Abstract
Current studies support the belief that high levels of performance and intellectual abilities are associated with increased brain size or volume. With few exceptions, this conclusion is restricted to studies of post-adolescent subjects and to cerebral cortex. There is evidence that “bigger is better” may not pertain to children and further, that there are areas of the brain in which larger structures are associated with cognitive deficits. In 50 preadolescent children (21 girls) a structural survey of the brain (VBM) was conducted to determine and locate areas in which gray matter volume was associated with poor cognitive performance. Only increased gray matter volume in particular areas of the basal ganglia and specifically the putamen were significantly associated with poor performance on tests of memory, response speed and a general marker and subtests of intelligence. Based on the VBM findings, volumetric analysis of basal ganglia structures were performed using FSL/FIRST. However, no significant changes in total volume of putamen or other basal ganglia structures were detected with this analysis. The disagreement between measures of localized gray matter differences and volumetric analysis suggested that there might be local regional deformity rather than widespread volumetric changes of the putamen. Surface analysis with FSL/FIRST demonstrated that bilateral outward deformation of the putamen, but especially the left, was associated with poor performance on several cognitive tests. Expansion of the globus pallidus and caudate nucleus also was associated with poor performance. Moreover a significant association was detected between a reliable test of language-free intelligence and topographically distinct outward and inward deformation of the putamen. Expansion and contraction of the putamen as a predictor of intelligence may explain why this association was not observed with measures of total volume. These results suggest that deformity is a sensitive measure of function, and that distortion of the basal ganglia may be a neurophenotype for risk of developmental impairment.
Keywords: Basal Ganglia, Putamen, Intelligence, Shape, Volume, Performance
Most studies in adults report that increased brain size or volume is associated with higher levels of intellectual abilities. Estimates suggest that increased gray matter volume can account for approximately 10% of the variation in adult intellectual ability (Toga and Thompson, 2005). The association between cognitive function and gray matter volume or volumetric analysis of subcortical structures in preadolescent children however is largely unexplored (Luders et al., 2011; Wilke et al., 2003). In a study of children between the ages of 5 and 18 years, IQ explained about 10% of variation in gray matter volume, primarily in the cingulate cortex and exclusively in the post-adolescent subjects (Wilke et al., 2003). Increased cortical thickness across a wide network of brain areas was associated with better performance on measures of general intelligence in a sample of subjects between the ages of 6 and 18, but the effects were primarily among the adolescent group (Karama et al., 2009) and were significant but not observed to be specific for cognitive domains, such as verbal reasoning, calculation or vocabulary (Karama et al., 2011). In a multi-site study, Luders et al. (2011) reported primarily negative correlations between callosal thickness and intelligence in subjects between the ages of 6-17, however there were no significant effects specific to the group of children between 6-8 years of age. A developmental shift from a negative association between intelligence and cortical thickness in early childhood to positive correlations later has been reported (Shaw et al., 2006) suggesting that the direction of the association between intelligence and cortical thickness may change with age. In support of this possibility, Wilke et al. (2003) reported modest positive associations between deep gray matter structures and intelligence in younger children but stronger and significant associations in the cingulate and higher brain structures in groups of adolescents and young adults.
These findings indicate that structure-function relations among preadolescent children are unclear at best and that the “bigger is better” hypothesis may not pertain to all areas of the brain. One candidate brain structure that has not been examined in detail among preadolescent children is the basal ganglia. In a group of adolescent and post-adolescent subjects, increased brain volume in the orbitofrontal cortex, cingulate gyrus, cerebellum, and thalamus were significantly associated with higher IQ scores, but high density in the caudate nucleus was associated with lower levels of intellectual function (Frangou et al., 2004). Complex findings were reported between cognitive function and basal ganglia shape in a recent study of late adolescent subject (Burgaleta et al, 2013). Patterns of expansion and inward displacement of subcortical areas including the putamen and caudate nucleus, especially in the right hemisphere, were associated with intellectual abilities. However another recent study (McDonald et al, 2013) in a large sample of children collected from six laboratories reported that positive correlations between intelligence and volumes of structures of the basal ganglia and striatum were significant in the left hemisphere and only in males.
There is evidence that impaired function among clinical groups is associated with larger structures in the basal ganglia, specifically the caudate and putamen. For instance bilateral enlargement of the putamen and the left caudate nucleus was observed in a large group of 3-4 year old autistic children (Estes et al., 2011). Similarly, basal ganglia volume was enlarged in schizophrenics, a group with well documented impaired executive function (Chakos et al., 1994; Hokama et al., 1995; Staal et al., 2000). Shape deformities in the basal ganglia have been reported in patients with obsessive-compulsive disorder including an outward deformity in the superior, anterior portion of the bilateral caudate and in the inferior, lateral portion of the left putamen (Choi et al., 2007). It is an important observation that medication history does not account for these associations. Structures of basal ganglia were increased in volume disproportional to total brain volume in medication-naive children and adolescents with autism (Langren et al., 2007) and in untreated and unaffected siblings of schizophrenic patients (Mamah et al., 2008). However there is evidence that children diagnosed with attention deficit hyperactivity disorder (ADHD) exhibit reduced volume of the caudate nuclei (Castellanos, 2002), perhaps related to delayed development arising at mid-adolescence (Silk et al, 2009).
The association between performance and basal ganglia size and structure is not resolved especially related to preadolescent subjects. Many, but not all, studies suggest that increased size and outward deformity of structures in the basal ganglia, especially the caudate nucleus and putamen are linked to poor performance in healthy subjects and to clinical conditions with behavioral and cognitive impairments. These effects, with one exception (Estes et al., 2011), have been reported in post-adolescent subjects. However as reviewed above, several studies reported positive associations between performance and reduced basal ganglia size in clinical populations. The initial purpose of this study is to determine if there are areas of the brain in which increased size is associated with poor cognitive performance among typically developing young children. Significant associations will be further assessed with specific topographical measures of size and shape.
METHODS
Participants
Participants included 50 children (21 girls) who were born at one of two hospitals in the greater Los Angeles area (UC Irvine Medical Center, or Long Beach Memorial Medical Center) and were recruited from ongoing protocols of development. Children were eligible for participation if they were between 6 and 10 (mean = 90.92 mo ±12.26) years of age, right handed (determined using a modified version of the Edinburgh Handedness Inventory (Oldfield, 1971) and products of a single birth. Our typically developing sample comprised children with a stable neonatal course (Median Apgar score = 9, range 7 to 10) and without known congenital, chromosomal, or genetic anomalies (e.g., trisomy 21). Participants had no evidence in the newborn period of intraventricular hemorrhage (determined by ultrasound), periventricular leukomalacia, and/or low-pressure ventriculomegaly. Further, at the current assessment, subjects had normal neurological findings including normal ventricle size and there was no evidence of emotional, physical or neurological problems reported in a structured interview using the MacArthur Health and Behavior Questionnaire (Armstrong & Goldstein, 2003). Parents and children gave informed consent and assent respectively for all aspects of the protocol, which was approved by the Institutional Review Board for protection of human subjects. Because age at testing and sex of the child (Muftuler et al., 2011) can influence brain structure and cognitive performance, both variables were controlled in all analyses.
MRI Acquisition
Structural MRI scans were acquired on a 3-T Philips Achieva system. To minimize head motion, padding was placed around the head. Ear protection was given to all children. To further increase compliance and reduce motion, children were fitted with headphones and allowed to watch the movie of their choice while in the scanner. Following the scanner calibration and pilot scans, a high resolution T1 anatomical scan was acquired in the sagittal plane with 1mm3 isotropic voxel dimensions. An Inversion-Recovery Spoiled Gradient Recalled Acquisition (IR-SPGR) sequence with the following parameters were applied: Repetition rate (TR)= 11ms, Echo Time (TE)= 3.3ms, Inversion Time (TI)= 1100ms, Turbo Field Echo factor (TFE)= 192, Number of slices: 150, no SENSE acceleration, Flip angle=18°. Acquisition time for this protocol was seven minutes.
After the images were acquired, initial quality control was performed by visual inspection of the acquired images while the subject was in the scanner. If there were noticeable motion artifacts, the scan was repeated.
Voxel Based Morphometry (VBM) Analysis
VBM is an automated structural analysis used to detect regional morphological differences in brain images obtained by MRI. Unlike volumetric tracing methods, VBM does not depend on user-defined brain regions, but rather presents a comprehensive localization of differences in gray matter volume between subjects (Ashburner and Friston, 2000).
MRI data was processed for VBM analysis using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/) (SPM5 The Wellcome Department of Imaging Neuroscience, University College London). The structural images were bias field corrected, and segmented using an integrated generative model (Ashburner and Friston, 2005). Unified segmentation involves alternating between segmentation, bias field correction, and normalization to obtain local optimal solutions for each process. The pediatric Cincinnati Children’s Hospital Medical Center a priori templates (Wilke et al., 2002) were used to segment and spatially normalize (affine and 16 iteration non-linear transformations) the children’s images. The resulting images were modulated to correct voxel signal intensities for the amount of volume displacement during normalization. The normalized and segmented images were averaged across the children’s datasets to produce sample specific gray matter, white matter, and CSF a priori templates. The process was then repeated using the sample specific a priori templates resulting in subject specific deformation maps for VBM analysis of 1mm isotropic voxels. The normalized, segmented, and modulated images were then smoothed using a 12 mm kernel to ensure that the data were normally distributed and to limit the number of false positive findings (Salmond et al., 2002). Coordinates of the most significant voxel within each cluster were converted from original Montreal Neurological Institute (MNI) coordinates to those of the Talairach brain atlas (Talairach and Tournoux, 1988) using the mni2tal utility (Matthew Brett 1999 GPL). Anatomical locations of the significant areas were based on the best estimate from the Talairach atlas using the Talairach Daemon Client (http://www.talairach.org/client.html).
Once the preprocessing was completed, the relation between performance on the neuropsychological tests and regional gray matter volume was determined using regression with child age, sex and total gray matter volume as covariates. Consequently, the analysis detected regional differences rather than overall, large-scale variations in gray matter. The statistics for VBM analyses were normalized to Z score. Clusters were considered significant if they contained at least 100 voxels and remained significant after False Discovery Rate (FDR) correction for multiple comparisons (p<.05) as recommended by Genovese and colleagues (2002).
Analytical Strategy
Initially, VBM analysis of gray matter morphology was implemented to survey areas of the brain associated with cognitive performance. Based on the findings from the VBM, direct measures of the shapes and volumes of subcortical structures were computed in areas of interest using FSL/FIRST software tools (FMRI Software Library [FSL] http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Those measures were used to inquire associations between cognitive performance and total volume or shape differences in subcortical structures of interest.
Subcortical Volume and Shape Analysis
For subcortical segmentation and shape analysis the FSL/FIRST software (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST) was used. FIRST is a model-based segmentation/registration tool proposed by Patenaude et al. (2011). It uses manually labeled brain image data as a training set that was provided by the Center for Morphometric Analysis (CMA), MGH, Boston. This training data comprises of 15 subcortical structures that were outlined by trained operators on T1 weighted MR images from 336 subjects. It contains a wide age range (4.2 to 72 years) and both normal and pathologic brains. Therefore, the variations seen in the typically developing brain was captured by this large database.
The software for analysis uses a two-stage affine process to register each brain image onto an MNI152 template. The first stage of this registration is performed on the whole brain. In the second stage, this intermediate brain image is weighted by a subcortical mask and affine registration is applied one more time in order to achieve optimal registration of the subcortical structures. Once the registration is estimated, inverse transformation is calculated and applied to the MNI152 template to bring it to the native space so that the original image voxels do not have to be interpolated. After a general registration is achieved, Bayesian Active Appearance Model (AAM) is used to register and segment each subcortical structure precisely. AAM is an extension of Active Shape Model (ASM; Cootes et al., 1995) and it incorporates intensity information in addition to the geometrical shape model. Each subcortical structure is parameterized as a surface mesh and then modeled as a point distribution. The meshes can be reconstructed either in standard (useReconMNI option) or native space (useReconNative option). In our experience, mesh generation in native space produced slightly more accurate results. Hence that option was used for the analyses presented here. The useRigidAlign option was selected to remove pose effects but scaling was not incorporated to preserve original sizes to ensure that volumetric differences were preserved.
The developers of the FSL software applied this method to the extensive training set and eigenvectors of deformations were calculated. To segment the subcortical structures, the software searches through eigenvectors to find the most probable shape instance. The software performs the shape and appearance deformations along the eigenvectors, which represent a wide range of possible brain shapes and sizes as described above. Using a linear combination of these eigenvectors, the 3D mesh surface of a particular subcortical structure is deformed from the mean shape for the best registration onto the corresponding structure in the subject’s brain. Once this registration is done, the location of each vertex on the surface is recorded. This approach assumes that each vertex corresponds to the same anatomical location across subjects. Therefore, the mean shape of, and deviations from the mean, for each subject can be calculated and vertex-by-vertex statistical analyses can be performed to investigate various effects on the shape.
Once the brain images are processed through the FSL/FIRST pipeline, each segmentation was inspected and found satisfactory. Then, statistical analyses were first conducted to study associations between putamen volume and cognitive performance measures. Then similar analyses were conducted on a per-vertex basis using multivariate GLM to study shape differences. The results, adjusted for age, sex and total gray matter volume are shown at p<.05 FDR corrected. In addition, volumetrics for the subcortical structures were derived using the scripts included in the FIRST module.
Behavioral Measures
Cognitive ability was assessed in two ways. First, children were assessed with Performance subtests of a standardized intelligence measure (WISC IV). This assessment provides a language-free, performance based assessment of general intelligence as well as an assessment of specific aptitudes. Second, children were evaluated with a series of tasks selected to measure cognitive domains not specifically addressed in tests of general intelligence including declarative memory, motor and executive functioning. These specific domains have established relations with focused areas of the brain.
Standardized Intelligence Measures
Children were evaluated with a standardized measure, the Perceptual Reasoning Scale of the Wechsler Intelligence Scale For Children (WISC-IV). The Perceptual Reasoning Scale (PRS) measured perceptual and fluid reasoning, spatial processing, and visual motor integration and required 25-35 minutes to complete. This instrument was standardized, validated, and accepted within the developmental and pediatric community. The three subtests that comprise the PRS are Block Design, Picture Concepts, and Matrix Reasoning. These subtests were selected because they are highly correlated with general intelligence (Baron, 2004; Raven et al., 1998; Wechsler, 2002) and they are relatively culturally fair and language free (Baron, 2004).
For each subtest a raw score was calculated by summing the total number of points obtained including baseline items. The raw scores for each of the three subtests were converted to a scaled score by reference to a developmental table. Conventional scoring of the Perceptual Reasoning Scale creates a composite index by summing the scaled scores for each of the three subtests.
Block Design
Block Design measures the ability to analyze and synthesize abstract visual stimuli. It involves nonverbal concept formation, visual perception and organization, simultaneous processing, and visual-motor coordination (Cooper, 1995; Groth-Marnat, 1997; Sattler, 2001). In this task, the child viewed a constructed model or a picture in a stimulus book, and used red and white blocks to recreate the design within a specific time limit. Across successive trials the design becomes more complex. Block Design contains 14 items and the task was discontinued when the child missed three consecutive items.
Picture Concepts
This task measured abstract, categorical reasoning ability (Deák and Maratsos, 1998; Shulman et al., 1995). The child was presented with two or three rows of pictures and asked to choose one picture from each row to form a group with a common characteristic. With successive trials the common characteristic becomes increasingly abstract. Picture Concepts contains 28 items and is discontinued when five consecutive errors are made.
Matrix Reasoning
Matrix Reasoning provides a reliable measure of visual information processing and abstract reasoning (Raven et al., 1998). For each trial, the child viewed an incomplete matrix and selected the missing portion from five response options. Matrix Reasoning contained 35 items and was discontinued when the child failed to give a correct response on four of the last five items. The four types of matrices that comprised this task are: continuous and discrete pattern completion, classification, analogical reasoning, and serial reasoning.
Computerized neuropsychological tests
Established tasks were adapted for computer application to a pediatric population. Responses were recorded with specialized colored response buttons or a light pen. All task instructions were prerecorded for standardized presentation and at the start of the session children were oriented to the system with a series of exercises designed to familiarize them with all components of the system. Prior to the start of each task the child was given performance dependent practice trials to ensure they could perform the task.
Go/No-Go Task
A variant of the Continuous Performance Test (CPT), the Go/No-Go Task required the execution of an anticipated motor response or its active inhibition. A child’s inhibitory ability is a measure of impulsivity and is associated with executive functioning. Participants were primed to press a button as quickly as possible in response to the presentation of every letter of the alphabet, except for the letter “X”. In this task, there were two blocks of trials. The first block consisted of 25 trials containing 100 percent target stimuli, used to prime participants to respond to the target stimuli. The second block was the response inhibition condition, consisting of 50 trials containing 25 target stimuli (Go trials) and 25 non-target stimuli (No-Go trials). Decision speed for correct responses was the primary variable for this assessment.
Set Shifting Test (SST)
This executive function task measured the ability to first learn a simple pattern and then abandon it when it was no longer reinforced, in favor of a new pattern. Specifically, children were instructed to “guess” into which doghouse an animated dog would move. After a choice was made, the dog moved according to a preprogrammed pattern. The first pattern was “single alternation” (Left, Right, Left, Right). After the child correctly learned this pattern, as indicated by guessing the correct house 10 times in a row, the pattern changed to “double alternation” (Left, Left, Right, Right). Total percent correct was the primary score derived from this test.
Continuous Recognition Memory Test (CRMT)
The Continuous Recognition Memory Test (CRMT) employed nonverbal, visual stimuli (both concrete and abstract) to assess the ability to discriminate between previously presented (“old”) and “new” stimuli. The CRMT included pictures of common objects that could be verbally labeled (“concrete” stimuli) and shapes that were not easily labeled verbally (“abstract” stimuli). Half of the 210 trials displayed concrete and half of the trials displayed abstract stimuli and were presented for 500 msec, with 2000 msec intervening between lags. Eighty stimuli each were presented twice at either a lag of two or five items. The remaining trials comprised 50 distracter stimuli (not analyzed) that either were not repeated or were repeated at non standard intervals. Scores derived from this test for this analysis were percent correct for the abstract items.
Finger Tapping Test (FTT)
The FTT required the child to tap a button with his/her index finger as fast as possible for 10 seconds without moving his/her arm or wrist. The FTT is used to evaluate lateralized motor ability as well as the capacity to sustain motor functioning over a short time. This task provides important information because it allows the separation of pure motor speed from reaction times in the cognitive tasks. A total of 6 trials are administered (3 for each hand). Scores derived from this test were the mean number of taps for each hand.
Performance on each of the tests is presented in Table 1. Data are presented for the total sample and boys and girls separately. There were no significant differences between boys and girls on any of the measures. Despite the large number of tests administered the association was low and nonsignificant for all the tests in the computerized battery. Subtests of the WISC shared ~25% variance and between 62%-75% variance with WISC percentile.
TABLE 1. MEAN PERFORMANCE AND (STANDARD DEVIATION) FOR THE TOTAL COHORT (N=50) AND FOR GIRLS AND BOYS SEPARATELY.
| VARIABLES | TOTAL SAMPLE | GIRLS | BOYS |
|---|---|---|---|
| AGE (MONTHS) | 90.9 (12.3) | 91.2 (11.6) | 90.7 (12.9) |
| GO/NO-GO REACTION TIME | 526.3 (75.2) | 529.2 (79.8) | 524.2 (73.1) |
| SINGLE ALTERNATION | 11.98 (2.8) | 12.2 (3.3) | 11.8 (2.5) |
| DOUBLE ALTERNATION | 26.2 (14.1) | 23.8 (12.4) | 28.1 (15.2) |
| TAPPING DOMINANT HAND | 34.1 (6.4) | 32.7 (5.4) | 35.2 (7.0) |
| TAPPING NON-DOMINANT HAND | 30.2 (5.2) | 28.8 (3.9) | 31.2 (5.8) |
| CONTINUOUS MEMORY OLD ABSTRACT | 64.9 (39.5) | 58.5 (53.7) | 70.1 (22.3) |
| CONTINUOUS MEMORY OLD CONCRETE | 69.0 (39.9) | 64.1 (55.9) | 72.9 (19.8) |
| WISC PERCEPTUAL REASONING INDEX | 107.8 (16.1) | 108.1 (9.8) | 107.7 (19.6) |
| WISC PERCEPTUAL REASONING PERCENTILE | 63.8 (29.6) | 67.6 (21.3) | 61.0 (34.5) |
| WISC BLOCK DESIGN | 10.5 (2.8) | 10.7 (2.2) | 10.3 (3.2) |
| WISC PICTURE COMPLETION | 11.8 (3.1) | 12.2 (2.8) | 11.5 (3.4) |
| WISC MATRIX REASONING | 11.5 (3.5) | 11.0 (2.8) | 11.9 (3.9) |
RESULTS
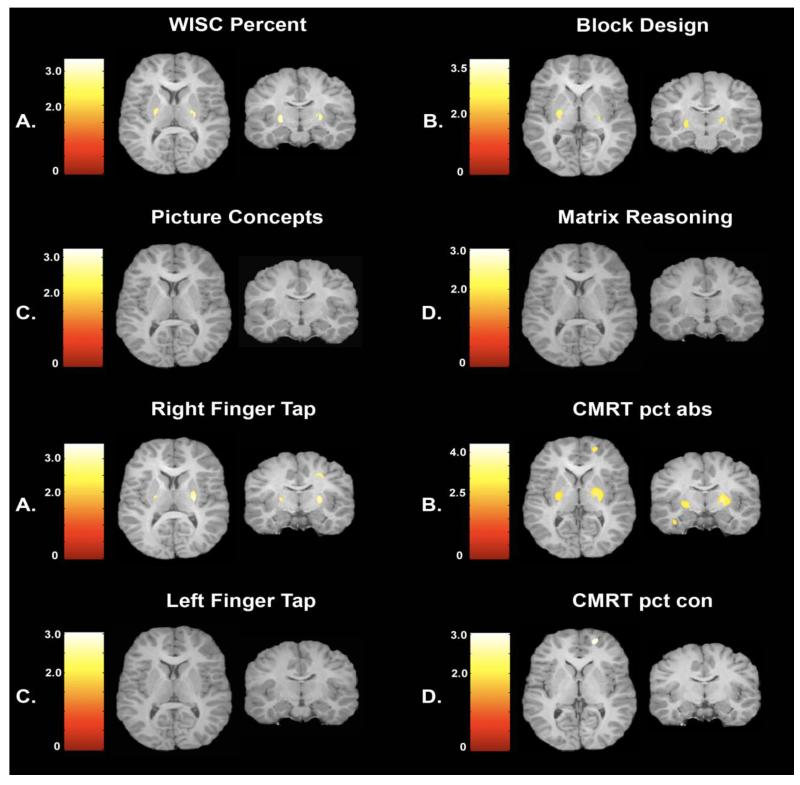
VBM analysis of gray matter morphology initially was implemented to address the central question of whether specific areas of increased gray matter volume were associated with impaired cognitive performance in typically developing preadolescent children. Regression analyses indicated that only areas of increased gray matter volume in the basal ganglia and specifically the putamen were significantly associated with impaired performance. Significant associations illustrated in Figure 1, indicate that lower scores on the WISC measure of intelligence (p< 0.05) and poorer scores on the Block Design (p< 0.05) from the WISC (which contributed significantly to the WISC score) were associated with larger gray matter volumes in the putamen. Slower response speed measured from the dominant hand (p<0.05) and poorer performance on the abstract (difficult) stimuli in the test of declarative memory (CRMT; p< 0.05) also were significantly associated with increased gray matter of the putamen. No significant negative associations were observed between gray matter volume and performance on Picture Concepts, Matrix Reasoning, non-dominant Finger Tap and the concrete (easy) trials of the CMRT.
Figure 1.
VBM analysis illustrating areas of the basal ganglia, primarily the putamen in which greater gray matter volume is associated with impaired performance. Significant relations are observed for WISC score (PRI), Block design, right finger tapping and CMRT percentage correct for abstract stimuli.
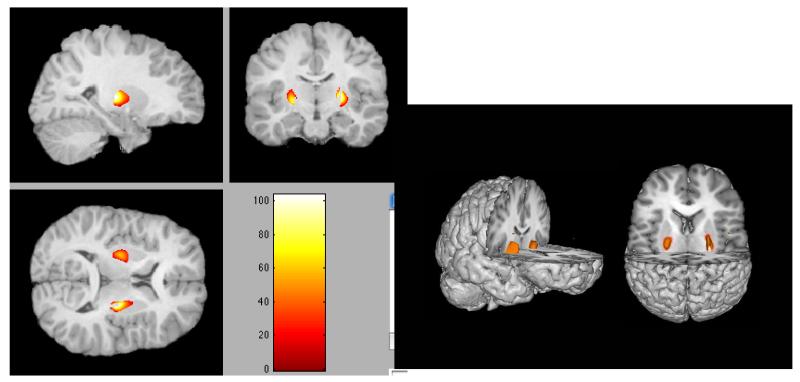
The statistical maps that indicated brain areas with higher gray matter volume associated with impaired cognitive performance on the four tasks listed above were loaded into Xjview with a p<.05 uncorrected threshold. This analysis detected areas of the brain in which there was overlapping significance (e.g., Haier et al., 2008). The only area to survive this analysis was the putamen. The finding was associated with a low p value and unlikely due to chance. If the performance measures had been completely independent (there was a positive relation between two of the tests) the probability of this occurrence would have been close to p< .054. Figure 2 illustrates that poor performance across all tests was associated with increased volume only in the basal ganglia and primarily the putamen.
Figure 2.
Common areas of the basal ganglia, primarily the putamen in which increased volume is associated with impaired cognitive performance.
Based on the findings from the VBM analysis, we expected that volumes in the putamen/basal ganglia would be largest in poor performing children. However, one criticism of imaging procedures including VBM is that errors of misregistration can result from abnormal anatomy and/or systematic shape differences in structures (Mechelli et al., 2005). We directly assessed with geometric algorithms, whether volume of the putamen was associated with cognitive performance. There were no significant correlations between any of the tests and total volume or between performance and the volume of any basal ganglia structures (p’s all > .10).
The inconsistent findings between VBM and volumetric analysis suggested a different approach. Examination of Figure 1 indicated that the increased gray matter volume in the putamen associated with poor performance was topographically distributed. About 30% of the putamen volume was associated with performance, localized primarily in the dorsal medial area. The possibility that the VBM effects were localized and related to the shape or regional deformity of the putamen was assessed with FSL/FIRST and GLM modeling.
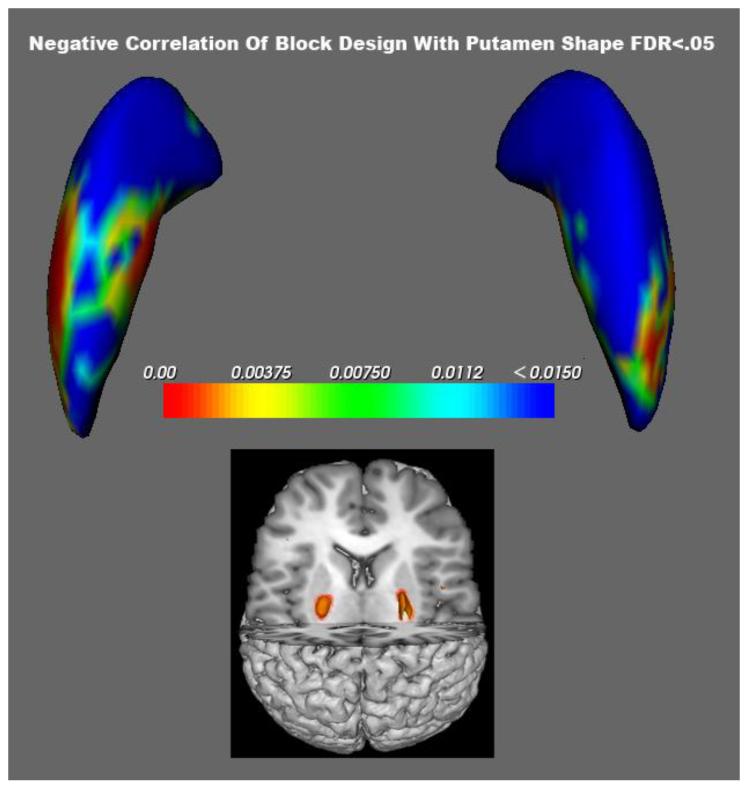
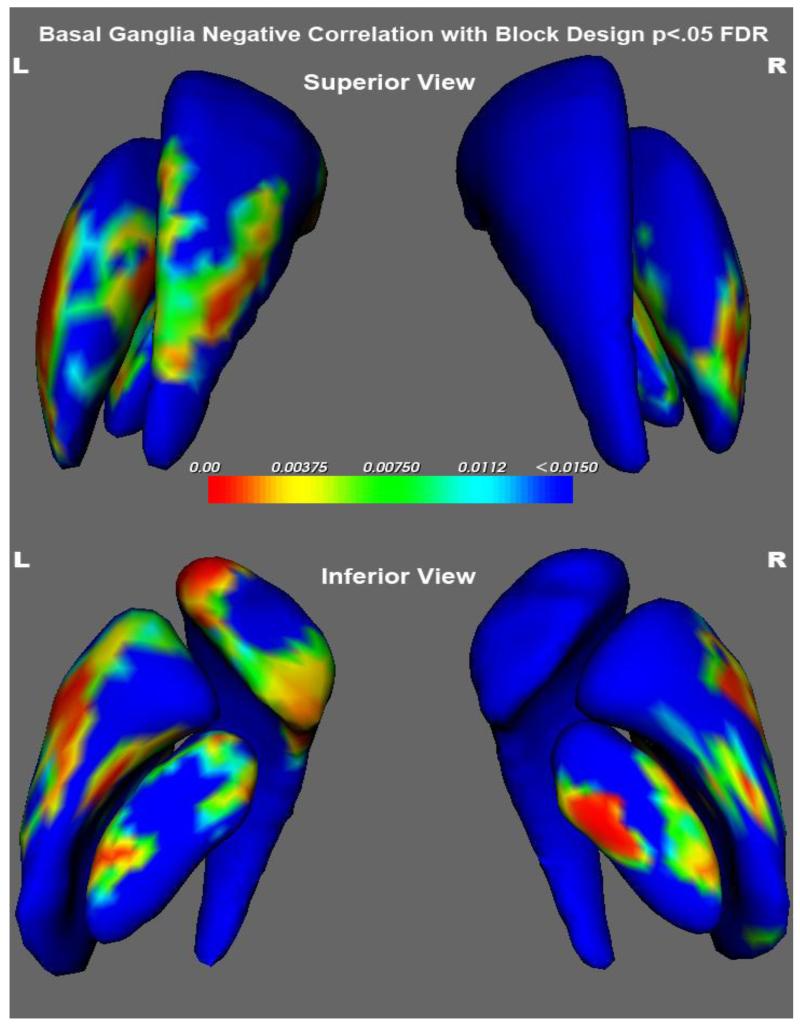
Bilateral shape of the putamen was significantly associated (GLM, all FDR corrected) with performance of the Block Design, Matrix Reasoning and the Perceptual Index of the WISC. As presented in Table 2, a remarkable percentage of the putamen was expanded in association with poor test performance. Figure 3 shows the relation between the VBM volume findings (insert) and the results of the shape analysis for Block Design. Impaired performance of the Block Design subtest was associated with greater volume and expanded shape of the putamen. The figure clearly shows that poor cognitive performance was associated with greater expansion of the left putamen (50.6%) than the right (26%) structure. Figure 4 shows that in addition to the putamen, areas of the globus pallidus and caudate nucleus also were significantly expanded in association with poorer performance on the block design subtest (Table 2).
TABLE 2. OUTWARD DEFORMATION OF THE PUTAMEN ASSOCIATED WITH IMPAIRED COGNITIVE PERFORMANCE.
| BLOCK DESIGN | F RATIO | DF | P VALUE | % OF STRUCTURE DEFORMED |
|---|---|---|---|---|
| L-Putamen | 3.415 | (3, 45) | 0.025 | 50.623 |
| R-Putamen | 4.032 | (3, 45) | 0.013 | 26.012 |
| MATRIX REASONSING | ||||
| L-Putamen | 3.031 | (3,45) | 0.039 | 77.882 |
| R-Putamen | 3.305 | (3,45) | 0.029 | 57.788 |
| PERCEPTUAL INDEX | ||||
| L-Putamen | 3.063 | (3,45) | 0.038 | 78.972 |
| R-Putamen | 3.251 | (3,45) | 0.030 | 62.305 |
| OUTWARD DEFORMATION OF GLOBUS PALLIDUS AND CAUDATE NUCLEUS ASSOCIATED WITH IMPAIRED PERFORMANCE ON THE BLOCK DESIGN SUBTEST | ||||
| L-Globus Pallidus | 3.659 | (3, 45) | 0.019 | 38.629 |
| R-Globus Pallidus | 3.322 | (3, 45) | 0.028 | 57.321 |
| L-Caudate | 3.827 | (3, 45) | 0.015 | 32.474 |
| R-Caudate | Not significant | |||
Figure 3.
Areas from green to blue survive FDR p<.05 correction and show where larger areas of the putamen correlate negatively with performance on Block Design. Comparison with VBM findings in the insert.
Figure 4.
Top panel is a superior view and the bottom panel an inferior view of the basal ganglia. The structures are colored coded to illustrate significance (p values are coded with hot colors reflecting higest levels of significance). Significance indicates that areas of expansion are associated with poorer cognitive performance.
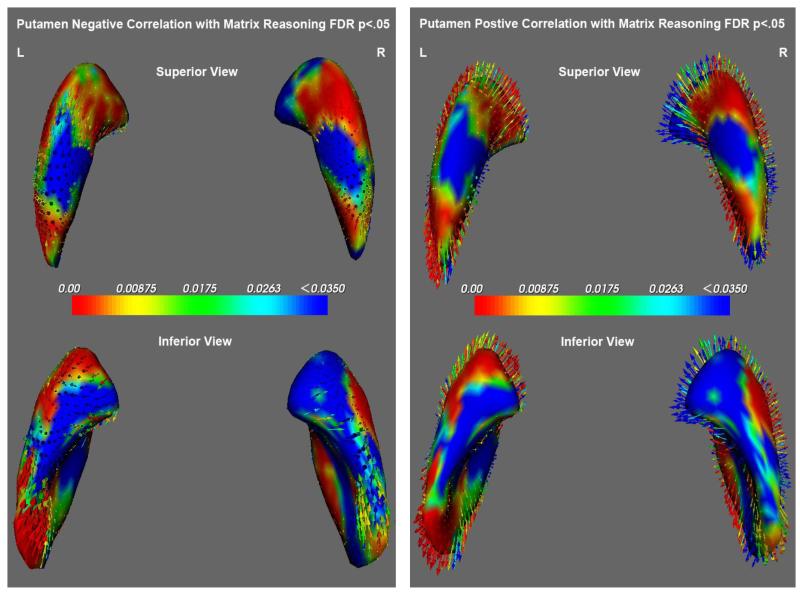
There were no significant associations between measures of gray matter volume or volume and the Matrix Reasoning subtest but complex and significant relations were discovered with analysis of shape. As seen in figure 5, topographically discrete areas of expansion and compression of the putamen were associated with performance on the Matrix reasoning. Significant (F3,45=3.03, p=.04 [Left], F3,45=3.31, p=.03 [Right]) expansion of the head and tail and compression of the dorsal and ventral areas of the putamen were associated with good performance.
Figure 5.
Illustrates the negative relations (compression-inward arrows) and positive relations (expansion-outward arrows) of the putamen with performance on the Matrix Reasoning subtest of the WISC.
None of the computerized performance measures were associated with shape of the basal ganglia.
DISCUSSION
The principal purpose of this study was to determine if there are areas in the preadolescent brain in which bigger is not always better. The general consensus is that increased gray matter in a large range of brain areas is associated with better cognitive performance, but this conclusion has been restricted largely to post-adolescent subjects (Karama et al., 2009; Luders et al., 2011; Wilke et al., 2003). No studies have examined brain-behavior associations in an exclusive, typically developing preadolescent subject population and the few studies that have included children among their samples have not observed significant associations between brain size and performance in the younger subgroups (Karama et al., 2009, 2011; Luders et al., 2011; Wilke et al., 2003). The evidence from the present study, utilizing three different methods of analysis, indicate that larger or outward surface deformation of the areas of the basal ganglia and primarily expansion of the medial dorsal putamen is associated with impaired cognitive function in typically developing young children.
The association between size and shape of the basal ganglia and cognitive performance may be surprising because it is widely accepted that the basal ganglia are responsible for sensorimotor coordination, including response selection and initiation (Grahn et al., 2009) and are centers of integration for the execution of habitual or automatic movements (Herrero et al., 2002). Dysfunction of basal ganglia is associated with neurological disorders (Romero et al., 2008) especially those with profound motor impairment including Parkinson’s and Huntington’s disease (HD) (Albin et al., 1995; DeLong, 1990; DeLong and Wichmann, 2007; Graybiel et al., 2000; Wichmann and DeLong, 2011). However, consistent with our findings of cognitive but not motor associations with structures of the basal ganglia, critical motivational, motor planning, procedural learning, associative and cognitive functions independent from motor skill or function have been reported for this brain area (Alexander et al., 1986; Brown et al., 1999; Ducharme et al., 2011; Knowlton et al., 1996; Nakano, 2000; Nicola, 2007; Rolls, 1994; Salinas et al., 2000; Yin and Knowlton, 2006). Moreover, electrophysiological studies have demonstrated that cells of the putamen and caudate nucleus are active when primates perform an associative learning task that does not require a motor response (Romero et al., 2008).
Through complex cortical connections with prefrontal association cortex and limbic cortex, the basal ganglia are thought to play a role in cognitive function that is similar to their role in motor control. That is, the basal ganglia are involved in selecting and enabling various cognitive, executive, or emotional programs that are stored in cortical areas. The putamen and caudate nucleus are the main recipients of afferents arising from the entire cerebral cortex and from the intralaminar nuclei of the thalamus (Kreitzer and Malenka, 2008). The projections from the cortex to basal ganglia are topographic. For instance the frontal lobe projects predominantly to the caudate head and the putamen. Specifically, the most dorsal aspect of the lateral prefrontal cortex (DLPC) projects to the dorsal and central part of the caudate nucleus, and the ventral DLPC projects to the ventral and central caudate nucleus. The medial PFC preferentially projects to the medial caudate nucleus, nucleus accumbens and ventral putamen and the orbital PFC projects to central and lateral parts of the caudate nucleus and to the ventromedial putamen (Tanji and Hoshi, 2008). Thus the basal ganglia may modulate a network of cortical and thalamic projections and pathways that contribute to the significant associations with behavior reported here.
The association between performance on the matrix reasoning test and basal ganglia deformation was especially interesting. Of the tests administered to the children, it was the only one in which both compression and expansion of the putamen and caudate nucleus were bilaterally and topographically related to improved performance. The finding that both expansion and compression were related to this test may explain why associations with performance were not observed with measures of size or volume. The matrix reasoning test based on the Raven’s Progressive Matrices (Raven, 1976), is a widely accepted test of non-verbal fluid reasoning and intelligence that examines cognitive flexibility to infer rules and perform high-level abstractions. The neural substrates for these abilities include a frontoparietal network (Soulières et al., 2009), and as suggested here, structures of the basal ganglia. It is possible that performance on the Matrix Reasoning test in part reflects the integrity of topograhic frontal connections with the caudate and putamen.
The findings with Matrix Reasoning and the results in general invites further discussion about the differences in the methods employed in this study to examine structure-functions relations. There was disagreement among the approaches regarding the associations between various structures and performance. With VBM, larger areas of the basal ganglia and especially the putamen, were associated with poorer motor performance with the dominant hand, visual memory, block design and a general index of intellectual ability. All of these associations disappeared and no new ones were generated when a specific, geometric analysis of volume was applied. Thus, with VBM, the conclusion would be that a larger putamen was associated with impairment in several specific abilities and with general intelligence. When differences in total volume of subcortical structures with respect to performance were investigated, the conclusion would be that there are no associations between the putamen/basal ganglia and performance. Even though there are problems of misregistration with VBM because surface deformities can masquerade as variation in volume (Mechelli et al., 2005) in this case VBM alerted us to brain regions of interest to examine structure shape.
There may be several reasons that structure-function relations have not been observed among preadolescent subjects. It has been suggested that the absence of relations between measured behavioral performance and gray matter volume in preadolescent children is related to their “pre-pruning” brain inefficiency (Wilke et al., 2003). Adolescence has been associated with a period of rapid brain maturation during which there are significant reductions (pruning) of synaptic connections resulting in greater mental efficiency (Gogtay et al., 2004) and therefore much less “noise.” This argument suggests that the “pre-pruned” preadolescent nervous system is inefficient and highly variable. The variability, both within and between subjects obscures the ability to detect reliable associations between brain areas and function. Recent findings from our studies however, indicate that cortical thinning does occur bilaterally within a narrow age range of preadolescent children (Muftuler et al., 2011) and although puberty may be a primary trigger for brain maturation, it probably is a more gradual process and may not completely explain the absence of findings. A second, obvious possibility is that the basal ganglia simply have been overlooked in most previous studies of typically developing preadolescent children.
One conclusion from these findings is that shape and not size or volume of basal ganglia may be a more sensitive measure of the association with performance. If differences in a subcortical structure across subjects are focal and not distributed throughout the whole volume, then a measure of total volume might not be sensitive enough to detect those differences. On the other hand, a vertex-by-vertex surface analysis should capture those localized differences. Moreover, measures of shape, as opposed to measures of volume, provide the opportunity to assess associations between behavioral performance and distinctive topographic regions of the structures.
In summary, the findings from our study indicated that typically developing children with generally expanded shape of the putamen and caudate nucleus perform more poorly on tests of cognitive function. Deformity of the basal ganglia may be a neurophenotype that is associated with risk for cognitive impairment and perhaps syndromes that include impaired thinking and reasoning. This possibility is consistent with recent studies that reported deformity of structures in the basal ganglia was linked to developmental disorders such as autism and Attention Deficit Disorder (Estes et al., 2011; Mamah et al., 2008; Qiu et al., 2009, 2010) and other psychiatric conditions in adults that are associated with impaired cognitive function (Choi et al., 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Armstrong JM, Goldstein LH. Manual for the MacArthur Health and Behavior Questionnaire (HBQ 1.0) MacArthur Foundation Research Network on Psychopathology and Development, University of Pittsburgh; Pittsburgh, PA: 2003. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. doi: http://dx.doi.org/10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. doi: http://dx.doi.org/10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baron IS. Neuropsychological evaluation of the child. Oxford University Press; New York, NY: 2004. [Google Scholar]
- Burgaleta M, MacDonald PA, Martinez K, Roman FJ, Alvarez-Linera J, Gonzalez AR, Karama S, Colom R. Subcortical regional morphology correlates with fluid and spatial intelligence. Human Brain Mapping. 2013 doi: 10.1002/hbm.22305. DOI 10.1002/hbm.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bullock D, Grossberg S. How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J Neurosci. 1999;19:10502–10511. doi: 10.1523/JNEUROSCI.19-23-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX. Anatomic magnetic resonance imaging studies of attention-defict/hyperacitivty disorder. Dialogues in Clin Neurosci. 2002;4:444–448. doi: 10.31887/DCNS.2002.4.4/fxcastellanos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim SH, Yoo SY, Kang DH, Kim CW, Lee JM, Kwon JS. Shape deformity of the corpus striatum in obsessive-compulsive disorder. Psychiatry Res. 2007;155:257–264. doi: 10.1016/j.pscychresns.2007.02.004. doi: 10.1016/j.pscychresns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Cooper S. The clinical use and interpretation of the Wechsler intelligence scale for children-revised. C.C. Thomas; Springfield, IL: 1995. [Google Scholar]
- Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models: Their training and application. Comput Vis Image Underst. 1995;61:38–59. doi: http://dx.doi.org/10.1006/cviu.1995.1004. [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Deák GO, Maratsos M. On having complex representations of things: preschoolers use multiple words for objects and people. Dev Psychol. 1998;34:224–240. doi: 10.1037//0012-1649.34.2.224. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, Karama S. Right anterior cingulate cortical thickness and bilateral striatal volume correlate with Child Behavior Checklist aggressive behavior scores in healthy children. Biol Psychiatry. 2011;70:283–290. doi: 10.1016/j.biopsych.2011.03.015. doi: http://dx.doi.org/10.1016/j.biopsych.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G, Dager SR. Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res. 2011;4:212–220. doi: 10.1002/aur.193. doi: 10.1002/aur.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SC. Mapping IQ and gray matter density in healthy young people. Neuroimage. 2004;23:800–805. doi: 10.1016/j.neuroimage.2004.05.027. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. doi: http://dx.doi.org/10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: Neuropsychological studies. Behav Brain Res. 2009;199:53–60. doi: 10.1016/j.bbr.2008.11.020. doi: http://dx.doi.org/10.1016/j.bbr.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: A new hypothesis. Trends Neurosci. 2000;23:S71–77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of psychological assessment. 3rd ed. J. Wiley; New York, NY: 1997. [Google Scholar]
- Haier RJ, Head K, Head E, Lott IT. Neuroimaging of individuals with Down’s syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage. 2008;39:1324–1332. doi: 10.1016/j.neuroimage.2007.09.064. doi: 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro J. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, McCarley RW. Caudate, putamen, and globus pallidus volume in schizophrenia: A quantitative MRI study. Psychiatry Res. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab’bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. doi: http://dx.doi.org/10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Evans AC. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. NeuroImage. 2011;55:1443–1453. doi: 10.1016/j.neuroimage.2011.01.016. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62:262–266. doi: 10.1016/j.biopsych.2006.09.040. doi: http://dx.doi.org/10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Narr KL, Zamanyan A, Chou YY, Gutman B, Toga AW. The link between callosal thickness and intelligence in healthy children and adolescents. Neuroimage. 2011;54:1823–1830. doi: 10.1016/j.neuroimage.2010.09.083. doi: 10.1016/j.neuroimage.2010.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PA, Ganjavi H, Collins DL, Evans AC, Karama S. Investigating the relation between striatal volume and IQ. Brain Imaging and Behavior. 2013 doi: 10.1007/s11682-013-9242-3. online, DOI 10.1007/s11682-013-9242-3. [DOI] [PubMed] [Google Scholar]
- Mamah D, Harms MP, Wang L, Barch D, Thompson P, Kim J, Csernansky JG. Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biol Psychiatry. 2008;64:111–120. doi: 10.1016/j.biopsych.2008.01.004. doi: http://dx.doi.org/10.1016/j.biopsych.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev. 2005;1:105–113. [Google Scholar]
- Muftuler LT, Davis EP, Buss C, Head K, Hasso AN, Sandman CA. Cortical and subcortical changes in typically developing preadolescent children. Brain Res. 2011;1399:15–24. doi: 10.1016/j.brainres.2011.05.018. doi: http://dx.doi.org/10.1016/j.brainres.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K. Neural circuits and topographic organization of the basal ganglia and related regions. Brain Dev. 2000;22:S5–16. doi: 10.1016/s0387-7604(00)00139-x. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH. Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2010;49:539–551. 551.e531–534. doi: 10.1016/j.jaac.2010.02.012. doi: 10.1016/j.jaac.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, Mostofsky SH. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Raven Progressive Matrices. The Psychological Corporation; Toronto, CA: 1976. [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven’s progressive matrices and vocabulary scales. Oxford Psychologists Press; Oxford, UK: 1998. [Google Scholar]
- Rolls ET. Neurophysiology and cognitive functions of the striatum. Rev Neurol (Paris) 1994;150:648–660. [PubMed] [Google Scholar]
- Romero MC, Bermudez MA, Vicente AF, Perez R, Gonzalez F. Activity of neurons in the caudate and putamen during a visuomotor task. Neuroreport. 2008;19:1141–1145. doi: 10.1097/WNR.0b013e328307c3fc. [DOI] [PubMed] [Google Scholar]
- Salinas E, Opris I, Zainos A, Hernandez A, Romo R. Motor and non-motor roles of the cortico-basal ganglia circuitry. In: Miller R, Wickens J, editors. Brain dynamics and the striatal complex: Conceptual advances in brain research. Harwood Academic; Amsterdam, Netherlands: 2000. pp. 237–256. [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston KJ. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: cognitive applications. 4th ed. J.M. Sattler; San Diego, CA: 2001. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shulman C, Yirmiya N, Greenbaum CW. From categorization to classification: A comparison among individuals with autism, mental retardation, and normal development. J Abnorm Psychol. 1995;104:601–609. doi: 10.1037//0021-843x.104.4.601. [DOI] [PubMed] [Google Scholar]
- Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. Structural development of the basal ganglia in attention deficit hyperactivity disorder: A diffusion tensor imaging study. Psychia Res: Neuroimage. 2009;172:220–225. doi: 10.1016/j.pscychresns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Soulières I, Dawson M, Samson F, Barbeau EB, Sahyoun CP, Strangman GE, Mottron L. Enhanced visual processing contributes to matrix reasoning in autism. Hum Brain Mapp. 2009;30:4082–4107. doi: 10.1002/hbm.20831. doi: 10.1002/hbm.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. Thieme; New York, NY: 1988. [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annu Rev Neurosci. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III) Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Wichmann T, Delong MR. Deep-brain stimulation for basal ganglia disorders. Basal Ganglia. 2011;1:65–77. doi: 10.1016/j.baga.2011.05.001. doi: 10.1016/j.baga.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Sohn JH, Byars AW, Holland SK. Bright spots: Correlations of gray matter volume with IQ in a normal pediatric population. NeuroImage. 2003;20:202–215. doi: 10.1016/s1053-8119(03)00199-x. doi: http://dx.doi.org/10.1016/S1053-8119(03)00199-X. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]