Abstract
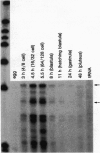
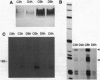
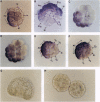
In the sea urchin embryo, the lineage founder cells whose polyclonal progenies will give rise to five different territories are segregated at the sixth division. To investigate the mechanisms by which the fates of embryonic cells are first established, we looked for temporal and spatial expression of homeobox genes in the very early cleavage embryos. We report evidence that PlHbox12, a paired homeobox-containing gene, is expressed in the embryo from the 4-cell stage. The abundance of the transcripts reaches its maximum when the embryo has been divided into the five polyclonal territories--namely at the 64-cell stage--and it abruptly declines at later stages of development. Blastomere dissociation experiments indicate that maximal expression of PlHbox12 is dependent on intercellular interactions, thus suggesting that signal transduction mechanisms are responsible for its transcriptional activation in the early cleavage embryo. Spatial expression of PlHbox12 was determined by whole-mount in situ hybridization. PlHbox12 transcripts in embryos at the fourth, fifth, and sixth divisions seem to be restricted to the conditionally specified ectodermal lineages. These results suggest a possible role of the PlHbox12 gene in the early events of cell specification of the presumptive ectodermal territories.
Full text
PDF

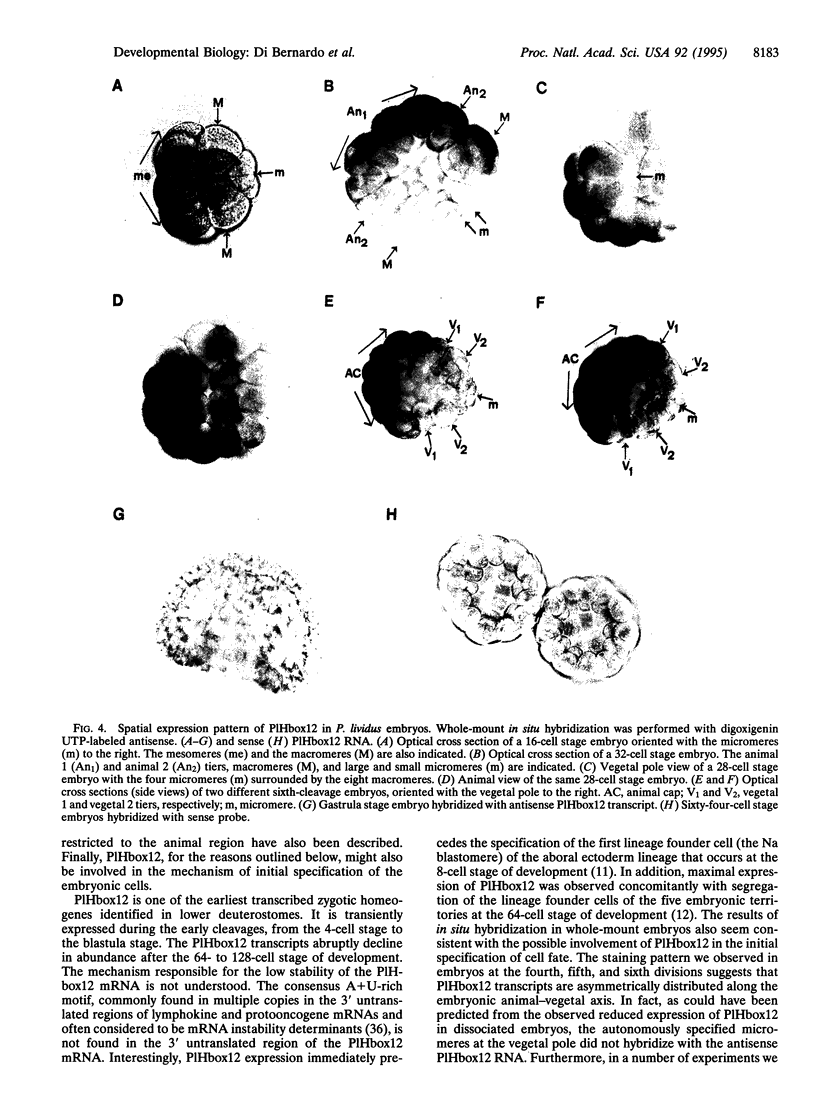

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Dolecki G. J., Gagnon M. L., Lum R., Wang G., Yang Q., Humphreys T., Angerer R. C. Progressively restricted expression of a homeo box gene within the aboral ectoderm of developing sea urchin embryos. Genes Dev. 1989 Mar;3(3):370–383. doi: 10.1101/gad.3.3.370. [DOI] [PubMed] [Google Scholar]
- Baumgartner S., Bopp D., Burri M., Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during Drosophila embryogenesis. Genes Dev. 1987 Dec;1(10):1247–1267. doi: 10.1101/gad.1.10.1247. [DOI] [PubMed] [Google Scholar]
- Benson S., Sucov H., Stephens L., Davidson E., Wilt F. A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. I. Authentication of the cloned gene and its developmental expression. Dev Biol. 1987 Apr;120(2):499–506. doi: 10.1016/0012-1606(87)90253-3. [DOI] [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. Segregation of oral from aboral ectoderm precursors is completed at fifth cleavage in the embryogenesis of Strongylocentrotus purpuratus. Dev Biol. 1990 Jan;137(1):77–85. doi: 10.1016/0012-1606(90)90009-8. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Fraser S. E., Britten R. J., Davidson E. H. The oral-aboral axis of a sea urchin embryo is specified by first cleavage. Development. 1989 Aug;106(4):641–647. doi: 10.1242/dev.106.4.641. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Hough-Evans B. R., Britten R. J., Davidson E. H. Lineage and fate of each blastomere of the eight-cell sea urchin embryo. Genes Dev. 1987 Mar;1(1):75–85. doi: 10.1101/gad.1.1.75. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. How embryos work: a comparative view of diverse modes of cell fate specification. Development. 1990 Mar;108(3):365–389. doi: 10.1242/dev.108.3.365. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Lineage-specific gene expression and the regulative capacities of the sea urchin embryo: a proposed mechanism. Development. 1989 Mar;105(3):421–445. doi: 10.1242/dev.105.3.421. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Molecular biology of embryonic development: how far have we come in the last ten years? Bioessays. 1994 Sep;16(9):603–615. doi: 10.1002/bies.950160903. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. Spatial mechanisms of gene regulation in metazoan embryos. Development. 1991 Sep;113(1):1–26. doi: 10.1242/dev.113.1.1. [DOI] [PubMed] [Google Scholar]
- Di Bernardo M., Russo R., Oliveri P., Melfi R., Spinelli G. Expression of homeobox-containing genes in the sea urchin (Parancentrotus lividus) embryo. Genetica. 1994;94(2-3):141–150. doi: 10.1007/BF01443428. [DOI] [PubMed] [Google Scholar]
- Di Carlo M., Romancino D. P., Montana G., Ghersi G. Spatial distribution of two maternal messengers in Paracentrotus lividus during oogenesis and embryogenesis. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5622–5626. doi: 10.1073/pnas.91.12.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecki G. J., Humphreys T. An engrailed class homeo box gene in sea urchins. Gene. 1988 Apr 15;64(1):21–31. doi: 10.1016/0378-1119(88)90477-5. [DOI] [PubMed] [Google Scholar]
- Dolecki G. J., Wang G., Humphreys T. Stage- and tissue-specific expression of two homeo box genes in sea urchin embryos and adults. Nucleic Acids Res. 1988 Dec 23;16(24):11543–11558. doi: 10.1093/nar/16.24.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolecki G. J., Wannakrairoj S., Lum R., Wang G., Riley H. D., Carlos R., Wang A., Humphreys T. Stage-specific expression of a homeo box-containing gene in the non-segmented sea urchin embryo. EMBO J. 1986 May;5(5):925–930. doi: 10.1002/j.1460-2075.1986.tb04305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R., Smouse D., Capaci T. M., Spradling A. C., Perrimon N. The orthodenticle gene encodes a novel homeo domain protein involved in the development of the Drosophila nervous system and ocellar visual structures. Genes Dev. 1990 Sep;4(9):1516–1527. doi: 10.1101/gad.4.9.1516. [DOI] [PubMed] [Google Scholar]
- Gan L., Mao C. A., Wikramanayake A., Angerer L. M., Angerer R. C., Klein W. H. An orthodenticle-related protein from Strongylocentrotus purpuratus. Dev Biol. 1995 Feb;167(2):517–528. doi: 10.1006/dbio.1995.1046. [DOI] [PubMed] [Google Scholar]
- Ghiglione C., Lhomond G., Lepage T., Gache C. Cell-autonomous expression and position-dependent repression by Li+ of two zygotic genes during sea urchin early development. EMBO J. 1993 Jan;12(1):87–96. doi: 10.1002/j.1460-2075.1993.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice G., Mutolo V. Reaggregation of dissociated cells of sea urchin embryos. Adv Morphog. 1970;8:115–158. doi: 10.1016/b978-0-12-028608-9.50007-7. [DOI] [PubMed] [Google Scholar]
- Hanes S. D., Brent R. DNA specificity of the bicoid activator protein is determined by homeodomain recognition helix residue 9. Cell. 1989 Jun 30;57(7):1275–1283. doi: 10.1016/0092-8674(89)90063-9. [DOI] [PubMed] [Google Scholar]
- Harkey M. A., Whiteley H. R., Whiteley A. H. Differential expression of the msp130 gene among skeletal lineage cells in the sea urchin embryo: a three dimensional in situ hybridization analysis. Mech Dev. 1992 May;37(3):173–184. doi: 10.1016/0925-4773(92)90079-y. [DOI] [PubMed] [Google Scholar]
- Lepage T., Gache C. Early expression of a collagenase-like hatching enzyme gene in the sea urchin embryo. EMBO J. 1990 Sep;9(9):3003–3012. doi: 10.1002/j.1460-2075.1990.tb07493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage T., Ghiglione C., Gache C. Spatial and temporal expression pattern during sea urchin embryogenesis of a gene coding for a protease homologous to the human protein BMP-1 and to the product of the Drosophila dorsal-ventral patterning gene tolloid. Development. 1992 Jan;114(1):147–163. doi: 10.1242/dev.114.1.147. [DOI] [PubMed] [Google Scholar]
- Livingston B. T., Wilt F. H. Lithium evokes expression of vegetal-specific molecules in the animal blastomeres of sea urchin embryos. Proc Natl Acad Sci U S A. 1989 May;86(10):3669–3673. doi: 10.1073/pnas.86.10.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston B. T., Wilt F. H. Phorbol esters alter cell fate during development of sea urchin embryos. J Cell Biol. 1992 Dec;119(6):1641–1648. doi: 10.1083/jcb.119.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston B. T., Wilt F. H. Range and stability of cell fate determination in isolated sea urchin blastomeres. Development. 1990 Mar;108(3):403–410. doi: 10.1242/dev.108.3.403. [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science. 1993 Feb 19;259(5098):1134–1138. doi: 10.1126/science.8438164. [DOI] [PubMed] [Google Scholar]
- Reynolds S. D., Angerer L. M., Palis J., Nasir A., Angerer R. C. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992 Mar;114(3):769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Mallamaci A., Stornaiuolo A., D'Apice M. R., Nigro V., Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993 Jul;12(7):2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., D'Apice M. R., Nigro V., Casanova J., Graziani F., Acampora D., Avantaggiato V. Orthopedia, a novel homeobox-containing gene expressed in the developing CNS of both mouse and Drosophila. Neuron. 1994 Jul;13(1):83–101. doi: 10.1016/0896-6273(94)90461-8. [DOI] [PubMed] [Google Scholar]
- Stephens L., Kitajima T., Wilt F. Autonomous expression of tissue-specific genes in dissociated sea urchin embryos. Development. 1989 Oct;107(2):299–307. doi: 10.1242/dev.107.2.299. [DOI] [PubMed] [Google Scholar]
- Sucov H. M., Hough-Evans B. R., Franks R. R., Britten R. J., Davidson E. H. A regulatory domain that directs lineage-specific expression of a skeletal matrix protein gene in the sea urchin embryo. Genes Dev. 1988 Oct;2(10):1238–1250. doi: 10.1101/gad.2.10.1238. [DOI] [PubMed] [Google Scholar]
- Treisman J., Harris E., Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991 Apr;5(4):594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]