69.9%, P < 0.0001) and plasma GSH/GSSG ratio (1.25 vs. 1.34, P = 0.07) than females. As shown in Table 2, the plasma GSH/GSSG ratio was negatively correlated with age in both folate groups. In support of previous findings from our group [37], plasma cobalamin was positively correlated with urinary %MMA. Total As exposures in well water, urine, and blood were positively correlated with %InAs and %MMA and negatively correlated with urinary %DMA, consistent with inhibition or saturation of AS3MT by InAs and MMA [16].
Table 2.
Spearman correlations of continuous sample characteristics with plasma glutathione variables and urinary As metabolite percentages.
| Total sample, N=376 | ||||
|---|---|---|---|---|
| Plasma GSH/GSSG | Urinary %InAs | Urinary %MMA | Urinary %DMA | |
| Age | –0.23*** | –0.17** | 0.15** | 0.01 |
| BMIa | –0.12* | –0.05 | –0.18** | 0.14** |
| Plasma cobalaminb | –0.15** | –0.08 | 0.13* | –0.04 |
| Water As | 0.09 | 0.21*** | 0.21*** | –0.26*** |
| Urinary As/Cr | 0.05 | 0.25** | 0.21*** | –0.27*** |
| Blood As | 0.03 | 0.25*** | 0.30*** | –0.35*** |
| 0.5 | ||||
| Folate deficient, N=110 | ||||
| Age | –0.29*** | –0.24** | 0.23** | –0.01 |
| BMI | –0.19* | –0.08 | –0.22** | 0.20** |
| Plasma cobalamin | –0.17 | 0.05 | 0.13 | –0.10 |
| Water As | 0.18 | 0.21* | 0.29*** | –0.27*** |
| Urinary As/Cr | 0.13 | 0.27** | 0.23* | –0.30** |
| Blood As | 0.05 | 0.29** | 0.36** | –0.38*** |
| 0.5 | ||||
| Folate sufficient, N=266 | ||||
| Age | –0.22*** | –0.17** | 0.09 | 0.05 |
| BMIc | –0.09 | –0.03 | –0.14* | 0.10 |
| Plasma cobalamind | –0.14* | –0.13* | 0.14* | –0.02 |
| Water As | 0.03 | 0.20** | 0.14** | –0.22** |
| Urinary As/Cr | 0.01 | 0.18** | 0.18** | –0.23** |
| Blood As | 0.01 | 0.22** | 0.25*** | –0.31*** |
N=375
N=370
N=265
N=260
p<0.05
p<0.01
p<0.0001.
In adjusted linear regression models stratified by plasma folate status (Table 3), the plasma GSH/GSSG ratio was negatively associated with urinary %MMA (B ± SE, -5.21 ± 1.58, P = 0.004) and positively associated with urinary %DMA (B ± SE, 6.53 ± 2.66, P = 0.04) in the folate-deficient stratum, indicating that a more reduced plasma GSH/GSSG ratio is associated with increased As methylation capacity. No significant associations were observed in the folate-sufficient stratum, and the interactions by folate status were significant for %MMA (P = 0.002) and %DMA (P = 0.01). The plasma GSH/GSSG ratio was not associated with urinary %InAs in adjusted models in either folate stratum (folate-deficient, P = 0.62; folate-sufficient, P = 0.47). The plasma GSH/GSSG ratio was positively associated with urinary SMI in the folate-deficient group (folate-deficient, B ± SE, 0.49 ± 0.14, P = 0.002; folate-sufficient, B ± SE, -0.04 ± 0.07, P = 0.98; P interaction = 0.001). The change in R2 values for urinary SMI models were 7.7% in the folate-deficient group and 0.0% in the folate-sufficient group; the change in R2 represents the percentage of the variance in urinary SMI explained by the plasma GSH/GSSG ratio after adjustment for covariates. Additionally, in the folate-deficient group, the plasma GSH/GSSG ratio was negatively associated with total bAs concentrations (B ± SE, -0.39 ± 0.18, P = 0.02). Similar patterns of association were observed when plasma GSH Eh, as calculated by the Nernst equation, was used as the predictor: in adjusted models, a more oxidized plasma GSH Eh was associated with decreased urinary SMI (P = 0.03), increased urinary %MMA (P = 0.03), decreased urinary %DMA (P = 0.17), and increased bAs concentrations (P = 0.08) in the folate-deficient stratum.
Table 3.
Regression coefficients for associations between log-transformed plasma GSH/GSSG ratio and arsenic methylation capacity indicators, stratified by plasma folate status.
| Outcome | Model | Total sample N=376 | Folate deficient N=110 | Folate sufficient N=266 | P interactionb | ||||
|---|---|---|---|---|---|---|---|---|---|
| B±SE | B+SE | R2 (%) | Δ in R2 (%) | B±SE | R2 (%) | Δ in R2 (%) | |||
| Urinary %MMA | Adj. for gender, age, and wAsc | –1.03±0.70 | –4.60±1.51** | 29.6 | 6.4 | –0.18±0.77 | 19.2 | 0.0 | 0.001 |
| Extended modela | –0.81±0.71 | –5.21±1.58** | 36.3 | 7.1 | 0.17±0.78 | 24.8 | 0.0 | 0.002 | |
| Urinary %DMA | Adj. for gender, age, and wAsc | 0.09±1.16 | 4.93±2.56† | 15.2 | 2.9 | –0.92±1.29 | 12.0 | 0.2 | 0.04 |
| Extended modela | 0.28±1.20 | 6.53±2.66* | 23.4 | 4.5 | –0.89±1.33 | 13.7 | 0.1 | 0.01 | |
| Urinary SMIc | Adj. for gender, age, and wAsc | 0.07±0.07 | 0.41±0.14** | 29.9 | 6.3 | –0.01±0.07 | 16.9 | 0.0 | 0.007 |
| Extended modela | 0.06±0.07 | 0.49±0.14** | 37.1 | 7.7 | –0.04±0.07 | 23.0 | 0.0 | 0.001 | |
| Blood Asc | Adj. for gender, age, and wAsc | –0.04±0.08 | –0.26±0.17 | 61.6 | 0.8 | 0.02±0.09 | 62.4 | 0.0 | 0.15 |
| Extended modela | –0.10±0.08 | –0.39±0.18* | 63.5 | 1.6 | –0.01±0.09 | 64.1 | 0.0 | 0.06 | |
Adjusted for gender, age, log-transformed water As, cigarette smoking (ever/never), betelnut chewing (ever/never), television ownership, plasma cobalamin, and BMI; N=110 in folate deficient, N=259 in folate sufficient
p-value from Wald test for group difference in the regression coefficient.
Log-transformed.
p<0.10
p<0.05
p<0.01.
To examine whether concentrations of plasma GSH or GSSG were independently associated with As methylation capacity, we built regression models with GSH and GSSG concentrations as predictors of urinary As metabolites, shown in Table 4. Since plasma GSH and GSSG were positively correlated with one another (overall sample, Spearman r = 0.29, P < 0.0001), we included both variables in the models simultaneously. After adjustment for covariates, in the folate-deficient stratum, plasma GSH was positively associated with urinary SMI (B ± SE, 0.41 ± 0.18, P = 0.04), while plasma GSSG was negatively associated with urinary SMI (B ± SE, -0.53 ± 0.15, P = 0.002). Again, no significant associations were observed in the folate-sufficient stratum.
Table 4.
Regression coefficients for associations between log-transformed plasma GSH and plasma GSSG concentrations and arsenic methylation capacity indicators, stratified by plasma folate status.
| Outcome | Predictor | Total sample N=376 | Folate-deficient N=110 | Folate-sufficent N=266 | P interactionb |
|---|---|---|---|---|---|
| B±SE | B±SE | B±SE | |||
| Urinary %MMA | Plasma GSHa | –0.02±0.88 | –4.75±2.00* | 1.16±0.98 | 0.008 |
| Plasma GSSGa | 1.59±0.87† | 5.46±1.72** | 0.93±1.02 | 0.0003 | |
| Urinary %DMA | Plasma GSHa | –0.60±1.50 | 4.93±3.37 | –1.24±1.68 | 0.10 |
| Plasma GSSGa | –1.13±1.48 | –7.40±2.90* | 0.50±1.76 | 0.02 | |
| Urinary SMIc | Plasma GSHa | –0.04±0.08 | 0.41±0.18* | –0.14±0.09 | 0.006 |
| Plasma GSSGa | –0.15±0.08† | –0.53±0.15** | –0.07±0.10 | 0.01 | |
| Blood Asc | Plasma GSHa | –0.10±0.10 | –0.34±0.22 | –0.05±0.12 | 0.25 |
| Plasma GSSGa | 0.09±0.10 | 0.41±0.19* | –0.03±0.12 | 0.05 |
Adjusted for gender, age, log-transformed water As, cigarette smoking (ever/never), betelnut chewing (ever/never), television ownership, plasma cobalamin, BMI, and other plasma variable (GSH or GSSG); N=110 in folate deficient, N=259 in folate sufficient.
p-value from Wald test for group difference in the regression coefficient.
Log-transformed.
p<0.10
p<0.05
p<0.01.
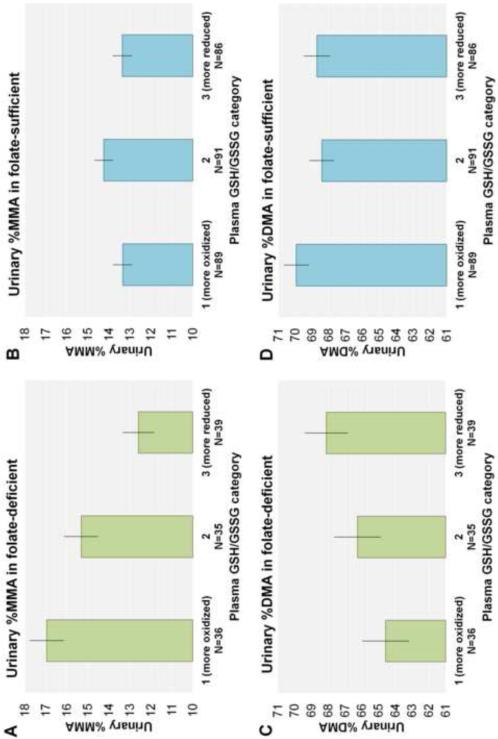
Least squares mean urinary %MMA and %DMA by plasma GSH/GSSG tertiles, stratified by plasma folate status, are presented in Figure 2. In the folate-deficient stratum, the most oxidized plasma GSH/GSSG category (Category 1, ratios of 0.39-1.06) had a 4.4% higher adjusted mean urinary %MMA compared to the least oxidized plasma GSH/GSSG category (Category 3, ratios of 1.42-2.92) (P = 0.0001) (Figure 2A). Category 2 (ratios of 1.07-1.41) had a 2.7% increase in mean urinary %MMA compared to Category 3 (P = 0.01) (Figure 2A). Adjusted mean urinary %MMA did not differ by plasma GSH/GSSG category in the folate-sufficient stratum (Figure 2B). Similarly, Category 1 had a 3.5% lower mean urinary %DMA compared to Category 3 in the folate-deficient (P = 0.07) (Figure 2C), but no differences by category were observed in the folate-sufficient group (Figure 2D).
Figure 2. Adjusted mean urinary %MMA and %DMA by plasma GSH/GSSG tertile, stratified by plasma folate status.
Bars represent mean ± SE urinary %MMA and %DMA for each plasma GSH/GSSG tertile, adjusted for gender and well water As.
DISCUSSION
The primary objective of this study was to examine the associations of the plasma GSH/GSSG ratio with indicators of As methylation capacity (urinary %MMA, %DMA, and SMI) and to further examine whether these associations were modified by plasma folate status. In the folate-deficient stratum, we observed that the plasma GSH/GSSG ratio was negatively associated with urinary %MMA and positively associated with urinary %DMA and urinary SMI. No significant associations were observed in the folate-sufficient stratum, and the interaction by folate status was statistically significant. Additionally, the plasma GSH/GSSG ratio was negatively associated with total bAs concentrations in the folate-deficient stratum. Our findings suggest that a more reduced plasma GSH redox state is associated with increased As methylation capacity and decreased bAs concentrations, and plasma folate status is a modifier of these associations.
We found that an oxidized plasma GSH redox state was negatively associated with As methylation capacity, with concentrations of plasma GSH and GSSG having independent—and opposite—associations with urinary As metabolite percentages. We speculate that these observations might be explained by S-glutathionylation, i.e., direct binding of GSH and GSSG to AS3MT [26]. In vitro studies show that biotinylated GSH and GSSG bind to recombinant human AS3MT and that binding of GSH is antagonized by GSSG (David Thomas, unpublished observations, 2013). These interactions are consistent with roles for GSH and GSSG and the GSH/GSSG ratio in regulation of AS3MT activity.
There are several potential explanations why the association between plasma GSH/GSSG and As methylation capacity was only observed in the folate-deficient group. First, it is possible that S-glutathionylation of AS3MT, if present, might have a regulatory purpose. For example, GSSG inhibits the Na,K-ATPase enzyme through S-glutathionylation of Cys residues in its ATP binding site, but this inhibition does not occur when ATP concentrations are above a certain threshold [38]. The authors speculated that S-glutathionylation of Na,K-ATPase is a regulatory mechanism to prevent irreversible loss of ATP under ATP-deficient conditions [38]. It is possible that an analogous mechanism occurs with AS3MT, where exposure to GSSG under folate-deficient conditions increases S-glutathionylation of redox-sensitive Cys residues near the SAM binding site, thereby conserving SAM. Interestingly, the AS3MT Cys residues that were previously identified as redox-sensitive, Cys156 and Cys206, are located in the SAM binding pocket [39]. Alternatively, mathematical models estimate that hepatic SAM concentrations during folate-sufficient conditions are markedly above the Km for AS3MT [40], and it is possible that GSH does not measurably modulate the AS3MT methylation rate when SAM concentrations are not limiting. While we cannot definitively establish whether one of these mechanisms explains our observations, it would be of interest to explore these potential mechanisms in experimental models.
It is also possible that our observed associations are explained by differential biliary transport of As metabolite species. For example, efflux of AsIII and MMAIII from hepatocytes to the bile via multidrug resistance protein 2 (Mrp2) requires the formation of As-GSH conjugates [41]. If plasma GSH redox state indeed reflects biliary As excretion, we would expect that plasma GSH redox would be associated with urinary As concentrations. However, we did not observe significant associations between plasma GSH, GSSG, or GSH/GSSG ratio with total urinary As or urinary As metabolite concentrations (InAs, MMA, or DMA) (data not shown). This suggests that the plasma GSH redox state is more strongly associated with the proportion of urinary As metabolites for a given As exposure. The absence of associations between plasma GSH redox and urinary As concentrations also suggests that our observations are less likely to be explained by reverse causality, e.g., inhibition of glutathione reductase (GR) by As metabolites seen at very high As concentrations in vitro [42-44] and in vivo [45].
We are aware of only one other study that examined the associations between GSH and urinary As metabolites in humans [46]. Xu et al. found that total non-protein sulfhydryls (primarily GSH + GSSG) measured in whole blood was strongly associated with decreased urinary %MMA and increased urinary %DMA in children and adults in Inner Mongolia, China [46]. In our study, we did not observe any significant associations between whole blood GSH or the sum of whole blood GSH and GSSG and urinary As metabolite percentages, either in the overall sample or within the folate strata (data not shown). The reason for this discrepancy is unclear, but it might be related to differing laboratory methodologies used to measure GSH or differences in the study populations. Conversely, numerous studies have examined the associations of genetic variants in the glutathione-S-transferase (GST) family of enzymes with urinary As metabolite profiles, although no consistent associations have been observed [47-57]. It is possible that the inconsistent findings might be due to differences in folate nutritional status among the populations included in these studies.
Our study has several limitations. First, we used plasma measurements of GSH, GSSG, and folate as proxies for liver measurements of GSH, GSSG, and SAM. Since the liver is the primary site of As methylation, we would ideally measure GSH, GSSG, and SAM concentrations in hepatic tissue. However, mathematical models of hepatic folate and GSH metabolism indicate that plasma biomarkers might be informative: based on model predictions, plasma folate is strongly related to liver SAM [58], and plasma GSH/GSSG tracks liver GSH/GSSG over a range of steady-state oxidative stress (H2O2) concentrations [59]. Second, evidence from Currier et al. suggests that our method for measuring urinary As metabolites, HPLC-ICP-MS, might underestimate the concentrations of the trivalent methylated As metabolites, i.e., MMAIII and DMAIII [60]. Finally, due to the cross-sectional nature of our study, we are unable to establish the directionality of the relationship between plasma GSH redox status and As methylation capacity.
In conclusion, we found that an oxidized plasma GSH/GSSG redox state was associated with decreased As methylation capacity (increased urinary %MMA, decreased urinary %DMA, and decreased urinary SMI) and increased total bAs among folate-deficient Bangladeshi adults. GSH/GSSG redox might be one of several mechanisms controlling intracellular AS3MT activity and might contribute to the large interindividual variation in As methylation capacity observed in humans.
Highlight.
Arsenic (As) is methylated to MMA and DMA in reductant-dependent reactions.
We examined interactions of GSH redox and folate status on As methylation capacity.
Plasma GSH, GSSG and folate and urinary As metabolites were measured in 376 adults.
Low GSH/GSSG was associated with higher %MMA and lower %DMA in folate deficiency.
Oxidized GSH redox and low folate may interact to reduce As methylation capacity.
ACKNOWLEDGEMENTS
We would like to thank Drs. David Thomas and Michael Reed for their assistance with the preparation of this manuscript. This work was supported by grants RO1 CA133595, RO1 ES017875, P42 ES10349, P30 ES09089, and T32 CA009529-24 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Cancer Institute, or the National Institutes of Health.
Abbreviations
- As
arsenic
- AS3MT
arsenic(III)-methyltransferase
- ATO
arsenic trioxide
- bAs
blood arsenic
- BMI
body mass index
- uCr
urinary creatinine
- CV
coefficient of variation
- FOX
folate and oxidative stress study
- GFAA
graphite furnace atomic absorption
- GSH
glutathione
- GSSG
glutathione disulfide
- ICP-MS
inductively coupled mass spectrometry
- SAM
S-adenosylmethionine
- SMI
secondary methylation index
- uAs
urinary arsenic
- uDMA
urinary dimethylarsinic acid
- uInAs
urinary inorganic arsenic
- uMMA
urinary monomethylarsonic acid
- wAs
water As
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests Declaration: The authors declare no competing interests.
REFERENCES
- 1.Ahmed MF, et al. Epidemiology. Ensuring safe drinking water in Bangladesh. Science. 2006;314(5806):1687–8. doi: 10.1126/science.1133146. [DOI] [PubMed] [Google Scholar]
- 2.IARC Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
- 3.Navarro Silvera SA, Rohan TE. Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control. 2007;18(1):7–27. doi: 10.1007/s10552-006-0057-z. [DOI] [PubMed] [Google Scholar]
- 4.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat Res. 2003;533(1-2):37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, et al. A novel S-adenosyl-L-methionine:arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277(13):10795–803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- 6.Chiang PK, et al. S-Adenosylmethionine and methylation. FASEB J. 1996;10(4):471–80. [PubMed] [Google Scholar]
- 7.Hughes MF, et al. Arsenic (+3 oxidation state) methyltransferase genotype affects steady-state distribution and clearance of arsenic in arsenate-treated mice. Toxicol Appl Pharmacol. 2010;249(3):217–23. doi: 10.1016/j.taap.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Gebel TW. Arsenic methylation is a process of detoxification through accelerated excretion. International Journal of Hygiene and Environmental Health. 2002;205(6):505–508. doi: 10.1078/1438-4639-00177. [DOI] [PubMed] [Google Scholar]
- 9.Styblo M, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74(6):289–99. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 10.Petrick JS, et al. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol. 2001;14(6):651–6. doi: 10.1021/tx000264z. [DOI] [PubMed] [Google Scholar]
- 11.Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol Toxicol. 2001;89(1):1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–22. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(1):1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DJ. Unraveling arsenic--glutathione connections. Toxicol Sci. 2009;107(2):309–11. doi: 10.1093/toxsci/kfn257. [DOI] [PubMed] [Google Scholar]
- 15.Jones DP. Radical-free biology of oxidative stress. American Journal of Physiology - Cell Physiology. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song X, et al. New insights into the mechanism of arsenite methylation with the recombinant human arsenic (+3) methyltransferase (hAS3MT). Biochimie. 2010;92(10):1397–406. doi: 10.1016/j.biochi.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Waters SB, et al. Glutathione modulates recombinant rat arsenic (+3 oxidation state) methyltransferase-catalyzed formation of trimethylarsine oxide and trimethylarsine. Chem Res Toxicol. 2004;17(12):1621–9. doi: 10.1021/tx0497853. [DOI] [PubMed] [Google Scholar]
- 18.Waters SB, et al. Endogenous reductants support the catalytic function of recombinant rat cyt19, an arsenic methyltransferase. Chem Res Toxicol. 2004;17(3):404–9. doi: 10.1021/tx0342161. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DJ, et al. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232(1):3–13. [PMC free article] [PubMed] [Google Scholar]
- 20.Ding L, et al. Methylation of arsenic by recombinant human wild-type arsenic (+3 oxidation state) methyltransferase and its methionine 287 threonine (M287T) polymorph: Role of glutathione. Toxicol Appl Pharmacol. 2012;264(1):121–30. doi: 10.1016/j.taap.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challenger F. Biological methylation. Adv Enzymol Relat Subj Biochem. 1951;12:429–91. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- 22.Challenger F. Biological methylation. Chemical Reviews. 1945;36(3):315–361. [Google Scholar]
- 23.Hayakawa T, et al. A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79(4):183–91. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, et al. Rapid equilibrium kinetic analysis of arsenite methylation catalyzed by recombinant human arsenic (+3 oxidation state) methyltransferase (hAS3MT). J Biol Chem. 2012;287(46):38790–9. doi: 10.1074/jbc.M112.368050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fomenko DE, et al. High-Throughput Identification of Catalytic Redox-Active Cysteine Residues. Science. 2007;315(5810):387–389. doi: 10.1126/science.1133114. [DOI] [PubMed] [Google Scholar]
- 26.Dalle-Donne I, et al. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Kombu RS, et al. Dynamics of glutathione and ophthalmate traced with 2H-enriched body water in rats and humans. American Journal of Physiology - Endocrinology And Metabolism. 2009;297(1):E260–E269. doi: 10.1152/ajpendo.00080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MN, et al. Chronic Arsenic Exposure and Blood Glutathione and Glutathione Disulfide Concentrations in Bangladeshi Adults. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Z, et al. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379(3):512–8. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 30.Van Geen A, et al. Reliability of a commercial kit to test groundwater for arsenic in Bangladesh. Environ Sci Technol. 2005;39(1):299–303. [PubMed] [Google Scholar]
- 31.van Geen A, et al. Monitoring 51 community wells in Araihazar, Bangladesh, for up to 5 years: implications for arsenic mitigation. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(12):1729–40. doi: 10.1080/10934520701564236. [DOI] [PubMed] [Google Scholar]
- 32.Vela NP, Heitkemper DT, Stewart KR. Arsenic extraction and speciation in carrots using accelerated solvent extraction, liquid chromatography and plasma mass spectrometry. Analyst. 2001;126(7):1011–7. doi: 10.1039/b102420p. [DOI] [PubMed] [Google Scholar]
- 33.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17(4):381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 34.Hall M, et al. Blood arsenic as a biomarker of arsenic exposure: results from a prospective study. Toxicology. 2006;225(2-3):225–33. doi: 10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Jones DP, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275(2):175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 36.Jones DP, et al. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33(9):1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 37.Hall MN, et al. Influence of cobalamin on arsenic metabolism in Bangladesh. Environ Health Perspect. 2009;117(11):1724–9. doi: 10.1289/ehp.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrushanko IY, et al. S-glutathionylation of the Na,K-ATPase catalytic alpha subunit is a determinant of the enzyme redox sensitivity. J Biol Chem. 2012;287(38):32195–205. doi: 10.1074/jbc.M112.391094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, et al. Functional evaluation of Asp76, 84, 102 and 150 in human arsenic(III) methyltransferase (hAS3MT) interacting with S-adenosylmethionine. FEBS Letters. (0) doi: 10.1016/j.febslet.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 40.Lawley S, et al. Mathematical model insights into arsenic detoxification. Theoretical Biology and Medical Modelling. 2011;8(1):31. doi: 10.1186/1742-4682-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leslie EM. Arsenic–glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs). Journal of Inorganic Biochemistry. 2012;108(0):141–149. doi: 10.1016/j.jinorgbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Styblo M, Thomas DJ. In vitro inhibition of glutathione reductase by arsenotriglutathione. Biochem Pharmacol. 1995;49(7):971–7. doi: 10.1016/0006-2952(95)00008-n. [DOI] [PubMed] [Google Scholar]
- 43.Styblo M, et al. Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol. 1997;10(1):27–33. doi: 10.1021/tx960139g. [DOI] [PubMed] [Google Scholar]
- 44.Chouchane S, Snow ET. In vitro effect of arsenical compounds on glutathione-related enzymes. Chem Res Toxicol. 2001;14(5):517–22. doi: 10.1021/tx000123x. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez VM, et al. Glutathione Reductase Inhibition and Methylated Arsenic Distribution in Cd1 Mice Brain and Liver. Toxicological Sciences. 2005;84(1):157–166. doi: 10.1093/toxsci/kfi057. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, et al. Association of oxidative stress with arsenic methylation in chronic arsenic-exposed children and adults. Toxicol Appl Pharmacol. 2008;232(1):142–9. doi: 10.1016/j.taap.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Chiou HY, et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997;386(3):197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- 48.Caceres DD, et al. Polymorphism of glutathione S-transferase (GST) variants and its effect on distribution of urinary arsenic species in people exposed to low inorganic arsenic in tap water: an exploratory study. Arch Environ Occup Health. 2010;65(3):140–7. doi: 10.1080/19338240903390354. [DOI] [PubMed] [Google Scholar]
- 49.Schlawicke Engstrom K, et al. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect. 2007;115(4):599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindberg AL, et al. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115(7):1081–6. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paiva L, et al. Association between GSTO2 polymorphism and the urinary arsenic profile in copper industry workers. Environ Res. 2010;110(5):463–8. doi: 10.1016/j.envres.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Steinmaus C, et al. Genetic polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and metabolism of arsenic. J Toxicol Environ Health A. 2007;70(2):159–70. doi: 10.1080/15287390600755240. [DOI] [PubMed] [Google Scholar]
- 53.Chung CJ, et al. Polymorphisms in one-carbon metabolism pathway genes, urinary arsenic profile, and urothelial carcinoma. Cancer Causes Control. 2010;21(10):1605–13. doi: 10.1007/s10552-010-9589-3. [DOI] [PubMed] [Google Scholar]
- 54.Agusa T, et al. Individual variations in arsenic metabolism in Vietnamese: the association with arsenic exposure and GSTP1 genetic polymorphism. Metallomics. 2012;4(1):91–100. doi: 10.1039/c1mt00133g. [DOI] [PubMed] [Google Scholar]
- 55.Hwang YH, et al. Genetic polymorphism of As3MT and delayed urinary DMA excretion after organic arsenic intake from oyster ingestion. J Environ Monit. 2010;12(6):1247–54. doi: 10.1039/c000844c. [DOI] [PubMed] [Google Scholar]
- 56.Ahsan H, et al. Arsenic Metabolism, Genetic Susceptibility, and Risk of Premalignant Skin Lesions in Bangladesh. Cancer Epidemiology Biomarkers & Prevention. 2007;16(6):1270–1278. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]
- 57.Marnell LL, et al. Polymorphisms in the human monomethylarsonic acid (MMA V) reductase/hGSTO1 gene and changes in urinary arsenic profiles. Chem Res Toxicol. 2003;16(12):1507–13. doi: 10.1021/tx034149a. [DOI] [PubMed] [Google Scholar]
- 58.Duncan TM, Reed MC, Nijhout HF. The relationship between intracellular and plasma levels of folate and metabolites in the methionine cycle: a model. Mol Nutr Food Res. 2013;57(4):628–36. doi: 10.1002/mnfr.201200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reed MC, et al. A mathematical model of glutathione metabolism. Theor Biol Med Model. 2008;5:8. doi: 10.1186/1742-4682-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Currier J, et al. Comparative oxidation state specific analysis of arsenic species by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry and hydride generation-cryotrapping-atomic absorption spectrometry. J Anal At Spectrom. 2013;28(6):843–852. doi: 10.1039/C3JA30380B. [DOI] [PMC free article] [PubMed] [Google Scholar]