Abstract
Background
The current study was designed to determine the effect of short-term moderate intensity exercise training (MEX) on arterial stiffness in patients with chronic kidney disease (CKD) stage 3.
Study Design
The study was a randomized controlled trial with a parallel group design.
Setting & Participants
Testing and training sessions were performed at Springfield College. Forty-six (treatment group, n=25; control group, n=21) CKD patients with diabetes and or hypertension completed the study.
Intervention
The aerobic training program consisted of 16 weeks of supervised exercise training at 50%–60% peak oxygen uptake (VO2peak), 3 times per week, while the control group remained sedentary. Identical testing procedures were performed following the 16-week intervention.
Outcomes
The primary outcome was arterial stiffness. Secondary outcomes were aerobic capacity, various blood parameters (endothelin 1 [ET-1], nitrate/nitrite, high-sensitivity C- reactive protein), and health-related quality of life (HRQoL).
Measurements
Arterial stiffness was assessed with aortic pulse wave velocity (aPWV), aerobic capacity by VO2peak, blood parameters by ELISAs, and HRQoL by SF-36. Subjects attended four sessions before being randomized to either the treatment or control groups. Subjects gave consent during the first session while a graded exercise test, with the measurement of VO2peak, was completed during the second session. During sessions three and four, aPWV was measured at rest prior to 40-min of either MEX or seated rest. A venous blood sample was taken prior to exercise or rest and participants completed the SF-36 questionnaire.
Results
Sixteen weeks of training led to an 8.2% increase in VO2peak for the treatment group (p =0.05) but no changes in aPWV.
Limitations
Randomization was not concealed and was violated on one occasion. Furthermore, the use of an indirect measurement of endothelial function and the short duration of the intervention are both limitations.
Conclusions
Short-term MEX does not alter arterial stiffness in CKD patients but it seems to reduce endothelin 1 levels.
Index words: Arterial stiffness, carotid-femoral arterial stiffness, pulse wave velocity, vasoactive balance, vascular function, short-term, aerobic training, endothelium, chronic kidney disease (CKD)
Cardiovascular disease (CVD) is the main cause of death in people with chronic kidney disease (CKD) 1,2. The traditional risk factors for the development of CVD in the general population do not completely explain the higher than normal prevalence of CVD in CKD3. The stiffness of central arteries has emerged as a powerful independent risk factor for CVD in persons with CKD2,4,5.
Elevated arterial stiffness is believed to be related to structural changes that occur within the media of the arterial wall caused by abnormal calcification6. The relative levels of key vasoactive substances is believed to affect vascular function and contribute to CVD. Endothelin 1 (ET-1) is a vasoconstrictor, the levels of which are elevated in some diseased populations7. In contrast, nitric oxide (NO) is a vasodilator, the levels of which are reduced in CKD8. Exercise training favorably alters the levels of these peptides and other vasoactive substances 9,10,11.
Sixteen weeks of aerobic training reduced central aortic pulse wave velocity (aPWV) in a sample of normotensive middle-aged sedentary men12. Twelve weeks of endurance training in hemodialysis patients decreased the central augmentation index (AIx)—a secondary measure of arterial stiffness 13. A year-long aerobic training program reduced AIx in a small sample of CKD patients14. Whether or not short-term exercise training, of the type utilized in rehabilitation programs, would improve aPWV in non–dialysis-dependent CKD patients and presumably reduce their risk of a future cardiac event has not been well studied. Therefore, the primary purpose of the present study was to ascertain the effect of short-term moderate-intensity aerobic exercise on aPWV in patients with CKD stage 3 who had diabetes (DM) and or hypertension (HTN) as the primary cause of their CKD. Secondary purposes of the study were to determine the impact of the exercise program on biomarkers that are known to affect vascular function and health-related quality of life (HRQoL) of our study sample. We hypothesized that the 16-week training program would lead to a reduction in aPWV in patients with CKD stage 3 and this reduction would be associated with favorable changes in the selected biomarkers and some components of HRQOL.
METHODS
Trial Design and Participants
The current study was a randomized clinical trial with a parallel group design. Patients with kidney disease were included if they were between the ages of 35–70 years, had an estimated glomerular filtration rate (GFR; calculated by the MDRD (Modification of Diet in Renal Disease) Study equation) of 30–59 ml/min/1.73 m2 and had either DM or HTN as the primary cause of their CKD. Potential participants were identified from the database of patients at a private nephrology practice. Individuals were excluded if they were smokers, if they were currently (i.e., within the previous 3-months) involved in a structured exercise program, if they had atrial fibrillation, or if they had any absolute contraindication to exercise as defined by the American College of Sports Medicine 15. Any patient with a prior cardiovascular diagnosis was cleared by their cardiologist before they were permitted to participate. All testing and training sessions occurred at Springfield College.
Exercise Intervention
At the end of the fourth session, participants were randomly assigned to either the treatment or the control group. Individuals who were assigned to treatment were prescribed 16 weeks of aerobic training, 3 times per week, at Springfield College. Most participants started with 15–30 minutes of continuous aerobic exercise utilizing a variety of apparati. They gradually progressed toward a goal of 55 minutes, composed of 5-minutes of warm-up, 45-minutes of conditioning, and 5-minutes of cool-down. Participants worked at 50%–60% of the VO2peak achieved during the graded exercise test (GXT). Heart rates (HR) and rate of perceived exertion were monitored to make sure that participants stayed within their prescribed intensity ranges. Individuals assigned to the treatment group were allowed to make up missed sessions within a two-week period. Participants randomized to the control group were told to follow the instructions of their nephrologist or primary care physician but not to start a structured exercise program.
Outcomes
The primary outcome for this study was aPWV, assessed by applanation tonometry using a SphygmoCor device (AtCor Medical, Sydney, Australia). aPWV was assessed at baseline during sessions three and four prior to randomization and in an identical manner following 16 weeks of training during sessions seven and eight. For statistical purposes, baseline aPWV was calculated as the mean of assessments taken before the 40 minute exercise bout and before the equivalent control period during sessions three or four. At week 16, (from sessions seven and eight) similar averages for aPWV were used. The AIx at a heart rate of 75 bpm (AIx at 75 bpm) was calculated in a similar way to aPWV for both baseline pre-intervention and following the 16 week intervention period.
Secondary outcome measures were blood parameters used to assess vascular status and systemic inflammation: endothelin 1 (ET-1, Biomedica, GmbH, Austria), nitrate/nitrite (NOx, R&D Systems, Minneapolis, MN), and high-sensitivity C-reactive protein (hsCRP, MP Biomedicals, Orangeburg, NY). Fasting blood samples were taken at rest during baseline sessions three and four prior to the intervention and at rest, post intervention during sessions seven and eight. All blood parameters were processed and then stored in a −80°C freezer and later analyzed using commercial ELISA kits. The coefficient of variation for these assays were all <10%. The HRQOL was assessed using the 36-Item Short Form Health Survey (SF-36)16.
Testing Sessions
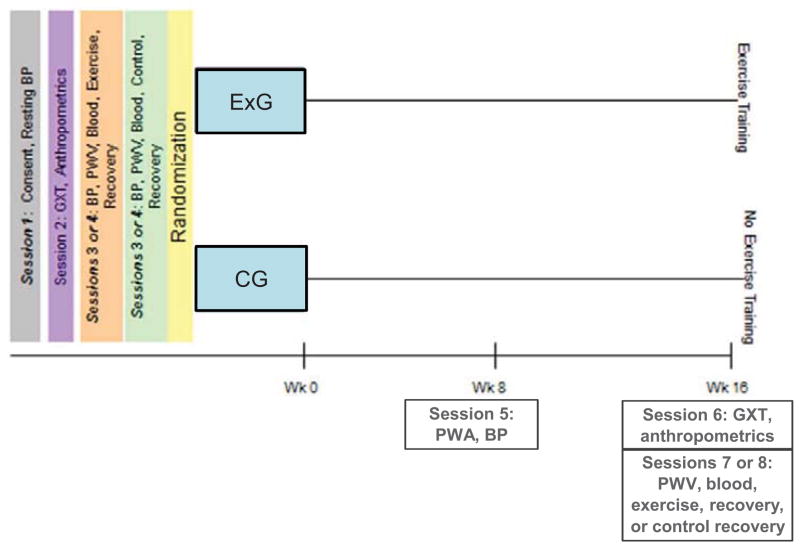
Each participant attended four sessions before being randomized to either the treatment or control groups. During the first session, participants read and signed a consent form that had been approved by the institutional review board of Springfield College. Resting blood pressures (systolic and diastolic) were taken in duplicate after a 5-minute rest period, using an automated sphygmomanometer (Tango; Sun Tech Medical, Morrisville, NC). A third reading was taken if the two readings differed by more than 6 mmHg. During the second session, anthropometric variables were assessed using standard procedures.17. Participants were administered a GXT using a computerized metabolic cart (MAX-II, Physio-Dyne, Quogue, NY). The Modified Bruce protocol was used during this test. The GXT was used to determine peak oxygen uptake (VO2peak) and to develop an appropriate exercise prescription. Approximately one week later, participants returned to the laboratory for their third session. The third and fourth baseline testing sessions were performed in random order and were separated by at least 72 hours. These sessions were identical with the exception that during one visit participants exercised for 40 minutes, while during the control session they sat quietly for an equal period of time. Sessions three and four were performed at the same time of day for each participant (in the morning) and environmental conditions were consistent between research sessions.
Participants came to sessions three and four (considered baseline sessions) following an overnight fast including abstinence from caffeine for at least 8 hours and alcohol for 10 hours18. Participants also collected their urine for a full 24-hour period prior to these sessions. Throughout the study, participants took their medications as prescribed. Participants completed 3-day diet logs prior to attending these baseline sessions and prior to sessions seven and eight following the 16-week training program. They were instructed to replicate their food intake each time they came to the laboratory for these testing sessions.
On testing days, when participants arrived at the laboratory, they were prepped and asked to lie supine for at least 10 minutes in accordance with the recommendations for the assessment of arterial stiffness.18 Then AIx at 75 bpm and aPWV measures were taken in duplicate using applanation tonometry. Values that did not meet the quality control guidelines, as defined by the manufacturer, were rejected and the reading was repeated. Throughout the study, standardized procedures were followed by all technicians19.
Following the assessment of baseline aPWV, a blood sample was taken via venipuncture and processed. Samples were stored at −80°C until analyses were performed. After giving the blood sample, participants warmed up for 5 minutes and walked for 30 minutes on a treadmill at 50%–60% of their VO2peak. Participants then completed a 5-minute cool-down. They completed the SF-36 during the control session prior to the 16-week intervention (i.e., baseline) and during session eight after the intervention (post-training).
Follow-up Testing
Participants were retested at week 8 for some variables (weight, systolic and diastolic blood pressures, and Aix at 75 bpm) and for all variables after 16 weeks, using identical procedures as at baseline. Session six was a repeat of the GXT while sessions seven and eight were a repeat of sessions three and four. Testing occurred at least 48 hours following the last training session for those assigned to the treatment group (See Figure 1).
Figure 1.
Overview of Study
Sample Size
A power analysis (G*power version 3.03) was performed using PWV data from a published study20. The following PWV data were used to compute expected mean differences in this variable: before training, 9.37±3.4 (standard error of the mean) m/s; after training, 8.71 ± 3.2 m/s. Using these data, and assuming a correlation of 0.8 between pre-intervention and post-intervention measures, an effect size of 0.76 was computed. With power set at 0.80, and the α level at 0.05, approximately 16 subjects were expected to be needed to detect a significant change in the treatment group. The addition of an equivalent control group brought the total subjects required to 32 (i.e., 16 per group) to detect a significant difference in PWV. We added additional subjects to this number to account for anticipated dropouts.
Randomization
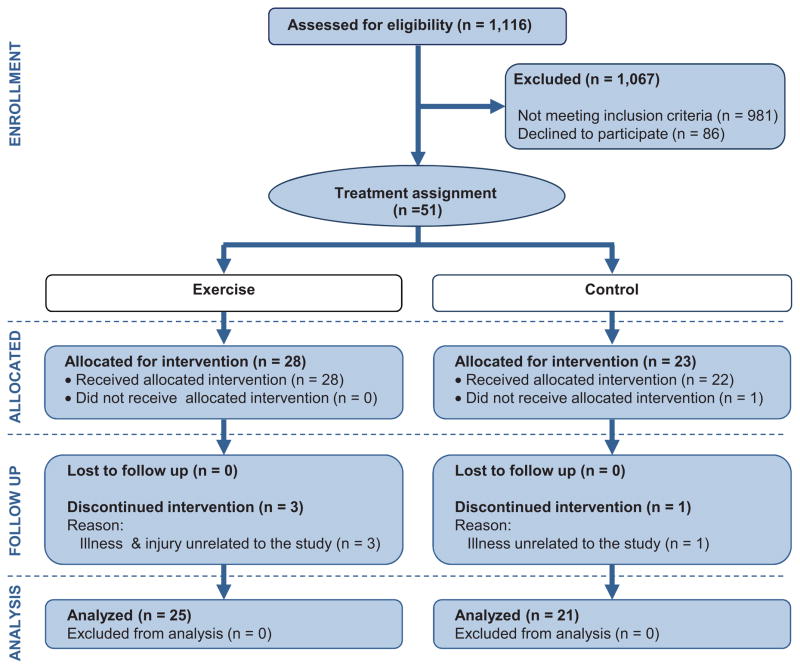
An on-line random generator (www.randomization.com) was used to determine the allocation of 50 participants into 25 blocks. Participants were made aware of their assignment at the completion of session 4. The principal investigator (S.H.) was responsible for obtaining the randomization sequence and he, along with the Research Coordinator (J.J.), supervised participant enrollment. As subjects were added to the study, they were assigned the next available number in the sequence. When a subject dropped out, we replaced that person with the next new person. This occurred 3 times for the treatment group. One individual from the control group dropped out towards the end of the study and was not replaced. Subjects were only told of their assignment after completing session 4.
Statistical Methods
A series of analyses of covariances (ANCOVAs), with condition as the independent variable and age and baseline measures as covariates, were used to determine the effect of the 16-week intervention on the primary (aPWV,) and secondary outcome variables (ET-1, nitrite/nitrate, hsCRP, VO2peak, HRQOL scores). For the analysis of AIx at 75 bpm, systolic and diastolic blood pressures and 2×2 ANCOVAs were used. The independent variables were condition (exercise or control) and time (week 8 or 16) with age and baseline readings for each of the variables as covariates. Prior to data analysis, all variables were screened for normality, and appeared to meet assumptions. The α level was set at the 0.05 level and SPSS version 19 (SPSS Inc., Chicago, IL) was used for all analyses.
RESULTS
Patient Characteristics
Forty-six participants completed the study. Fifty one patients were assigned to the treatment (n=28) or control (n=23) group, (see Figure 2). The physical characteristics of the participants and pertinent clinical information are presented in Table 1. There were no differences in any of these variables at baseline. Analyses were conducted on all non-missing data; if a participant missed an assessment, they were excluded from that particular analysis.
Figure 2.
Consort Flow Diagram of Study
Table 1.
Characteristics of Study Sample at Baseline.
| Variable | Treatment (n= 25) | Control (n=21) | p value |
|---|---|---|---|
| Age (y) | 58.0 (8.0) | 57.1 (9.0) | 0.7 |
| Height (cm) | 169.7 (10.5) | 170.3 (10.5) | 0.8 |
| Weight (kg) | 101.7 (24.9) | 104.8 (29.8) | 0.7 |
| Waist (cm) | 108.7 (13.3) | 107.4(16.9) | 0.8 |
| Sex | 0.8 | ||
| Female | 9 | 7 | |
| Male | 16 | 14 | |
| Disease status | 0.7 | ||
| HTN | 15 | 10 | |
| DM | 1 | 1 | |
| HTN and DM | 9 | 10 | |
| Heart rate (bpm) | 64.3 (8.9) | 65.5 (12.0) | 0.7 |
| SBP (mm Hg) | 126.4 (17.7) | 132.9 (19.0) | 0.2 |
| DBP (mm Hg) | 79.5 (10.1) | 77.8 (11.9) | 0.6 |
| Cholesterol (mg/dl) | 191.7 (40.6) | 182.7(49.0) | 0.5 |
| eGFR (ml/min/1.73 m2) | 47.0 (12.0) | 48.3 (12.7) | 0.8 |
| Urine protein (g/24 h) | 0.5 (1.2) | 1.6 (3.7) | 0.3 |
Note: Values for categorical variables are given as number; values for continuous variables, as mean ± standard deviation. Conversion factor for cholesterol in mg/dL to mmol/L, ×0.02586.
DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; SBP, systolic blood pressure
Exercise Intervention Responses
Compliance was computed as the number of sessions attended out of a possible 48 expressed as a percentage. The average compliance to exercise was 96.9%±7.2% (standard deviation). The weekly time spent exercising in the treatment group was 128.7 ± 25.9 minutes. This was associated with a weekly caloric expenditure of 733.1 ± 380.7 kcal.
Peak Exercise Data
The 16-week exercise training program led to an increase in VO2peak in the treatment group of 1.6 mL/kg/min (or 8.2%; p=0.05), whereas over time the VO2peak in the control group declined by 0.5 ml/kg/min (or approximately 2.8%). Apart from peak HR, there were no differences in other exercise data after the 16-week intervention. There were no statistically significant differences in body composition post- versus pre-intervention in the treatment and control groups (p = 0.6) (Table 2).
Table 2.
Anthropometric, physiological and nutritional variables of study participants at baseline and after 16-week intervention.
| Variable | Treatment | Control | Adj Mean Diff (95% CI)* | p value** | ||
|---|---|---|---|---|---|---|
| Baseline | 16 wk | Baseline | 16 wk | |||
| BMI(kg/m2) | 34.9 (8.0) | 34.5 (7.8) | 36.5 (8.9) | 36.2 (8.9) | −0.24 (−090, 0.41) | 0.5 |
| Body Fat (%) | 35.9 (9.5) | 34.7 (9.7) | 37.4 (8.6) | 37.7 (8.7) | −1.43 (−2.95, 0.09) | 0.1 |
| SBP (mm Hg) | 126.4 (17.8) | 124.5 (15.9) | 133.7 (19.2) | 128.4 (25.3) | 2.02 (−7.54, 11.58) | 0.9 |
| DBP mm Hg) | 79.5 (10.2) | 77.8 (10.6) | 79.1 (10.7) | 75.3 (14.7) | 2.62 (−2.62, 7.85) | 0.1 |
| Pulse pressure (mm Hg) | 38.5 (10.9) | 38.9 (9.1) | 43.7 (14.5) | 44.7 (13.9) | −2.42 (−6.91, 2.06) | 0.3 |
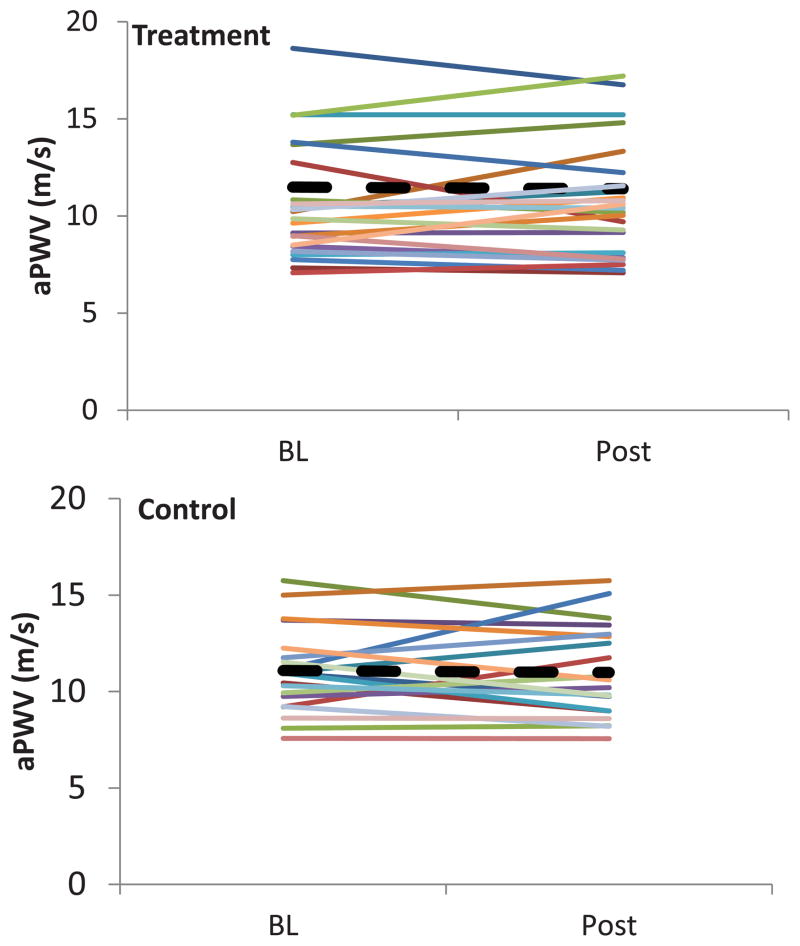
| Aortic PWV(m/s) | 10.7 (2.9) | 10.8 (2.9) | 11.1 (2.2) | 10.98 (2.4) | 0.14 (−0.74, 1.02) | 0.8 |
| VO2peak (ml/kg/min) | 19.6 (6.7) | 21.2 (7.7) | 18.0 (6.0) | 17.5 (5.7) | 2.29 (0.12, 4.47) | 0.04§ |
| Peak RER | 0.96 (0.2) | 0.99 (0.09) | 0.99 (0.14) | 0.96 (0.13) | 0.04 (−0.03, 0.11) | 0.2 |
| Peak SBP (mm Hg) | 179.4 (29.4) | 173.1 (26.1) | 178.7 (27.7) | 167.4 (30.8) | 5.00 (−11.14, 21.13) | 0.5 |
| Peak heart rate (bpm) | 134.4 (25.8) | 136.8 (24.7) | 126.7 (18.9) | 122.2 (18.6) | 8.23 (0.32, 16.14) | 0.04§ |
| AIx at 75 bpm | 22.6 (11.2) | 20.9 (11.1) | 17.1 (11.0) | 16.4 (7.7) | 0.34 (−2.89, 3.57) | 0.8 |
| hsCRP (mg/L) | 6.0 (3.7) | 5.9 (4.5) | 5.6 (2.96) | 5.4 (3.3) | 0.14 (−1.50, 1.78) | 0.9 |
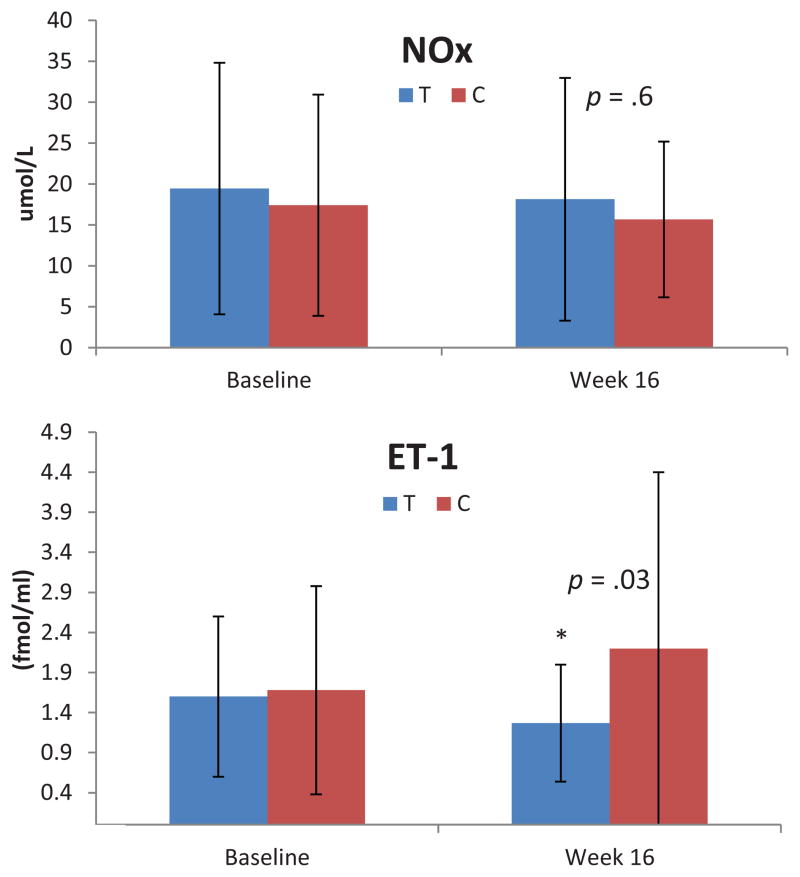
| ET-1* (fmol/ml) | 1.6 (1.0) | 1.3 (0.7) | 1.7 (1.3) | 2.2 (2.2) | −0.83 (−1.56, − 0.10) | 0.03§ |
| NO (μmol/L) | 19.4 (15.3) | 18.1 (14.8) | 17.4 (13.5) | 15.7 (9.5) | 1.69 (−5.39, 8.77) | 0.6 |
| NO:ET-1 ratio | 12.3 (7.11) | 15.93 (13.1) | 14.1 (15.0) | 8.8 (6.8) | 7.80 (1.12, 14.17) | 0.02§ |
| Calories (kcal) | 2149.3 (722.1) | 2200.5 (849.6) | 2077.2 (568.5) | 1985.1 (411.7) | 183.05 (−162.46, 528.87) | 0.3 |
| Sodium (mg) | 3643.7 (1273.2) | 3714.4 (1432.7) | 3612.9 (1550.4) | 3512.6 (1289.8) | 186.38 (−217.73, 590.49) | 0.4 |
Note: Unless otherwise indicated, values are given as mean ± standard deviation.
Adj, adjusted; AIx, central augmentation index; BMI, body mass index; diff, difference; CI. confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; RER, respiratory exchange ratio; SBP, systolic blood pressure; VO2peak, peak oxygen uptake; NO, nitric oxide; ET-1, endothelin 1;
hsCRP, high-sensitivity C-reactive protein;
95% CI for difference between adjusted means of treatment and control groups at 16 weeks, controlling for age and baseline assessment.
p value for difference between treatment and control groups at 16 weeks analyzed by either a 1 way or 2×2 analysis of covariance (see text for details for each variable).
indicates significant difference between treatment and control groups at week 16 controlling for age and baseline values.
Arterial Stiffness
The 16 week exercise training program did not affect aPWV (p = 0.8; partial η2 = 0.002. (see Figure 3). Furthermore, results of the 2×2 ANCOVA revealed that there was no significant difference between the treatment and control groups for AIx at 75 bpm (p = 0.8; partial η2 = 0.02) or interaction between time of assessment and condition (p = 0.6; partial η2 = 0.01).
Figure 3.
Individual plots of pulse wave velocity (a PWV) at baseline and following the 16 week intervention, p = 0.8, ---- represents mean values for each group.
Blood Parameters
ET-1 data were obtained on 44 subjects at both pre and post testing times. One subject in the control group had an extremely high level at both times (> 18.0 fmol/ml), and this level was recoded to 1 U more than the next highest level at each time. Two subjects had ET-1 levels that were below the detectable range of the assay kit. Results of an ANCOVA analysis revealed a significant difference between the treatment and control groups at week 16 (p = 0.03; partial η2 = 0.1); ET-1 decreased over the 16-week intervention in the treatment group while there was an increase in the control group (see Figure 4). There were no statistically significant group differences for NOx (p = 0.9; partial η2 = 0.01) or hsCRP (p = 0.9; partial η2 = 0.01), (see Table 2).
Figure 4.
Nitrate/nitrite (NOx), endothelin-1 (ET-1) and NOx:ET-1 ratio. T = Treatment group, C = Control group. * = p <0.05
Ratio of NOx/ET-1
As described in Beck et al9, we examined ratios of NOx to ET-1 values as an indicator of vasoactive balance. Two ET-1 values that were below the detectable range were deleted from the analysis in addition to three outliers (values > 100, much greater than 3 standard deviation above the mean). The results of an ANCOVA on the remaining observations revealed a significant difference between the treatment and control groups after the 16 week intervention (p = 0.02; partial η2 = 0.1). The NOx:ET-1 ratio increased in the treatment group but decreased in the control group (see Figure 3).
HRQoL
The SF-36 assessments were compared in a series of ANCOVAs. Group differences on each subscale were examined through an ANCOVA analysis, with SF-36 subscale values post exercise used as the dependent variable and age and the SF-36 subscale value at baseline prior to the 16-week intervention used as covariates. Scores for Physical Functioning (p = 0.02; partial η2 = 0.2), Vitality (p = 0.05; partial η2 = 0.1) and Bodily Pain (p = 0.02; partial η2 = 0.02) were higher in the treatment group than the control group at session eight, indicating an improvement in these variables (See Table 3).
Table 3.
Means for SF-36 scales by group for control and session-8 analysis.
| Variable | Treatment | Control | Adj Mean Diff (95% CI)* | p value** | ||
|---|---|---|---|---|---|---|
| Baseline | 16 wk | Baseline | 16 wk | |||
| Physical Functioning | 67.67 (25.27) | 79.67 (19.50) | 74.69 (17.17) | 65.93 (22.10) | 15.75 (3.07, 28.41) | 0.02§ |
| Physical Role Functioning | 78.33 (37.64) | 75.00 (35.35) | 57.81 (37.32) | 46.87 (44.60) | 19.77 (−7.43, 46.98) | 0.2 |
| Emotional Role Functioning | 88.89 (27.22) | 88.89 (27.22) | 64.58 (35.42) | 81.25 (27.13) | 0.45 (−21.44, 22.35) | 0.9 |
| Vitality | 55.33 (12.60) | 64.00 (19.29) | 52.50 (26.89) | 49.06 (27.52) | 12.96 (0.04, 28.88) | 0.05 |
| Mental Health | 82.40 (13.25) | 81.60 (15.55) | 80.75 (15.54) | 79.75 (12.60) | 1.10 (−8.34, 10.55) | 0.8 |
| Social Functioning | 87.50 (17.03) | 88.33 (17.34) | 71.09 (31.20) | 64.06 (28.82) | 13.43 (−1.83, 28.70) | 0.1 |
| Bodily Pain | 67.33 (25.55) | 77.17 (20.46) | 77.50 (16.76) | 65.78 (28.89) | 21.29 (3.54, 39.04) | 0.02§ |
| General Health | 52.33 (18.60) | 64.20 (14.43) | 53.44 (23.00) | 57.56 (20.65) | 10.06 (−0.99, 21.12) | 0.1 |
Note: Unless otherwise indicated, values are given as mean ± standard deviation.
Adj, adjusted; diff, difference; CI. confidence interval; SF-36, 36-Item Short Form Health Survey.
95% CI for difference between adjusted means of treatment and control groups at 16 weeks, controlling for age and baseline assessment.
p value for difference between treatment and control grups at 16 weeks analyzed by a 1-way analysis of covariance with age and baseline assessment as covariates.
indicates significant difference between treatment and control groups at week 16 controlling for age and baseline values.
Discussion
The present study was designed to ascertain the effect of short-term supervised moderate intensity aerobic training on aPWV in CKD stage 3. The 16- week exercise program did not change aPWV, but it led to a reduction in ET-1 and to a favorable vasoactive balance as evidenced by an increase in the NOx;ET-1 ratio9. We also found that the intervention improved some aspects of HRQOL.
The 16-week exercise training study did not alter aPWV in this sample of patients with CKD stage 3. This is in contrast to the findings of Hayashi et al.12 who reported a decrease in aPWV following an exercise program of similar duration in 17 healthy, sedentary middle-aged men. The current study was adequately powered to detect a difference in arterial stiffness if one existed. However, it is possible that the exercise intensity could have been a factor since Hayashi et al.12 used a higher intensity (60%–75% heart rate reserve) in contrast to our 50%–60% VO2peak. Mustata et al.13, who aerobically trained hemodialysis patients twice weekly for 3 months, also used a higher intensity (60%–80% maximum heart rate [≈50%–70% VO2peak]) and found that this led to a reduction in arterial stiffness. However, Mustata et al.13 used AIx to assess arterial stiffness rather than aPWV, which is the preferred method. In the current study, both of these methods were used and gave similar results. 13
One explanation for the difference between our findings and those reported by Hayashi et al.12 could relate to the physical structure of the arterial wall in CKD patients. Arterial stiffness and remodeling are known to occur as GFR declines with medial calcification believed to be linked to the observed increased stiffness2, 20. Furthermore, there is a reduction in the relative amount of elastin, concomitant with an increase in abnormal collagen, possibly mediated by chronic inflammation6. These structural alterations in the vessel walls of patients with CKD stage 3 may partially explain the differences observed in this study compared to the findings reported by Hayashi et al.12
It is therefore possible that by the time a CKD patient progresses to stage 3 of the disease, their arteries have become so stiff that a 16-week exercise intervention would not evoke any improvement. Recently, Howden et al. 21 reported similar findings even after 12-months of exercise training. Coupled with our findings, the results reported by Howden et al. 21 might suggest that lifestyle interventions should be initiated earlier in the disease process prior to the development of irreversible structural changes in the arterial wall.
The most noteworthy finding of this study is the 20.6% reduction in ET-1 that occurred in the treatment group. This reduction in ET-1 following exercise training is consistent with other researchers who have reported similar results in persons with prehypertension or hypertension 7,9. In the current study, 16 weeks of training had no effect on the stable markers of NO. A similar finding was reported by Hansen et al. in a 16-week training study involving hypertensive individuals.22 However, this is in contrast to the findings of Beck et al.9 who studied non-CKD pre-hypertensive individuals, in an 8-week training program that resulted in a similar reduction (≈20%) in ET-1 as we observed but this was associated with an increase (≈25%) in NOx. The production of NO is known to be blunted in CKD, with researchers reporting increases in NO synthase inhibitors 6, 8. Furthermore, the high oxidative stress state in CKD is believed to inactivate NO, and therefore the measurement of circulating NOx may not fully reflect the biologic activity of endogenous NO in this population22, 24.
The exercise program led to an improvement in vasoactive balance as indicated by an increase in the NOx:ET-1 ratio. A favorable change in vasoactive balance, using metabolites of the arachidonic acid pathway, has also been observed in a sample of hypertensive individuals following 16 weeks of exercise training. However, unlike in the current study, these changes were associated with a reduction in blood pressure23. A reciprocal relationship is believed to exist between ET-1 and NO in CKD, thereby producing a low NOx:ET-1 ratio that seems to be associated with poor cardiovascular outcomes24. Our finding of a favorable improvement in this ratio, with a low volume of exercise, could indicate a possible mechanism by which exercise training may be cardioprotective in CKD. In contrast to its impact on ET-1, the exercise intervention had no effect upon hsCRP. This observation is consistent with our previous findings and those reported by Wilund et al.25,26.
In the current study, HRQOL was assessed using the SF-36 form. The scores that we recorded at baseline prior to the intervention are similar to those reported by Padilla et al27 in a sample of CKD patients. Of particular note is the observation that our 16-week intervention led to significant improvements in self-reported Physical Functioning, Vitality, and Bodily Pain. An increase in any subscale score in the SF-36 represents an improvement in that health outcome, so the increase in Bodily Pain score actually represents an improvement (i.e., less) in this parameter. Our results support the recommendation that CKD patients should follow public health guidelines and exercise on a regular basis even if there is no appreciable improvement in some clinical markers, such as arterial stiffness, since some aspects of HRQOL will improve.
The current study was limited by the short exercise duration and the relatively small volume (128.7 ± 25.9 min; 733.1 ± 380.7 kcal) of exercise completed by the participants. However, even this relatively small exercise dose resulted in an increase in aerobic capacity (8.3%) in addition to a reduction in ET-1. The magnitude of the increase in aerobic capacity is consistent with results that we and others have found following similar training in this population (Howden et. al.21, Headley et al.25, Edemak et al.28). Perhaps these results would have been more marked if we had used a higher exercise intensity (≈60%–70% VO2peak) or longer duration. However, we chose an intensity range that was unlikely to produce adverse events, patients are more likely to use long-term, and is recommended for high risk patients15. We do acknowledge that it would have been ideal to have included a measure of endothelial function, and such a measurement should be included in future studies of this type.
In conclusion, short-term exercise training of a moderate intensity that leads to an improvement in aerobic capacity of patients with CKD stage 3 does not alter aPWV. However, this type of training seems to be effective in reducing ET-1 levels and it also improves some aspects of HRQOL. Future studies should be designed to further explore the impact of exercise training on other markers of vascular function and to determine if this intervention reduces the excess cardiovascular morbidity and mortality observed in the CKD population.
Acknowledgments
We acknowledge the contribution of the staff at Western New England Renal and Transplant Associates who helped with the recruitment of participants for this study. We also thank the administration at the Wellness Center at Springfield College for the use of the training facilities to complete this study. In addition, we thank Dr Mary Ann Coughlin for the assistance that she gave during the initial stages of this study.
Support: This study was supported by a grant from the National Institutes of Health (1R15HL096097-01).
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
N SECTION: Trial registration: www.ClinicalTrials.gov; study number: NCT01399489.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=17606856&loginpage=login.asp&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 2.Briet M, Pierre B, Laurent S, London GM. Arterial stiffness and pulse pressure in CKD and ESRD. [Accessed February 11, 2013];Kidney Int. 2012 82(4):388–400. doi: 10.1038/ki.2012.131. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22534962. [DOI] [PubMed] [Google Scholar]
- 3.Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23(3):282–289. doi: 10.1038/ajh.2009.240. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=20019670&site=ehost-live. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ML, Tomlinson La, Chapman TPE, Rajkumar C, Holt SG. Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. [Accessed January 31, 2013];Hypertension. 2010 55(5):1110–5. doi: 10.1161/HYPERTENSIONAHA.109.143024. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20212269. [DOI] [PubMed] [Google Scholar]
- 5.Peralta CA, DRJ, Katz R, et al. Association of Pulse Pressure, Arterial Elasticity, and Endothelial Function With Kidney Function Decline Among Adults With Estimated GFR >60 mL/min/1.73 m 2: The Multi-Ethnic Study of Atherosclerosis (MESA) 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gusbeth-Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. [Accessed May 22, 2013];Kidney Blood Press Res. 2007 30(2):97–107. doi: 10.1159/000100905. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17374960. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg M, Mortensen SP, Hellsten Y. Physical activity opposes the age-related increase in skeletal muscle and plasma endothelin-1 levels and normalizes plasma endothelin-1 levels in individuals with essential hypertension. [Accessed June 26, 2013];Acta Physiol (Oxf) 2013 207(3):524–35. doi: 10.1111/apha.12048. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23227981. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri N. Effect of chronic renal failure on nitric oxide metabolism. [Accessed July 25, 2013];Am J Kidney Dis. 2001 38(4):S74–S79. doi: 10.1053/ajkd.2001.27409. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0272638601022028. [DOI] [PubMed] [Google Scholar]
- 9.Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. [Accessed June 26, 2013];Exp Biol Med (Maywood) 2013 238(4):433–41. doi: 10.1177/1535370213477600. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23760009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. [Accessed February 11, 2013];Hypertension. 2011 58(5):943–9. doi: 10.1161/HYPERTENSIONAHA.111.176529. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21896936. [DOI] [PubMed] [Google Scholar]
- 11.Roque FR, Hernanz R, Salaices M, Briones AM. Exercise training and cardiometabolic diseases: focus on the vascular system. [Accessed November 30, 2013];Curr Hypertens Rep. 2013 15(3):204–14. doi: 10.1007/s11906-013-0336-5. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23519745. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol. 2005;55(4):235–239. doi: 10.2170/jjphysiol.S2116. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=16248931&loginpage=login.asp&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 13.Mustata S, Chan C, Lai V, Miller JA. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol JASN. 2004;15(10):2713–2718. doi: 10.1097/01.ASN.0000140256.21892.89. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=15466276&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 14.Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol. 2011;43(4):1133–1141. doi: 10.1007/s11255-010-9823-7. [DOI] [PubMed] [Google Scholar]
- 15.Thompson WR. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Lippincott Williams and Wilkins; 2010. p. 380. [Google Scholar]
- 16.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 17.Bellizzi V, Scalfi L, Terracciano V, et al. Early changes in bioelectrical estimates of body composition in chronic kidney disease. J Am Soc Nephrol JASN. 2006;17(5):1481–1487. doi: 10.1681/ASN.2005070756. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=16611719&loginpage=login.asp&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 18.Van Bortel LM, Duprez DA, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, task force III: Recommendations for user procedures. Am J Hypertens. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- 19.Wimmer NJ, Townsend RR, Joffe MM, Lash JP, Go AS. Correlation between pulse wave velocity and other measures of arterial stiffness in chronic kidney disease. Clin Nephrol. 2007;68(3):133–143. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=17915615&loginpage=login.asp&site=ehost-live. [PubMed] [Google Scholar]
- 20.Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T. Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. [Accessed February 11, 2013];Jpn J Physiol. 2005 55(4):235–9. doi: 10.2170/jjphysiol.S2116. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16248931. [DOI] [PubMed] [Google Scholar]
- 21.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH. Effects of Exercise and Lifestyle Intervention on Cardiovascular Function in CKD. [Accessed September 13, 2013];Clin J Am Soc Nephrol. 2013 8(9):1494–501. doi: 10.2215/CJN.10141012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23970136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumur Z, Niwa T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. [Accessed September 14, 2013];Am J Nephrol. 2009 29(6):551–7. doi: 10.1159/000191468. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19129694. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. [Accessed November 29, 2013];Hypertension. 2011 58(5):943–9. doi: 10.1161/HYPERTENSIONAHA.111.176529. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21896936. [DOI] [PubMed] [Google Scholar]
- 24.Kurita A, Matsui T, Ishizuka T, Takase B, Satomura K. Significance of plasma nitric oxide/endothelial-1 ratio for prediction of coronary artery disease. Angiology. 2005;56(3):259–64. doi: 10.1177/000331970505600304. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15889192. [DOI] [PubMed] [Google Scholar]
- 25.Headley S, Germain M, Milch C, et al. Exercise training improves HR responses and V O2peak in predialysis kidney patients. [Accessed February 11, 2013];Med Sci Sports Exerc. 2012 44(12):2392–9. doi: 10.1249/MSS.0b013e318268c70c. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22811032. [DOI] [PubMed] [Google Scholar]
- 26.Wilund K, Tomayko E, Wu E, et al. Inflammatory response to exercise training in chronic kidney disease. Med Sci Sport Exerc. 2009;41(5):S42. [Google Scholar]
- 27.Padilla J, Krasnoff J, Da Silva M, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008;21(4):550–559. Available at: http://ezproxy.spfldcol.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=18651545&site=ehost-live. [PubMed] [Google Scholar]
- 28.Eidemak I, Haaber AB, Feldt-Rasmussen B, Kanstrup I-L, Strandgaard S. Exercise Training and the Progession of Chronic Renal Failure. Nephron. 1997;75:36–40. doi: 10.1159/000189497. [DOI] [PubMed] [Google Scholar]