Abstract
BACKGROUND
The purpose of the current study was to define the myocellular changes and adaptation of the β-adrenergic receptor (β-AR) system that occur in the systemic right ventricle (RV) of children with hypoplastic left heart syndrome (HLHS).
METHODS
Explanted hearts from children with HLHS and non-failing controls were used for this study. HLHS patients were divided into 2 groups: “compensated” (C-HLHS), infants listed for primary transplant with normal RV function and absence of heart failure symptoms, and “decompensated” (D-HLHS), patients listed for transplant after failed surgical palliation with RV failure and/or refractory protein-losing enteropathy or plastic bronchitis.
RESULTS
Compared with non-failing control RVs, the HLHS RV demonstrated decreased sarcoplasmic reticulum calcium-adenosine triphosphatase 2a and α-myosin heavy chain (MHC) gene expression, decreased total β-AR due to down-regulation of β1-AR, preserved cyclic adenosine monophosphate levels, and increased calcium/calmodulin-dependent protein kinase II (CaMKII) activity. There was increased atrial natriuretic peptide expression only in the C-HLHS group. Unique to those in the D-HLHS group was increased β-MHC and decreased α-MHC protein expression (MHC isoform switching), increased adenylyl cyclase 5 expression, and increased phosphorylation of the CaMK target site on phospholamban, threonine 17.
CONCLUSIONS
The HLHS RV has an abnormal myocardial gene expression pattern, downregulation of β1-AR, preserved cyclic adenosine monophosphate levels, and increased CaMKII activity compared with the non-failing control RV. There is MHC isoform switching, increased adenylyl cyclase 5, and increased phosphorylation of phospholamban threonine 17 only in the D-HLHS group. Although abnormal gene expression and changes in the β-AR system precede clinically evident ventricular failure in HLHS, additional unique adaptations occur in those with HLHS and failed surgical palliation.
Keywords: β-adrenergic receptor system, gene expression, right ventricle, hypoplastic left heart, syndrome
Hypoplastic left heart syndrome (HLHS) is the most common form of severe congenital heart disease and is uniformly fatal without intervention.1 Overall outcomes remain poor, and in a recent multicenter study, freedom from death or transplant at the age of 1 year in patients undergoing single ventricle reconstruction for lesions such as HLHS was only 68.7%.2 Because the right ventricle (RV) in a child with HLHS must provide cardiac output to the systemic circulation, chronic pressure and volume overload result in compensatory hypertrophy and can eventually lead to tricuspid valve regurgitation, ventricular dilation, and cardiac failure.
In adults, myocardial failure is characterized by recapitulation of a “fetal” gene program (FGP) as embryonically expressed genes are reactivated.3 This FGP is indicative of ventricular remodeling and is typified by increases in β-myosin heavy chain (β-MHC), B-type natriuretic peptide (BNP), and atrial natriuretic peptide (ANP), and coordinate decreases in α-MHC and sarcoplasmic reticulum calcium-adenosine triphosphatase 2a (SERCA2a). Although the effect of volume overload on gene expression is incompletely understood, changes in gene expression related to mitochondrial function, cardiac fibrosis, and apoptosis were observed in an animal model of pulmonary insufficiency.4 Specific to HLHS, 1 study demonstrated marked variations in the gene expression profile and messenger (m)RNA splicing in the RV of children with HLHS compared with the LV of normal controls.5 Selective evaluation of FGP expression has not been investigated in those with a single systemic RV.
Although the β-adrenergic system has been well-defined in adults and supports the rationale of β-blocker therapy for heart failure,6 the utility of β-blocker therapy in the HLHS population is currently unproven. The primary β-adrenergic receptors (β-AR) in the heart are β1-AR and β2-AR, which have a ratio of ~75:25 in adult and pediatric non-failing LV.7,8 β1-AR and β2-AR are both coupled to adenylyl cyclase (AC) through a stimulatory G protein (Gs). β-AR stimulation leads to activation of AC (primarily AC5 and AC6 in the heart), which converts adenosine 5′-triphosphate (ATP) to the potent second messenger, cyclic adenosine monophosphate (cAMP). cAMP subsequently activates protein kinase A (PKA), which phosphorylates phospholamban (PLB) at the serine 16 residue, decreasing SERCA2a inhibition and increasing sarcoplasmic reticulum calcium uptake, which increases myofilament sensitivity to calcium.9 Therefore, ACs play an important role in the positive inotropic response to β-AR stimulation by cAMP-mediated post-translational modification of contractile proteins. In adult heart failure, chronic adrenergic stimulation results in downregulation of the β1-receptor so that the ratio of β1-to-β2 is ~60:40, the β-ARs are uncoupled from the signaling system linked to Gs, and AC activity and cAMP production are decreased.10
Adaptation of the β-adrenergic system in volume overload heart failure is less clearly defined. In adults with chronic mitral regurgitation, some studies demonstrate activation of β-adrenergic signaling, whereas others demonstrate downregulation of β-AR density in those with mitral regurgitation and associated LV dysfunction.11,12 Mixed results have been found in animal studies as well, with no change in β-AR density in a dog tricuspid regurgitation model13 and upregulation of the β1-AR in a rat aortocaval shunt model.14
Calcium/calmodulin-dependent protein kinase II (CaM-KII) is activated in response to β1-AR stimulation. CaMKII levels and activity are increased in adult heart failure, which is important given its involvement in the regulation of calcium resulting in effects on muscle contraction and gene expression.15 CaMKII phosphorylates PLB at threonine-17 (PT17), which releases its inhibitory effect on SERCA2a, accelerating calcium transport.15 Understanding the balance of phosphorylation and dephosphorylation of signaling proteins associated with the β-adrenergic system is vital in defining the mechanisms involved in ventricular adaptation, calcium handling, and contractility.
The purpose of this study was to define the myocellular changes and adaptations that occur in the β-AR system in HLHS. We hypothesized that pathologic gene expression (in the form of recapitulation of the FGP), β-AR downregulation, and altered β-AR signaling would be present in HLHS RV compared with non-failing control RV.
Methods
This study used tissue samples that were selected from the University of Colorado Institutional Review Board-approved pediatric heart tissue bank (informed consent is obtained for all patients), which includes male and female patients of all races and ethnic background undergoing heart transplantation at Children’s Hospital Colorado.
Study cohort
Tissue from the non-failing RV (NF-RV) was obtained from 12 pediatric organ donors for whom no suitable recipient was available due to size or blood type mismatch and who had no history of cardiac dysfunction. RV tissue was also obtained from 26 cardiac transplant recipients with HLHS (Table 1). The HLHS patients were grouped as defined by a clinical state of compensated HLHS (C-HLHS) or decompensated HLHS (D-HLHS) at time of transplant.
Table.
RV samples were obtained from 12 non-failing controls and 26 patients with HLHS
| Subject ID |
Age (y) |
Gender | FGP | β -AR |
AC 5/6 |
β-AR binding |
α-MHC WB |
PLB WB |
PLB-Thr17 WB |
PLB- Ser16 WB |
cAMP | CaMK activity |
Inotrope | Digoxin | ACEI | BB | Diuretic | Palliation history/indication for transplant for D-HLHS group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF-RV | ||||||||||||||||||
| NF1 | 9 | M | X | X | X | X | X | X | X | X | + | − | − | + | − | |||
| NF2 | 7 | M | X | X | X | X | X | X | X | + | − | − | − | − | ||||
| NF3 | 13 | M | X | X | X | X | X | X | X | X | X | X | + | − | − | + | − | |
| NF4 | 3 | F | X | X | X | X | X | X | X | X | X | + | − | − | − | − | ||
| NF5 | 8 | F | X | X | X | X | X | X | X | X | X | X | − | − | − | − | − | |
| NF6 | 1.3 | F | X | X | X | X | X | X | X | X | X | X | + | − | − | − | − | |
| NF7 | 7 | M | X | X | X | X | X | X | X | X | X | X | − | − | − | − | − | |
| NF8 | 1.4 | M | X | X | X | X | X | X | X | X | X | − | − | − | − | − | ||
| NF9 | 12 | M | X | X | X | − | − | − | − | − | ||||||||
| NF10 | 14 | M | X | X | X | X | X | NA | NA | NA | NA | NA | ||||||
| NF11 | 8 | F | X | + | − | − | − | − | ||||||||||
| NF12 | 13 | F | X | X | X | X | NA | NA | NA | NA | NA | |||||||
| 11 | 10 | 8 | 6 | 7 | 9 | 8 | 9 | 8 | 9 | 6 | 0 | 0 | 2 | 0 | ||||
| C-HLHS | ||||||||||||||||||
| CH1 | 0.5 | M | X | X | X | X | X | − | − | − | − | − | PDA stent and PAB | |||||
| CH2 | 0.14 | F | X | X | X | X | X | X | X | − | − | + | − | − | PDA stent | |||
| CH3 | 0.22 | M | X | X | X | X | X | X | X | X | − | − | + | − | + | PDA stent | ||
| CH4 | 0.6 | F | X | X | X | X | X | X | X | − | − | + | − | + | Atrial septal, PDA stent and PAB | |||
| CH5 | 0.05 | F | X | X | X | X | X | X | X | X | X | − | − | − | − | + | On PGE | |
| CH6 | 0.17 | M | X | X | X | X | X | X | X | X | X | − | − | − | − | + | PDA stent | |
| CH7 | 0.07 | M | X | X | X | X | X | X | X | X | X | − | − | − | − | + | Atrial septal stent and on PGE | |
| CH8 | 0.17 | M | X | X | X | X | X | X | X | X | − | − | − | − | + | PDA stent | ||
| CH9 | 0.02 | F | X | X | − | − | − | − | − | On PGE | ||||||||
| CH10 | 0.1 | M | X | X | − | − | − | − | − | PDA stent | ||||||||
| CH11 | 0.1 | M | X | X | X | X | − | + | − | − | − | PDA stent | ||||||
| CH12 | 0.1 | F | X | X | X | − | − | + | − | + | On PGE | |||||||
| CH13 | 0.09 | M | X | X | X | X | − | − | + | − | + | On PGE | ||||||
| CH14 | 0.3 | F | X | X | X | X | − | − | − | − | + | PDA stent and PAB | ||||||
| 8 | 10 | 8 | 4 | 7 | 9 | 10 | 9 | 7 | 10 | 0 | 1 | 5 | 0 | 9 | ||||
| D-HLHS | ||||||||||||||||||
| DH1 | 7.9 | M | X | X | X | X | X | − | + | + | − | + | Fontan (h/o BT shunt)/PLE | |||||
| DH2 | 3.8 | M | X | X | + | + | − | − | + | Fontan (h/o BT shunt)/RV failure | ||||||||
| DH3 | 3.2 | M | X | X | X | X | X | + | + | + | − | + | Fontan/RV failure | |||||
| DH4 | 1.0 | F | X | X | X | X | X | X | X | X | X | X | − | + | + | − | + | Norwood with Sano shunt/RV failure |
| DH5 | 6.0 | F | X | X | X | X | X | X | X | X | X | X | + | − | − | − | + | Fontan (h/o Sano shunt)/PLE and RV failure |
| DH6 | 2.8 | F | X | X | X | X | X | X | X | X | X | X | + | + | + | − | + | Glenn (h/o Sano shunt)/RV failure |
| DH7 | 9.9 | M | X | X | X | X | X | X | X | X | X | + | − | + | − | + | Fontan/PLE and RV failure | |
| DH8 | 0.4 | M | X | X | X | X | X | X | X | X | X | + | − | + | − | + | Norwood with BT shunt/RV failure | |
| DH9 | 4.8 | M | X | X | X | X | + | − | + | − | + | Fontan (h/o Sano shunt)/PB and RV failure | ||||||
| DH10 | 0.7 | F | X | X | X | X | X | X | X | − | + | − | − | + | PDA stent and PAB/RV failure | |||
| DH11 | 1.3 | M | X | X | X | X | X | X | X | X | − | − | + | − | + | PAB/RV failure | ||
| DH12 | 7.5 | M | X | X | X | NA | NA | NA | NA | NA | Glenn (h/o BT shunt)/RV failure | |||||||
| 11 | 12 | 8 | 7 | 7 | 9 | 6 | 7 | 8 | 7 | 7 | 6 | 8 | 0 | 11 |
RV samples were obtained from 12 non-failing controls and 26 patients with HLHS. Of the HLHS cohort, 14 are defined as compensated (C-HLHS) and 12 are defined as decompensated (D-HLHS). The age, gender, specific studies performed on each patient sample, medications, palliation history and indications for transplant (for the D-HLHS group) are noted. Inotropes include dopamine (3mcg/kg/min in one patient) or milrinone (0.5–0.75mcg/kg/min in 7 patients) for the D-HLHS group and dopamine, vasopressin and/or norepinephrine for the NF-RV group. Duration of inotropic therapy in the D-HLHS group was a median of 98 days (range 30–385 days).
ACEI, angiotensin converting enzyme inhibitor; BT shunt, Blalock-Taussig shunt; BB, beta-blocker; F, female; h/o, history of; M, male; NA, information not available; PAB, surgically placed bilateral pulmonary artery bands; PDA, patent ductus arteriosus; PGE, prostaglandin E1 infusion; PLE, protein losing enteropathy; WB, western blot.
The 14 C-HLHS patients were infants listed for primary transplantation (based on parent preference) who had normal systolic RV function defined by echocardiogram, were not receiving inotropes, and had no heart failure signs or symptoms at the time of transplant. The 12 D-HLHS patients were listed for transplant due to failure of surgical palliation leading to the need for heart transplant. The D-HLHS group included patients with RV failure, which was defined as signs and symptoms of heart failure in the setting of abnormal systolic function on an echocardiogram, low cardiac output measured at catheterization (cardiac index < 2.5 liters/min/m2), and/or diastolic dysfunction of the RV (RV enddiastolic pressure > 12 mm Hg), with or without dependence on inotropic support, and/or the presence of protein-losing enteropathy (PLE) or plastic bronchitis resistant to medical therapy.
At the time of cardiac transplantation and for NF-RV donors, the explanted hearts were immediately cooled in ice-cold oxygenated Tyrode’s in the operating room. The ventricular tissue was rapidly dissected (immediately after removal from the patient), flash frozen, and stored at –80°C.
Selected gene expression and adaptations of the β-AR–mediated signaling in the RV of the 26 patients with HLHS were compared with the RV of 12 NF donor hearts. The HLHS group was analyzed after sub-grouping according to their clinical status of C-HLHS or D-HLHS, as described.
Real-time polymerase chain reaction
Total RNA was extracted by mirVana kit (Ambion, Austin, TX), and RNA (0.5 µg) was reverse transcribed into complementary DNA using I-script (Bio-Rad, Hercules, CA). Typically, 5 to 10 ng complementary DNA, 12.5 nmol/liter each primer, and Power Syber Green PCR Master Mix (Applied Biosystems/Life Technologies, Carlsbad, CA) were used in the real-time polymerase chain reactions. Reactions were performed using the ABI 7300 system. The primers have been previously described8 or are listed below. Change in expression was determined by the ΔΔCt method. Primers used were:
AC5 Forward 5′-CTCAACGACTCTACCTACGA; Reverse 5′-TTTCGTGCCCCTATCACCC
AC6 Forward 5′-ACGATTGGTAGCACCTACATG; Reverse 5′-ACAACAACTTCCACAGCCTC
β-AR density
β-AR density was determined using crude membrane preparations and [125I]-iodocyanopindolol ([125I]ICYP) binding by the Engel method.16 The membrane protein concentration was between 100 and 300 µg/ml, and the buffer for [125I]ICYP binding was 150 mmol/liter NaCl, 20 mmol/liter Tris (pH 7.5), and 1 mmol/liter ascorbic acid. Binding displaceable by 1 µmol/liter propranolol was considered specific binding. Experiments were conducted at 30°C for 120 minutes (105 minutes needed to reach equilibrium). The percentage of β1-AR was determined using 1 µmol/liter CGP20712A, which binds exclusively to β1-AR, and subtracting the percentage of β2-AR binding from total specific binding.
Anti-bodies
Total PLB and PLB serine 16 (A0101-12) and threonine 17 (A010-13) anti-bodies were purchased from Badrilla Ltd, Leeds, United Kingdom. The horseradish peroxidase (115-035-146) anti-mouse and anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories Inc, West Grove, Pennsylvania.
Western blots
Western blots were performed as previously described.17 Antibodies were diluted 1:15000 (PLB), 1:5000 (glyceraldehyde-3-phosphate dehydrogenase), and 1:50 (α-MHC) in 1X Tris-buffered saline (20 mmol/liter Tris, 500 mmol/L NaCl [pH 7.5]) containing 3% bovine serum albumin and 0.1% Tween and incubated with the blot overnight at 4°C.
cAMP assay
Enzyme-linked immunoassay for cAMP levels was performed by the Colorado Clinical and Translational Institute core facility at Children’s Hospital Colorado, Aurora, Colorado.
CaMKII activity assay
Nuclear and cytoplasmic protein fractions were prepared from frozen RV tissue using the NE-PER kit (Thermo Scientific, Rockford, IL). CaMKII activity was measured as described by Kirchhefer et al18 Briefly, 5 µg protein were combined with 20 µmol/liter Syntide 2 (Bachem, Torrance, CA) and 5 µCi [γ-32P] ATP. Total protein kinase activity was measured in the presence of 1 µmol/liter CaCl2 and 10 µg/ml calmodulin, and Ca2+-independent protein kinase activity was determined by the addition of 5 mmol/liter ethyleneglycotetraacetic acid. CaMKII activity was expressed as incorporation of picomoles per liter 32P per mg protein per minute.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc, La Jolla, CA). The Shapiro-Wilk normality test was performed to confirm that all data were normally distributed. All values are presented as the mean with the standard error. Disease and age comparisons were performed by Student’s t-test for all experimental outcomes. One-way analysis of variance was performed to evaluate for significant differences across all groups. When significance was detected, a Bonferroni post hoc analysis was performed to determine between-group differences. Statistical significance was set a priori at p < 0.05.
Results
Patient characteristics
The C-HLHS group consisted of infants listed for primary transplant and therefore were inherently younger (median age, 0.12 years; interquartile range [IQR], 0.1–0.6 years) than D-HLHS patients (median age, 3.5 years; IQR, 1.1–7.1 years). Because of the limited availability of infant donor heart tissue, the NF-RV group was older than both HLHS groups (median age, 8 years; IQR, 4–12.8 years). There was no difference in gender (42% female NF-RV vs 37% and 33% female for C- and D-HLHS, respectively; Table 1). The primary indication for transplant in the 12 D-HLHS patients (Table 1) included RV failure in 9, PLE in 3 (2 of these patients also had RV failure), and plastic bronchitis in 1 (this patient had RV failure); overall, 11 of the 12 D-HLHS patients had RV failure at the time of transplant. One patient was listed for transplant secondary to severe PLE who did not strictly meet the criteria for RV failure as defined in the Methods. No patient in the C-HLHS group was receiving inotropic support, whereas 7 of 12 (58%) of the D-HLHS group were dependent on inotropes at the time of transplant. None of the patients in this study were receiving mechanical circulatory support at the time of transplant.
HLHS RV has an abnormal myocardial gene expression pattern
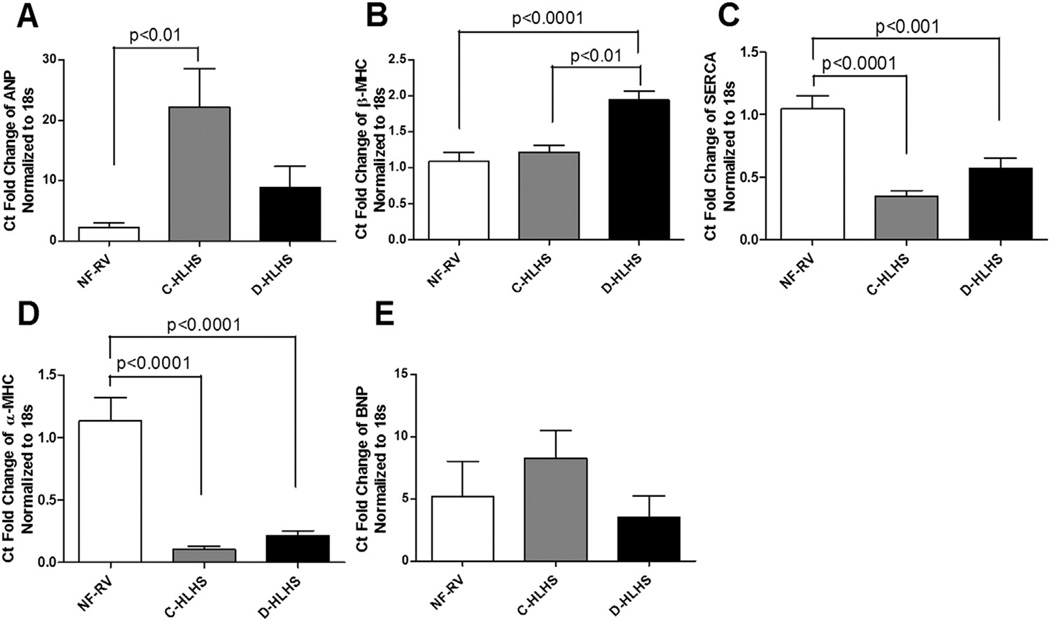
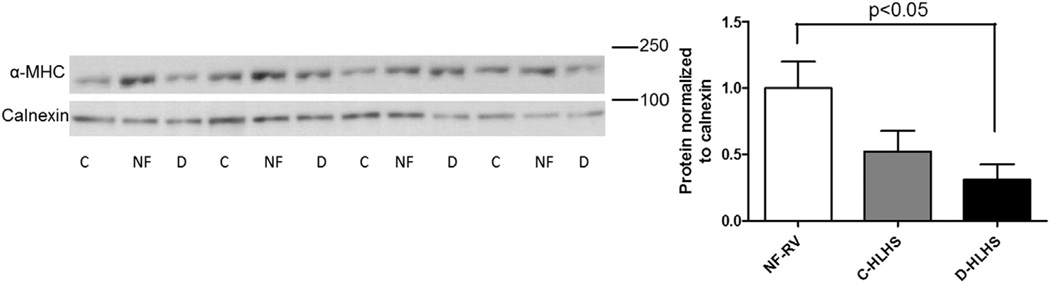
To investigate FGP expression in the RV of children with HLHS, mRNA expression of ANP, β-MHC, SERCA2a, α-MHC, and BNP was determined (Figure 1). All those with HLHS demonstrated a pathologic gene expression pattern, with decreased SERCA2a and α-MHC mRNA expression compared with NF-RV. BNP expression was unchanged in HLHS compared with NF-RV, but was increased in adult heart failure. Interestingly, ANP was increased in C-HLHS compared with NF-RV, whereas β-MHC was increased in the D-HLHS group compared with NF-RV and C-HLHS. Consistent with the decreased mRNA expression, protein levels of α-MHC were also decreased in D-HLHS RV compared with NF-RV (Figure 2). Although there was a trend toward decreased α-MHC protein in C-HLHS compared with NF-RV, this was not significant.
Figure 1.
Recapitulation of the fetal gene program (FGP) occurs in the hypoplastic left heart syndrome (HLHS) right ventricle (RV). Messenger (m)RNA expression was determined in tissue from non-failing RV (NF-RV) controls (n = 11) and in RV tissue from compensated (C)-HLHS (n = 8) and decompensated (D)-HLHS patients (n = 11). There was a pathologic gene expression pattern in HLHS with increased expression of (A) atrial natriuretic peptide (ANP) in C-HLHS and (B) β-myosin heavy chain (MHC) in D-HLHS, along with decreased expression of (C) sarcoplasmic reticulum calcium-adenosine triphosphatase (SERCA) and (D) α-MHC in both HLHS groups compared with NF-RV. (E) B-type natriuretic peptide (BNP) was unchanged in HLHS compared with NF-RV. Results are presented as the mean with the standard error (error bars).
Figure 2.
Western blot analysis demonstrates that protein expression of α-myosin heavy chain (MHC) is decreased in decompensated hypoplastic left heart syndrome (D-HLHS, n = 7) compared with non-failing right ventricle (NF-RV, n = 7) and compensated (C)-HLHS (n = 7). Results are presented as the mean with the standard error (error bars)
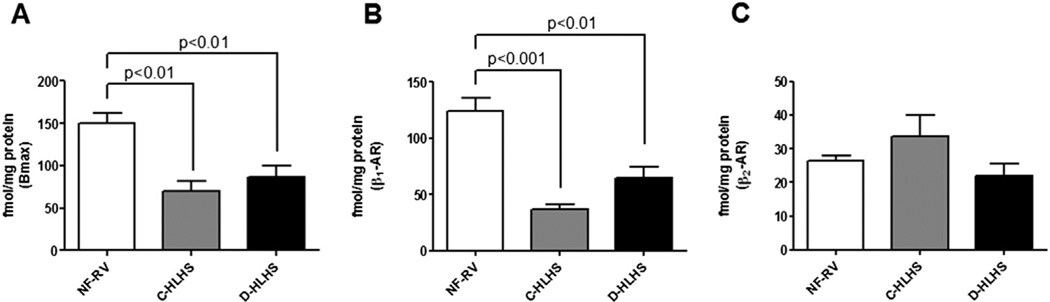
β1-AR is downregulated in HLHS RV
Similar to what has been found in adults and children with LV failure, binding assays demonstrated significant down-regulation of total β-AR (Bmax) in both HLHS groups compared with NF-RV (Figure 3A). The sub-type specific contribution to the decrease in β-AR in HLHS RV was determined, and a targeted decrease in β1-AR was found, whereas β2-AR expression remained unchanged compared with NF-RV (Figure 3B and C). An investigation of mRNA expression of the β-AR sub-types found no difference in the mRNA expression of β1-AR or β2-AR in HLHS RV compared with NF-RV (data not shown).
Figure 3.
β-Adrenergic receptor (β-AR) is downregulated in compensated hypoplastic left heart syndrome (C-HLHS, n = 4) and decompensated (D)-HLHS (n = 7) compared with non-failing right ventricle (NF-RV, n = 6). (A) Total β-AR (Bmax) is decreased in HLHS as determined by [125I]-iodocyanopindolol binding assay. The decrease of β-AR in both HLHS groups is due to (B) β1-AR downregulation with (C) preserved β2-AR expression. Results are presented as the mean with the standard error (error bars).
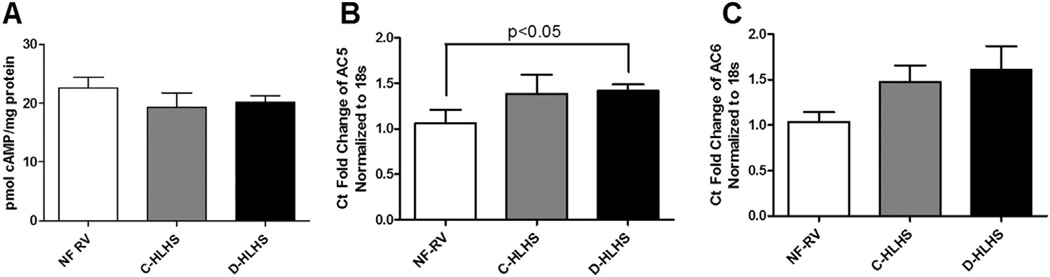
cAMP levels are unchanged in HLHS RV, whereas AC5 expression is increased only in those who receive a transplant after failed surgical palliation
β1-AR stimulation results in an increase in AC-mediated production of cAMP. In adult heart failure, the down-regulation and desensitization of β1-AR results in decreased cAMP production.10 Although β1-AR is downregulated in HLHS RV, cAMP levels were unchanged compared with NF-RV (Figure 4A). In an effort to understand the preserved cAMP levels in HLHS RV, we evaluated the most common AC isoforms in the heart, AC5 and AC6. Although AC6 mRNA expression was unchanged, AC5 expression was increased in the D-HLHS group compared with NF-RV (Figure 4B and C).
Figure 4.
Cyclic adenosine monophosphate (cAMP) levels and adenylyl cyclase (AC) 6 (AC6) expression are unchanged in hypoplastic left heart syndrome (HLHS) compared with the non-failing right ventricle (NF-RV), whereas expression of AC5 is increased in decompensated (D)-HLHS. (A) cAMP levels determined by enzyme-linked immunosorbent assay are unchanged in HLHS (NF-RV, n = 8; compensated [C]-HLHS, n = 7; D-HLHS, n = 8). Messenger RNA levels of (B) AC5 and (C) AC6 were determined by real-time polymerase chain reaction (NF-RV, n = 8; C-HLHS, n = 8; D-HLHS, n = 8). Results are presented as the mean with the standard error (error bars).
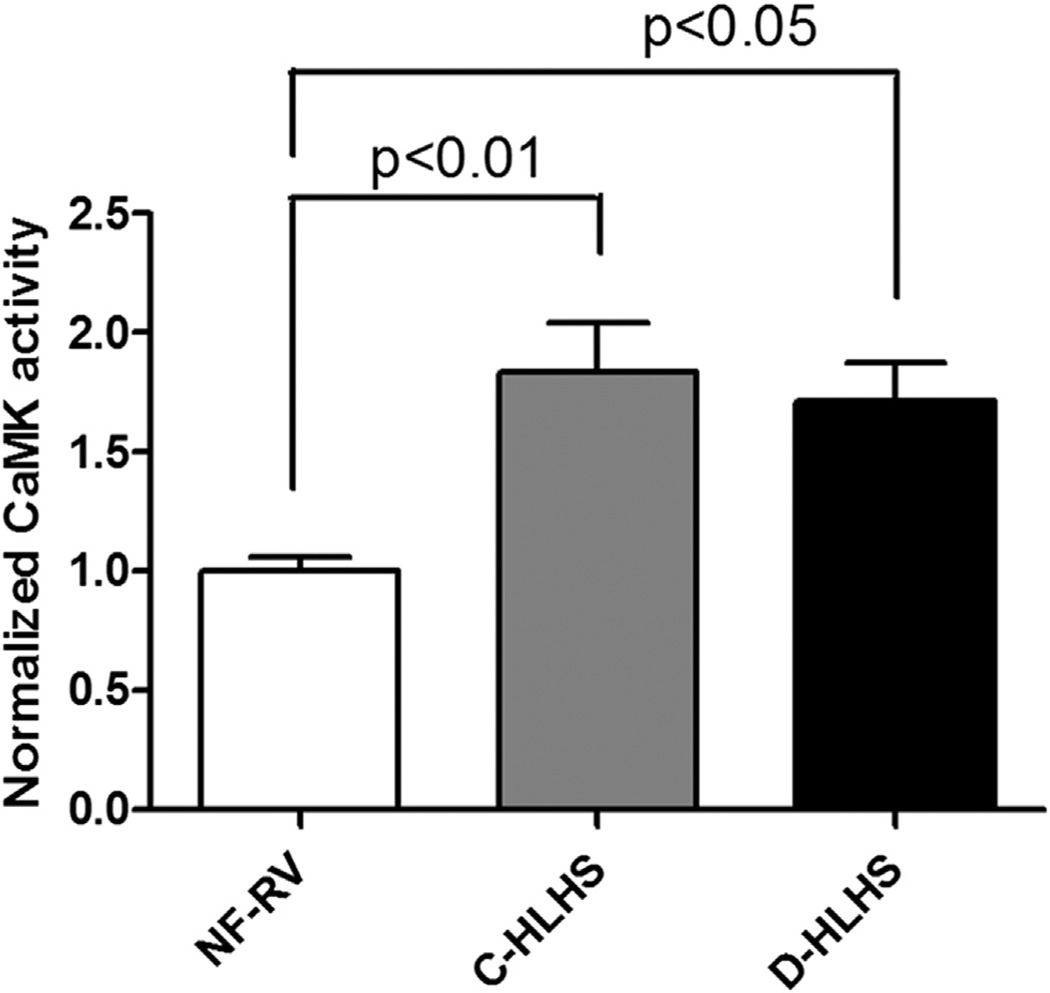
CaMKII activity is increased in HLHS, whereas phosphorylation of phospholamban at threonine 17 is increased only in those who receive a transplant after failed surgical palliation
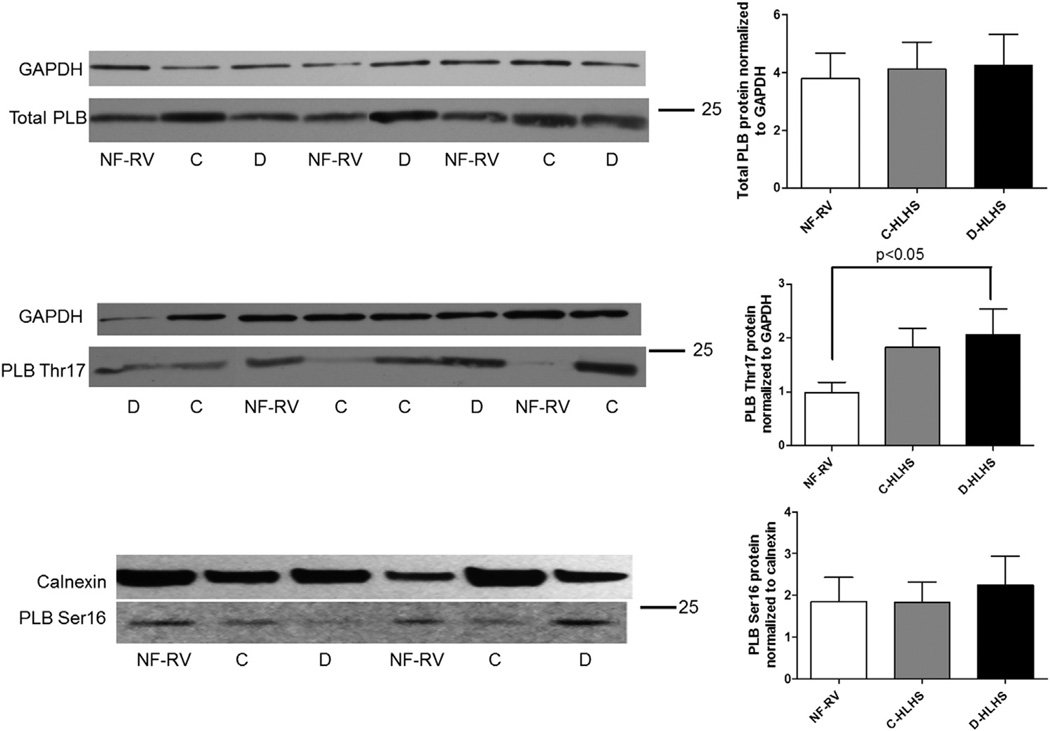
PKA and CaMKII are important downstream effectors of β1-AR stimulation, through regulation of gene transcription and coordination of excitation-contraction coupling. Similar to what is seen in adults with LV failure, CaMKII activity is increased in HLHS RV compared with NF-RV (Figure 5). Although PLB phosphorylation does not change in the C-HLHS group, phosphorylation of the CaMKII target site on PLB, PT17, is increased in D-HLHS compared with NF-RV (Figure 6B). This increased PLB phosphorylation is in stark contrast to what is seen in adult LV failure. Total PLB (Figure 6A) and phosphorylation of serine 16 (PKA target site, Figure 6C) are unchanged in HLHS RV compared with NF-RV.
Figure 5.
Calcium/calmodulin-dependent protein kinase II (CaMKII) activity is increased in hypoplastic left heart syndrome (HLHS) compared with non-failing right ventricle (NF-RV, n = 9; compensated [C]-HLHS, n = 10; decompensated [D]-HLHS, n = 7). Results are presented as the mean with the standard error (error bars).
Figure 6.
Phosphorylation of the calcium/calmodulin-dependent protein kinase (CaMK) target site on phospholamban (PLB), threonine 17 (PT17), is increased in decompensated hypoplastic left heart syndrome (D-HLHS) compared with the non-failing right ventricle (NF-RV). (A) By Western blot analysis, there was no difference in total PLB between HLHS (compensated [C]-HLHS, n = 9; D-HLHS, n = 9) and NF-RV (n = 9). (B) There was increased phosphorylation of PT17 in D-HLHS (n = 6) compared with NF-RV (n = 8) and C-HLHS (n = 10) (C) but no differences in phosphorylation of PLB at the protein kinase A (PKA) target site, Ser16, between groups (NF-RV, n = 9; C-HLHS, n = 10; and D-HLHS, n = 7). Results are presented as the mean with the standard error (error bars).
Discussion
Unfortunately, there are no proven medical therapies to preserve ventricular function or treat ventricular failure in patients with single-ventricle physiology. In 2007, Shaddy et al19 reported the results from the first and only randomized controlled trial of medical therapy, which investigated carvedilol (a non-specific β-blocker) in children with heart failure. The sub-set of patients with a single systemic RV (HLHS and variants) demonstrated a trend toward a detrimental effect in response to carvedilol. Although this trial had several limitations, including the heterogeneity of the children enrolled and the composite end points used, these results are in stark contrast to the beneficial effect of β-blocker therapy in adults with heart failure.20
The purpose of our study was to investigate myocellular adaptations and the β-adrenergic pathway in HLHS. Results of this study may begin to reconcile results of recent clinical trials with specimens from patients with HLHS. In general, the gene expression pattern in HLHS RV was indicative of a failing phenotype, consistent with what has been previously described in the adult failing RV and LV, with decreased SERCA2a in both HLHS groups and a trend toward increased ANP, although this was only significantly increased in the C-HLHS group.21 SERCA2a is the major calcium pump for the sarcoplasmic reticulum, and decreased SERCA2a activity would result in impaired calcium handling and diminished cardiac contractility and relaxation.22 Although BNP expression is increased in the LV and RV of adults with heart failure, BNP was not differentially expressed in HLHS RV compared with NF-RV. Interestingly, this suggests there may be age-related regulation of myocyte BNP expression, because we also found no increase in BNP mRNA expression in the LV of children with dilated cardiomyopathy.8 BNP is considered a good biomarker for heart failure in children; however, there may not be a correlation between circulating and myocyte expression.23
Investigation ofMHC expression revealed that α-MHC was decreased compared with NF-RV, whereas β-MHC was only increased in the D-HLHS group. This α-MHC to β-MHC isoform switch demonstrated in the D-HLHS group is consistent with what is described in adult LV and RV heart failure and could be physiologically relevant in the presence of systemic RV failure.21,24 Although β-MHC is the predominant isoform in human hearts, α-MHC has a more rapid contractile velocity and, despite its lower levels, is important in force generation of the heart. Owing to the hemodynamic stress imposed on a systemic RV, that there is at least partial recapitulation of the FGP, even in those with HLHS and no overt heart failure, is not surprising. Chronic pressure and volume overload may lead to a decrease in α-MHC, whereas in the D-HLHS group (nearly all of whom had concomitant RV failure), there was also a reciprocal increase in β-MHC. The downregulation of α-MHC and upregulation of β-MHC is thought to lead to decreased myosin adenosine triphosphatase activity, slowing the speed of contraction and contributing to myocardial dysfunction.21 One strategy for preventing systemic RV failure could target increasing α-MHC expression or prevent this isoform switch from occurring.
As seen in adults, total β-AR density is decreased in HLHS RV secondary to β1-AR sub-type downregulation with preserved β2-AR expression. However, this pattern is distinct from what we found in children with dilated cardiomyopathy and LV failure, where β1-AR and β2-AR levels are both decreased.8 Importantly, β-AR downregulation occurs even in those with HLHS and preserved RV function, suggesting that the presence of single-ventricle physiology influences adaptation of the β-AR system in children before the onset of clinically evident heart failure.
AC5 and AC6 are the 2 major AC isoforms in the human heart and are critical in the production of cAMP from ATP in response to β-AR stimulation. In adult heart failure and adult pulmonary hypertension, cAMP levels and AC activity are decreased.10 Overexpression of AC6 results in improved cardiac function in response to myocardial infarction, and therefore, activation of AC6 has been identified as a potential therapeutic target for heart failure.25 However, the opposite is true for AC5, with targeted deletion or pharmacologic inhibition of AC5 being protective in various animal models of cardiac stress.26,27 Children with HLHS demonstrate a unique pattern of expression, with unchanged cAMP levels and AC6 expression, but increased AC5 expression in the D-HLHS group compared with NF-RV. On the basis of these findings, AC5 inhibition could represent a potential therapeutic target for children with HLHS and RV failure.
β-AR stimulation leads to activation of CaMKII, which is involved in the development of myocardial hypertrophy, myocyte apoptosis, and heart failure in response to stress.15 Consistent with what has been previously demonstrated in adult LV failure, CaMKII activity is increased in HLHS compared with NF-RV. CaMKII is thought to mediate its effects through autophosphorylation as well as phosphorylation of calcium-handling proteins such as PLB. Although CaMKII activity is increased in adult heart failure, PLB is dephosphorylated at the CaMKII target site, PT17, presumably through elevated phosphatase activity. However, in D-HLHS there is increased PT17 phosphorylation, which leads to decreased inhibition of SERCA2a and therefore increased sarcoplasmic reticulum calcium content. Although some of the beneficial effects of β-blocker treatment in adults are thought to be a result of attenuation of CaMKII signaling, β-blockers in the D-HLHS population could prevent beneficial CaMKII-mediated phosphorylation of PLB.
Dephosphorylation of the serine 16 site of PLB is associated with decreased contractility, and as opposed to what is seen in adults with heart failure, there are no changes in serine 16 phosphorylation in D-HLHS or C-HLHS. In adult LV failure, dephosphorylation of PLB is presumed to be due to decreased cAMP levels and/or phosphatase activation. Because cAMP levels are preserved in HLHS, β-AR blockade may result in decreased cAMP levels and subsequent PLB-serine 16 dephosphorylation, which could be detrimental.
This study has some limitations that deserve consideration. First, we do not have detailed information about the indication for inotropic support in the control population. However, if anything, catecholamine stimulation of the NF-RV could theoretically have made it more difficult to detect differences between the control and HLHS RV.
Second, tissue bank–based studies such as this are cross-sectional by nature, so proof of mechanistic associations based on the results presented is not possible.
Third, the D-HLHS group was distinct in that all were exposed to ischemia–reperfusion insults in association with staged surgical palliation. Given that transplant was quite remote in timing from the palliative operations in the D-HLHS group (median, 18.5 months; range, 4–72 months from previous operation to transplant), changes were not likely to be a direct result of ischemia–reperfusion. However, we cannot rule out the possibility that exposure to repeated ischemia–reperfusion injury influenced the gene expression and β-AR system findings.
Finally, owing to limited numbers and the inability to obtain tissue on an older cohort of patients with C-HLHS, we were unable to determine the influence of age, prior surgical procedures, and type of heart failure on our findings.
In conclusion, this study describes the adaptation of the β-adrenergic system in HLHS. The results from this study demonstrate that selected gene expression and β-AR regulation and signaling in the systemic RV of children with HLHS differs from the normal, NF, pediatric RV and undergoes unique changes in the setting of failed surgical palliation. Downregulation of β1-AR, preserved cAMP levels, and increased CaMKII activity were observed in both HLHS groups studied. There is MHC isoform switching, increased AC5, and increased phosphorylation of PT17 only in those with failed surgical palliation. Because all but 1 of the D-HLHS group had systemic RV failure, we speculate that the unique myocellular and β-adrenergic system adaptations noted in that group are secondary to the presence of heart failure. The goal of this study was to characterize the molecular changes that occur in the hearts of children with HLHS and was not designed to identify effective medical therapies for treatment of RV failure in this population. However, our results show that the β-AR pathway is uniquely regulated in HLHS, and β-blocker therapy may inhibit functional contractile pathways. especially in those with HLHS in whom surgical palliation has failed. The combination of prior clinical trials and the results of this study will be relevant in planning future studies aimed at identifying the mechanisms involved in systemic RV failure and remodeling.
Acknowledgments
The α-MHC anti-body was a gift from Dr Eva Van Rooij. This work was supported by the National Institutes of Health (NIH)/National Institute of Child Health Development Grant K12-HD-068372 (to S.D.M.), NIH/National Heart Lung Blood Institute grants R21-HL-097123 (to S.D.M., B.L.S., and C.C.S.) and R01-HL-107715 (to B.L.S.), and by NIH/National Center for Advancing Translational Sciences, Colorado Clinical and Translational Sciences Institute Grant Number UL1-TR-000154. The contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Disclosure statement
C.S. discloses equity in miRagen Inc. B.S. receives research support from Forest Laboratories, Inc. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
References
- 1.Ferencz C, Loffredo RJ, Magee CA. Mount Kisco, NY: Futura; 1993. [Google Scholar]
- 2.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lompre AM, Schwartz K, d'Albis A, Lacombe G, Van Thiem N, Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979;282:105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- 4.Reddy S, Zhao M, Hu DQ, et al. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol. 2013;304:H1314–H1327. doi: 10.1152/ajpheart.00776.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricci M, Xu Y, Hammond HL, et al. Myocardial alternative RNA splicing and gene expression profiling in early stage hypoplastic left heart syndrome. PloS One. 2012;7:e29784. doi: 10.1371/journal.pone.0029784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol. 1997;80:26–40L. doi: 10.1016/s0002-9149(97)00846-1. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J. 2014;35:33–41. doi: 10.1093/eurheartj/ehs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 10.Bristow MR, Feldman AM. Changes in the receptor-G protein-adenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Res Cardiol. 1992;87(Suppl 1):15–35. doi: 10.1007/978-3-642-72474-9_2. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J, Yancey DM, Ahmed MI, et al. Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation. Circ Heart Fail. 2014;7:194–202. doi: 10.1161/CIRCHEARTFAILURE.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakagoshi N, Nakano S, Taniguchi K, Hirata N, Matsuda H. Relation between myocardial beta-adrenergic receptor and left ventricular function in patients with left ventricular volume overload due to chronic mitral regurgitation with or without aortic regurgitation. Am J Cardiol. 1991;68:81–84. doi: 10.1016/0002-9149(91)90715-w. [DOI] [PubMed] [Google Scholar]
- 13.Shah AS, Atkins BZ, Hata JA, et al. Early effects of right ventricular volume overload on ventricular performance and beta-adrenergic signaling. J Thorac Cardiovasc Surg. 2000;120:342–349. doi: 10.1067/mtc.2000.107278. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Sentex E, Saini HK, Chapman D, Dhalla NS. Upregulation of beta-adrenergic receptors in heart failure due to volume overload. Am J Physiol Heart Circ Physiol. 2005;289:H151–H159. doi: 10.1152/ajpheart.00066.2005. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel G, Hoyer D, Berthold R, Wagner H. (+/−)[125Iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- 17.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem. 2003;278:31233–31239. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 19.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 21.Lowes BD, Minobe W, Abraham WT, et al. Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J Clin Invest. 1997;100:2315–2324. doi: 10.1172/JCI119770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res. 2003;57:20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- 23.Lowenthal A, Camacho BV, Lowenthal S, et al. Usefulness of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide as biomarkers for heart failure in young children with single ventricle congenital heart disease. Am J Cardiol. 2012;109:866–872. doi: 10.1016/j.amjcard.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest. 1997;100:2362–2370. doi: 10.1172/JCI119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai NC, Tang T, Gao MH, et al. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okumura S, Takagi G, Kawabe J, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci U S A. 2003;100:9986–9990. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwatsubo K, Minamisawa S, Tsunematsu T, et al. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J Biol Chem. 2004;279:40938–40945. doi: 10.1074/jbc.M314238200. [DOI] [PubMed] [Google Scholar]