Abstract
Background
This study compared immediate versus delayed massage-like compressive loading on skeletal muscle viscoelastic properties following eccentric exercise.
Methods
Eighteen rabbits were surgically instrumented with peroneal nerve cuffs for stimulation of the tibialis anterior muscle. Rabbits were randomly assigned to a massage loading protocol applied immediately post exercise (n=6), commencing 48 hours post exercise (n=6), or exercised no-massage control (n=6). Viscoelastic properties were evaluated in vivo by performing a stress-relaxation test pre- and post-exercise and daily pre- and post-massage for four consecutive days of massage loading. A quasi-linear viscoelastic approach modeled the instantaneous elastic response (AG0), fast ( ) and slow ( ) relaxation coefficients, and the corresponding relaxation time constants τ1 and τ2.
Findings
Exercise increased AG0 in all groups (P<0.05). After adjusting for the three multiple comparisons, recovery of AG0 was not significant in the immediate (P=0.021) or delayed (P=0.048) groups compared to the control group following four days of massage. However, within-day (pre- to post-massage) analysis revealed a decrease in AG0 in both massage groups. Following exercise, increased and and τ1 decreased for all groups (P<0.05). Exercise had no effect on τ2 (P>0.05). After four days of massage, there was no significant recovery of the relaxation parameters for either massage loading group compared to the control group.
Interpretation
Our findings suggest that massage loading following eccentric exercise has a greater effect on reducing muscle stiffness, estimated by AG0, within-day rather than affecting recovery over multiple days. Massage loading also has little effect on the relaxation response.
Keywords: viscoelastic, skeletal muscle, massage, passive properties, compression
Introduction
Unaccustomed eccentric exercise (EEX) results in delayed onset muscle soreness, presenting clinically as pain, stiffness, and decreased range of motion. These symptoms are attributed to tissue inflammation and the disruption of the cellular components, thus altering the muscle’s structure and subsequently the tissue’s viscoelastic response (Lieber and Friden, 2002; McHugh et al., 1999; Page, 1995). Various approaches to characterize the viscoelastic properties of both human and animal skeletal muscle in response to EEX have been utilized (Chleboun et al., 1998; Howell et al., 1993; Jones et al., 1987; Pousson et al., 1990; Whitehead et al., 2001). Changes in muscle mechanical properties (particularly increased stiffness), increased tissue swelling, and decreased joint range of motion have been observed 4–6 days following unaccustomed EEX (Chleboun et al., 1998; Jones et al., 1987). Additional studies have estimated changes in human skeletal muscle stiffness by measuring joint torque-angle properties (Chleboun et al., 1993; Chleboun et al. 1998; Whitehead et al., 2001). Moreover, Howell et al. (1993) noted that passive elbow stiffness doubled immediately following EEX and remained elevated for 4 days. The increase in stiffness was estimated from the slope of the first 50 degrees of the torque-angle curves of the elbow flexors and is therefore not a measure of the tissue’s passive stiffness. In a recent study, Green et al. (2012) employed magnetic resonance elastography to directly measure changes in the in vivo elastic properties of the human triceps surae after EEX. The investigators noted that both the storage modulus (elastic component) and the shear loss modulus (viscous component) increased after exercise in the gastrocnemius and remained elevated for one week.
Massage therapies have been shown to reduce muscle soreness following unaccustomed exercise (Farr et al., 2002; Hilbert et al., 2003; Smith et al., 1994; Zainuddin et al., 2005). Several animal models and theoretical approaches have been developed to understand the effects of compressive loading on muscle mechanical properties. As a result, these pursuits have sought to provide possible mechanisms to explain the effects of these manual therapies on tissue function. Bosboom et al. (2001) performed a ramp and hold compression test on rat skeletal muscle and utilized an Ogden model to estimate both elastic and viscous material parameters that accurately described the muscle’s behavior under compressive loading. Van Loocke et al. (2008) employed a quasi-linear viscoelastic (QLV) model to investigate the time-dependent behavior of porcine skeletal muscle and found that the muscle’s viscoelastic behavior was dependent on both compression rate and fiber orientation. Haas et al. (2013) demonstrated in a rabbit model that recovery of isometric torque production following intense EEX was dependent on both magnitude and frequency of compressive loading intended to mimic clinical massage. In our lab’s previous work, Haas et al. (2012b) quantified the effects of massage-like compressive loading (MLL) on the recovery of muscle viscoelastic properties and noted that tissue stiffness, rather than relaxation, was more affected. Finally, our lab’s previous work has also noted that immediate and 48 hour delayed MLL had different effects on the recovery of both torque production and inflammatory cell infiltration following EEX (Haas et al., 2012a).
The purpose of the current study was to extend our previous work and to compare immediate and delayed MLL following a damaging bout of EEX and their changes in the viscoelastic properties of the muscle-tendon complex. We hypothesized that MLL applied immediately following exercise would have a greater recovery on the tissue’s viscoelastic properties compared to the same regimen started 48 hours post-exercise. Such information could guide clinicians in prescribing the optimal time for massage therapies following exercise in order to mitigate symptoms, such as prolonged muscle stiffness, of intense EEX.
Methods
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University. The rabbits were anesthetized using 5% isoflurane and maintained under anesthesia during all surgeries and experiments.
Surgical Procedure and Exercise Protocol
Eighteen skeletally mature New Zealand White female rabbits were surgically instrumented with bilateral deep fibular (peroneal) nerve cuffs (Koh and Leonard, 1996) for consistent and reproducible stimulation of the tibialis anterior (TA) muscle. The surgical procedure is detailed in Butterfield et al. (2008). The interfaces for the nerve cuffs were subdermal on the back of each rabbit so as not to interfere with normal ambulation and activity.
The rabbits were subjected to the exercise protocol seven days after surgery. As detailed in previous works (Butterfield et al., 2008; Haas et al., 2012a; Haas et al., 2012b; Haas et al., 2013), the rabbits were placed supine in a sling with one foot placed on a footplate attached to a Servo motor with a torque sensor (Figure 1). The EEX protocol consisted of seven sets of ten lengthening contractions, during which the TA was stimulated at a voltage three times the α-motoneuron threshold. Two minutes of rest preceded each set of contractions in order to minimize the effects of fatigue. The parameters of the lengthening contractions are detailed in Haas et al. (2012b).
Figure 1.
Exercise protocol setup. Animal was under anesthesia and placed supine in sling. The stimulator box controlled the pulse for stimulation of the tibialis anterior. The animal underwent 7 sets of 10 eccentric contractions with an external stimulation of three times the α-motoneuron threshold.
MLL Protocol
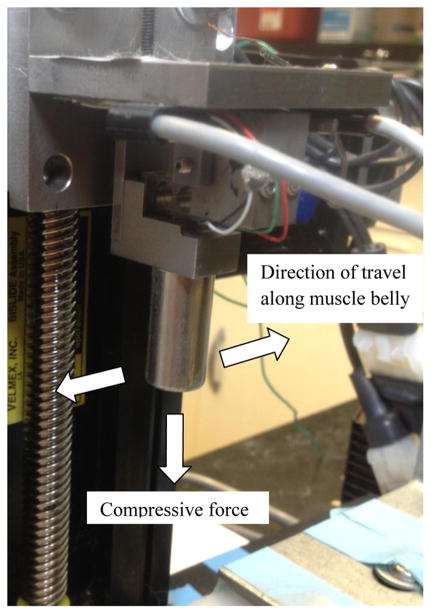
The rabbits were randomized into one of three groups: immediate MLL (n=6), 48 hour delayed MLL (n=6), or exercised no-MLL control (n=6). Each of the 18 rabbits had one hind limb exercised. MLL was applied immediately after exercise (day 1) in the immediate MLL group and 48 hours after exercise (day 3) in the delayed MLL group and daily (approximately 24 hours apart) for four consecutive days. MLL was not applied for the control group in order to assess the effects of natural healing four days after EEX. The rabbits were subjected to in vivo MLL using a customized device (Haas et al., 2012b, Wang et al., 2013). A mechanical tip was mounted on the end of the motorized device and connected to a force sensor (Figure 2). The tip compressed the tissue surface until a compressive force of 10 N was attained. The mechanical tip moved longitudinally along the muscle at 0.5 Hz for 15 minutes. These parameters were shown to produce optimal recovery of isometric torque after a bout of EEX (Butterfield et al., 2008; Haas et al. 2013).
Figure 2.
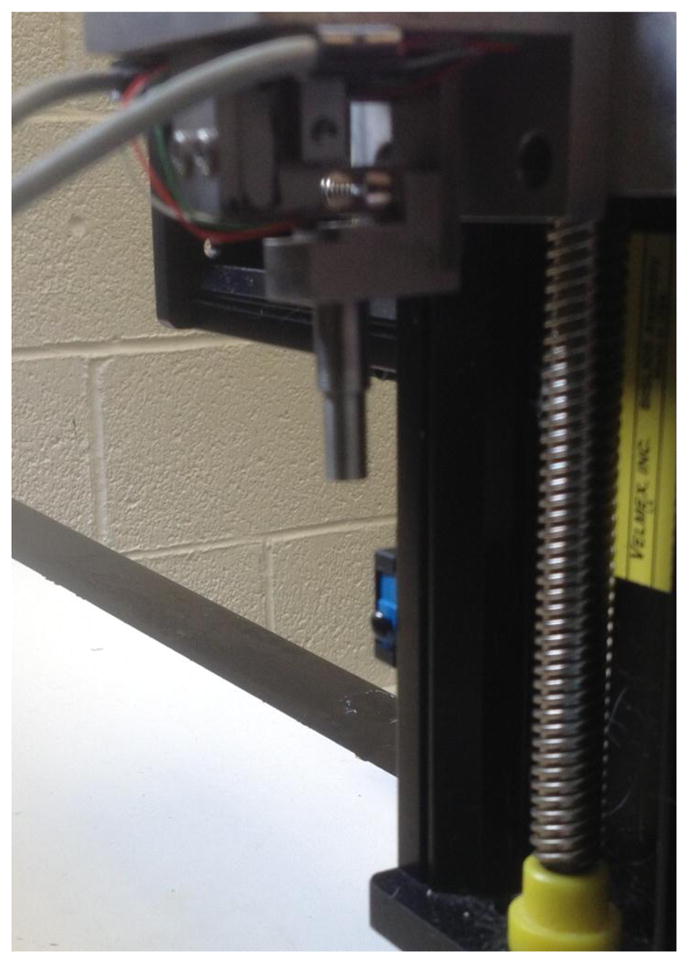
Cutomized MLL device. A mechanical tip was mounted on the end of the motorized device connected to force sensors. Data acquisition within Labview controlled compressive magnitude, frequency, and duration of MLL.
Evaluation of Muscle Viscoelastic Properties
The passive mechanical properties were evaluated in vivo using the same device that applied MLL (Figure 2). While the rabbits were anesthetized, the hind limb was secured on a footplate underneath the mechanical tip with the TA facing up. The viscoelastic properties were measured by a 300 sec compressive ramp-and-hold stress relaxation test. These measurements were taken pre-exercise and post-exercise on day 1 and pre- and post-MLL for all four days of the MLL protocol for the immediate and delayed MLL groups. On the final day for each group (day 5 for immediate MLL and day 7 for delayed MLL) a final ramp-and-hold test was performed in order to gauge the cumulative effects of the MLL intervention. In the no-MLL control group, the viscoelastic properties were only measured pre-exercise, post-exercise, and four days after exercise. Performing a stress-relaxation test on the control group every day would be a type of mechanical loading. Because we were unsure if this loading would affect the viscoelastic properties, and consequently would not allow us to evaluate the recovery of the exercised muscle due only to natural healing, we did not evaluate the daily passive mechanical properties in the control group.
Mathematical Modeling
The mathematical modeling used to model the viscoelastic properties was first proposed by Fung (1972) and later modified by Abramowitch and Woo (2004). Our previous work (Haas et al. 2012b) further details the mathematical modeling used to determine the viscoelastic parameters. Briefly, the stress relaxation was:
| (1) |
where the instantaneous elastic response was:
| (2) |
and the coefficient AG0 (kPa) represented the linear behavior of the elastic response, B represented the nonlinear behavior of the elastic response, and ε was the engineering strain. The reduced relaxation function G(t) was:
| (3) |
where k0, k1, and k2 were constants fitted by experimental data, t was time, and the time constants τ1 and τ2 were the time constants corresponding to the fast and slow relaxations, respectively. The fast time constant (τ1) was smaller than the slow time constant (τ2). The fast and slow relaxation coefficients, and , respectively, where:
| (6) |
| (7) |
After fitting the stress relaxation data, we compared the daily and cumulative effects of MLL or natural healing (exercise + no MLL) on AG0, and , τ1, and τ2.
Statistical analysis
In order to account for rabbit variability at pre-exercise, post-exercise, and post four days of MLL, a recovery index (RI) considering all three measurements was used to calculate the level of recovery for AG0, , τ1, and τ2. This analysis is described in detail in (Haas et al. 2013). The RI was calculated as:
| (8) |
The numerator measured the change in viscoelastic properties post four-day MLL protocol (four days post exercise in the no-MLL control) relative to pre-exercise and the denominator measured the same properties post-exercise relative to pre-exercise. This ratio quantified the amount of recovery due to either MLL or natural healing compared to the amount of damage induced by EEX. For instance, if the pre-exercise and post-MLL viscoelastic properties were the same, recovery was 100% and the RI = 1. An RI = 0 indicated no recovery in mechanical properties from post-exercise values.
AG0 data were first log transformed to reduce skewness and variance before the calculation of RI. One-way ANOVA tests were used to compare the RIs for AG0, , , τ1 and τ2 among the three tested groups, i.e. immediate MLL, delayed MLL, and control (exercised no-MLL) groups. The primary analyses were focused on the comparisons of the RIs. A Holm’s procedure (Holm, 1979) was used to control for multiple comparisons for each factor resulting in P=0.017 being regarded as significant for the smallest P-value and P=0.025 and P=0.05 being regarded as significant for subsequent comparisons. Daily changes (pre- to post-MLL) in each parameter were also performed and considered as secondary analyses. The P-values were adjusted for multiple comparisons using a Holm’s procedure where P=0.0125 was set as the significance level for the comparison with the smallest P-value. Accordingly, P=0.017, P=0.025, and P=0.05 were considered as significant for the subsequent within-day comparisons. Linear mixed effects models were used to take into account the correlation among observations from the same animal. SAS 9.2 (SAS Institute Inc., NC, USA) was used for all analyses.
Results
Instantaneous Elastic Response (AG0)
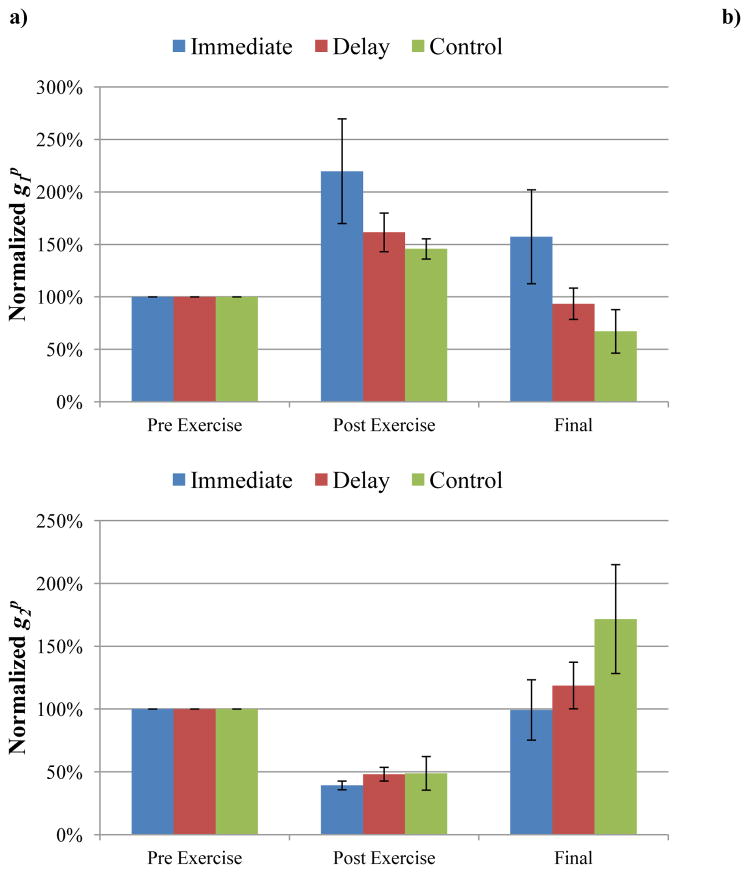
The instantaneous elastic response coefficient (AG0) increased immediately following EEX for all 18 animals within the three groups (immediate MLL, 48 hour delayed MLL, and no-MLL control groups, P=0.0007, 0.0035, and 0.0008, respectively), indicating that the muscle became stiffer. Each animal’s AG0 values were normalized by its pre-exercise value and then averaged within each group. The average post-exercise AG0 values were 289%, 431%, and 320% for the immediate, delayed, and no-MLL control groups, respectively. The final averages of AG0 for the immediate and delayed massage groups were 126% and 253% of pre-exercise AG0, respectively, indicating that AG0 values were decreasing from post-exercise values and approaching pre-exercise levels. The no-MLL control group final measurement (four days post-exercise) AG0 showed no change from post-exercise and was 328% of pre-exercise values.
The recovery indices (RI) of the final day AG0 were compared amongst the three groups and are shown in Table 1. Using a Holm’s procedure to adjust for the three group comparisons resulted in P=0.017 as being significant for the lowest P-value. Because of this adjusted significance level, there was no significant difference between the no-MLL control and immediate MLL groups (P=0.021). Since the first comparison was not significant, subsequent comparisons resulted in no significant differences between the no-MLL control and delayed MLL groups (P=0.048) and between immediate and delayed MLL groups (P=0.68). Despite this lack of statistical significance, the four-day MLL protocol resulted in an average 53% decrease in AG0 from post-exercise to the final measurement (day 5) in the immediate group and an average 41% decrease in AG0 from post-exercise to the final measurement (day 7) in the delayed group.
Table 1.
Average recovery index values (RI) for each group. The RI was calculated as where the ratio quantified the amount of recovery due to natural healing or MLL compared to the amount of change in the QLV parameters initiated by exercise. Note that an RI of 1 indicates full recovery and an RI of 0 indicates no recovery.
| AG0 |
|
|
τ1 | τ2 | |||
|---|---|---|---|---|---|---|---|
| Control | −0.157 | 1.727 | 2.037 | 0.615 | −0.512 | ||
| Delay | 0.596 | 1.061 | 1.479 | 0.262 | 0.473 | ||
| Immediate | 0.739 | 1.00 | 0.929 | 1.111 | 0.607 |
A secondary analysis of the intra-day differences (pre- to post-MLL) was performed to detect any differences in AG0 due to MLL. A representative figure demonstrating the normalized daily effects of MLL is shown in Figure 3. There were within-day decreases in AG0 between pre- and post-MLL in both the immediate and delayed groups on all days of MLL (Table 2). In the immediate group, there was a 55% decrease in AG0 from post-exercise (pre-MLL) to post-MLL on Day 1 (P=0.0008), which was significant after the adjustment for multiple comparisons. On Days 2 (P=0.0011) and 3 (P=0.0086), there were also significant decreases in AG0 following MLL after adjusting for multiple comparisons (51% and 48% decreases, respectively). On Day 4 there was a 31% decrease in AG0 following MLL, however this was not a significant decrease (P=0.062).
Figure 3.
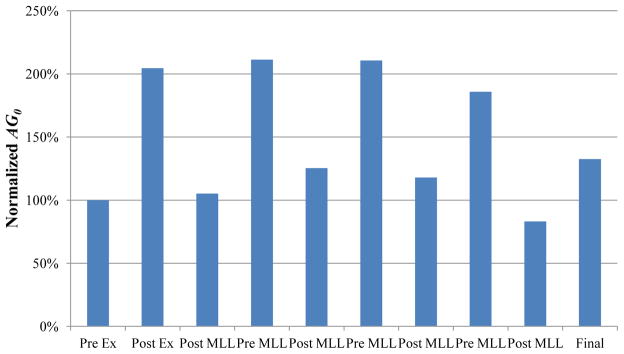
Representative normalized instantaneous elastic response coefficient (AG0) effects. Exercise doubled AG0 compared to pre-exercise. MLL produced a daily decrease in AG0. Final AG0 was approximately 130% of pre-exercise value.
Table 2.
Immediate and Delayed MLL Intra-day AG0 comparison. MLL decreased AG0 within the same day of application in both groups. There was a larger percent decrease in AG0 in both groups following MLL applied closer to EEX rather than later.
| Immediate Massage Group | Percent decrease Post MLL | P-value | Delayed Massage Group | Percent decrease Post MLL | P-value | ||
|---|---|---|---|---|---|---|---|
| Day 1 | Pre MLL | 55% | 0.0023 | Day 3 | Pre MLL | 37% | 0.0095 |
| Post MLL | Post MLL | ||||||
| Day 2 | Pre MLL | 51% | 0.0011 | Day 4 | Pre MLL | 41% | 0.034 |
| Post MLL | Post MLL | ||||||
| Day 3 | Pre MLL | 48% | 0.0086 | Day 5 | Pre MLL | 15% | 0.874 |
| Post MLL | Post MLL | ||||||
| Day 4 | Pre MLL | 31% | 0.062 | Day 6 | Pre MLL | 7% | 0.759 |
| Post MLL | Post MLL | ||||||
The intra-day AG0 decreased from pre-MLL to post-MLL in the delayed group, although the decreases were an average 21% less than those observed in the immediate group (Table 2). After adjusting for multiple comparisons, there was a significant decrease (P=0.0095) in AG0 pre-MLL to post-MLL on Day 3, which corresponded to a 37% decrease. However, there were no significant decreases on Days 4, 5, and 6 (P=0.034, 0.874, 0.759, respectively) despite decreases in AG0 of 41%, 15%, and 7%, respectively, between pre- and post-MLL.
Reduced Relaxation Response ( , τ1, , and τ2)
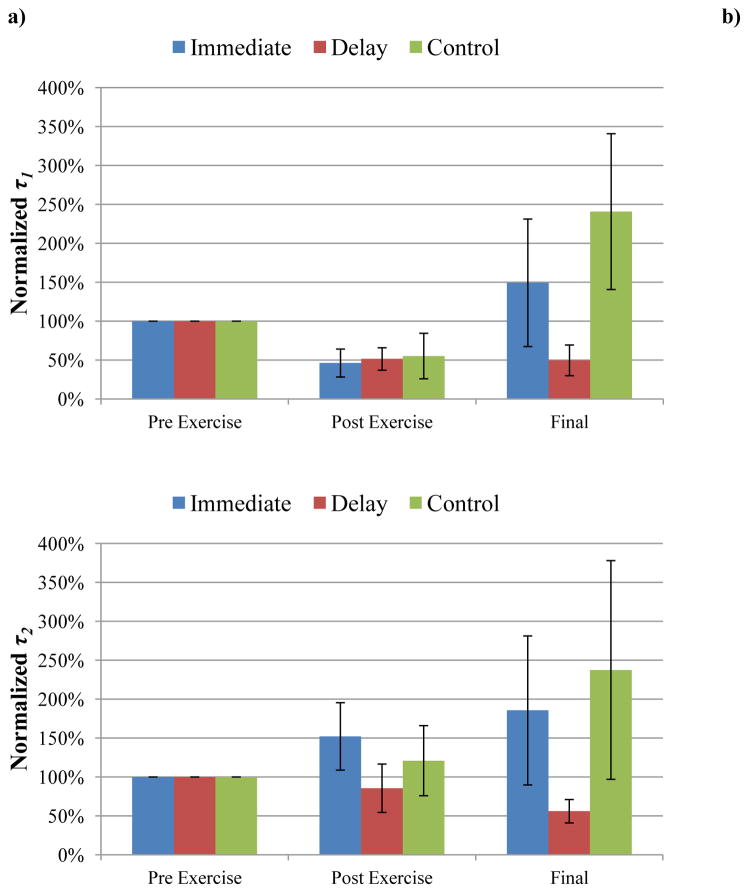
In the QLV model, fast relaxation was represented by and τ1. The fast relaxation coefficient ( ) increased in all three groups after exercise (P=0.0027, 0.0014, 0.0025 for the immediate, delayed, and no-MLL control groups, respectively). Following four consecutive days of MLL, the recovery indices of the final measurements were not significantly different amongst the three groups (P=0.444 for immediate group vs. control, P=0.498 for delayed group vs. control, and P=0.994 for immediate group vs. delayed group). This indicated that massage loading did not affect recovery over the four day period as indicated by the RI values (Figure 4, Table 1). Daily massage-like loading had a varied effect on in both groups resulting in no consistent trends of the within-day values. Consequently, after adjusting for multiple comparisons where P=0.0125 was regarded as significant for the lowest P-value, there were no significant differences in either the immediate (P=0.623, 0.389, 0.729, and 0.733 for Days 1, 2, 3 and 4, respectively) or the delayed groups (P=0.102, 0.0127, 0.134, and 0.793 for Days 3, 4, 5, and 6, respectively).
Figure 4.
Effects of MLL on altering and . a) was normalized by pre-exercise values. There was a significant increase in after exercise in all three groups (P<0.05). b) was normalized by pre-exercise values. There was a significant decrease in after exercise in all three groups (P<0.05). Note that there was no significant difference in recovery in either MLL group compared to controls, indicating that the MLL protocol had little effect on altering the fast and slow relaxation coefficients.
The fast time constant (τ1) decreased in all three groups following exercise (P=0.046, 0.032, and 0.043 for the immediate, delayed, and no-MLL control groups, respectively). Following four consecutive days of MLL, the recovery indices of the final τ1 measurements (Table 1) were not significantly different amongst all three groups (P=0.977 for immediate group vs. control, P=0.761 for delayed group vs. control, P=0.880 for immediate group vs. delayed group). Thus, MLL did not affect the recovery of τ1 (Figure 5). Daily massage-like loading had a varied effect on τ1 within the same day and we observed no consistent within-day (pre- to post-MLL) trends. Furthermore, after adjusting for multiple comparisons, there were no significant differences due to daily massage loading in either the immediate (P=0.159, 0.213, 0.159, and 0.866 for Days 1, 2, 3 and 4, respectively) or delayed groups (P=0.343, 0.581, 0.014, and 0.518 for Days 3, 4, 5, and 6, respectively).
Figure 5.
Effects of MLL on altering τ1 and τ2. a) τ1 was normalized by pre-exercise values. Note there was a significant decrease in τ1 (P<0.05) in all three groups after exercise. b) τ2 was normalized by pre-exercise values. Exercise had no effect on altering τ2 (P>0.05). There was no significant difference in recovery in either MLL group compared to controls, indicating that MLL had little effect on altering the fast and slow relaxation time constants.
The slow relaxation was represented by and τ2 in the mathematical model. The slow relaxation coefficient ( ) decreased in all three groups following exercise (P<0.0001 for the immediate group, P=0.027 for the delayed group, and P=0.035 for the no-MLL control). However, the recovery indices of the final measurements were not significantly different amongst the three groups (P=0.816 for immediate group vs. control, P=0.662 for delayed group vs. control, and P=0.962 for immediate group vs. delayed group), indicating massage loading had no effect on the recovery of (Figure 4). The RI values are shown in Table 1. Similar to , massage-like loading had a varied effect on within the same day (pre- to post-MLL) in both the immediate and delayed MLL groups. After adjusting for multiple comparisons, there were no significant differences in due to daily massage loading in the immediate (P=0.176, 0.757, 0.454, and 0.134 for Days 1, 2, 3 and 4, respectively) or delayed groups (P=0.611, 0.611, 0.017, and 0.310, for Days 3, 4, 5, and 6, respectively).
Unlike the previously mentioned relaxation parameters, exercise had no effect on the slow time constant τ2 (P=0.488 for the immediate, 0.106 for the delayed, and P=0.175 for the no-MLL control groups). Following four consecutive days of massage loading (Figure 5), there were no significant changes in the recovery indices of τ2 amongst the three groups (P=0.638 for immediate group vs. control, P=0.687 for delayed group vs. control, P=0.991 for immediate group vs. delayed group). The RI values are shown in Table 1. Similar to τ1, massage-like loading had a varied effect on τ2 within the same day (pre- to post-MLL) in both the immediate and delayed MLL groups. As a result, there were no consistent trends of the within-day values due to massage-like loading. After adjusting for multiple comparisons, there were no significant differences in the daily effects of massage loading on τ2 in the immediate (P=0.504, 0.093, 0.540, and 0.924 for Days 1, 2, 3, and 4, respectively) or delayed groups (P=0.0416, 0.676, 0.503, and 0.734 for Days 3, 4, 5, and 6, respectively).
Discussion
Following a bout of eccentric exercise, massage-like loading (MLL) had a greater effect on recovery of the muscle’s instantaneous elastic response coefficient (AG0) compared with its relaxation response ( , τ1 and τ2). Furthermore, these effects were more evident within the same day (pre- to post-MLL) rather than over the entire four day massage protocol.
With our protocol, one bout of EEX significantly increased the instantaneous elastic response coefficient (AG0) for all 18 animals in this study. This increase indicates an increase in muscle stiffness immediately following EEX, which is consistent with similar studies in both humans and animals (Chleboun et al., 1995; Chleboun et al., 1998; Haas et al., 2012b; Howell et al., 1993; Whitehead et al., 2001). Although there is increasing interest in the use of manual therapies such as massage for facilitating recovery from injury and intense exercise, possibly by decreasing tissue stiffness (Weerapong et al., 2005), little is known about the ideal time course for their implementation. Whereas many studies have investigated the effects of compression on muscle viscoelastic behavior (Bosboom et al., 2001; Palevski et al., 2006; Van Loocke et al., 2006, 2008, 2009), the current study builds upon these previous reports by investigating the in vivo mechanical response of rabbit skeletal muscle following an intense bout of EEX; subsequent interventions of compressive MLL applied immediately or 48 hours post-exercise; and the recovery of the tissue’s viscoelastic properties.
Neither immediate nor delayed MLL showed a significant effect on the recovery of AG0 following four days of MLL compared to no-MLL controls. Despite the lack of statistical significance, we did observe 53% and 41% decreases in AG0 from post-exercise to final day in the immediate and delayed MLL groups, respectively. In contrast, the no-MLL control AG0 final values showed no change from post-exercise and remained elevated four days after EEX. The percent decreases in AG0 in both the immediate and delayed groups indicate that both massage strategies reduced tissue stiffness compared to natural healing alone. The clinical importance of these findings remains unknown but worthy of investigation in a relevant human model.
Interestingly, we observed that both MLL strategies decreased AG0 within the same day (pre- to post-intervention) for all four days of tissue loading. However, the intra-day percent decreases in the delayed MLL group were not as great compared with the immediate MLL group. Additionally, MLL applied closer to EEX resulted in a greater within-day percent decrease in AG0. Furthermore, we observed a significant decrease in pre- to post-MLL AG0 in the first three days in the immediate group compared to only the first day in the delayed group. Based upon this within-day analysis, it appears that MLL has a more pronounced effect on decreasing muscle stiffness pre-MLL to post-MLL rather than having a sustained effect over the course of several days. This finding is in agreement with Chleboun et al. (1995), who investigated the effects of pneumatic compression on reducing tissue stiffness following exercise. In their study, exercise increased stiffness by 100%, but following five consecutive days of intermittent pneumatic compression, stiffness remained elevated by 60%. However, there were significant intra-day (pre-compression to post-compression) decreases two and three days post-exercise.
Exercise also had a pronounced effect on the muscle’s viscous behavior. The fast relaxation coefficient ( ) increased, while the slow relaxation coefficient ( ) decreased immediately following exercise. These findings support the Green et al. (2012) study which showed that the mean shear modulus, which corresponds to the viscous component of the muscle, was significantly greater one hour after EEX. We propose that the changes in the relaxation coefficients and after EEX are most likely due to the increase in fluid associated with the muscle’s inflammatory response. An alternative theory is that the increase in the loss modulus might be due to a localized increase in fluid and blood flow (Green et al., 2012).
We investigated the effects of EEX on the fast (τ1) and slow (τ2) relaxation time constants and observed that EEX decreased τ1 but had no effect on τ2. According to the QLV model proposed by Abramowitch and Woo (2004), τ1 corresponds to the initial slope of the relaxation and τ2 corresponds to the time to reach equilibrium. Lieber et al. (2011) proposed that many muscle components, including myoplasm, cross-bridges, and cytoskeletal proteins, contribute to the tissue’s observed viscosity, which the investigators found to be dependent on time, strain, and strain rate. However, it should be noted that the physical interpretation of the viscous modeling coefficients remain undetermined. Van Loocke et al. (2008) hypothesized that the viscoelastic component might be due to fluid movement within the muscle being constrained by the endomysium and perimysium layers when compressed in the cross-fiber direction. However, this theory is based on in vitro cuboid sections of porcine muscle subjected to ramp-and-hold relaxation tests and may not be applicable to in vivo studies. We hypothesize that the edema, inflammation, and structural damage to the tissue that accompany muscle injury would have a greater effect on the initial relaxation (τ1) rather than the slow relaxation (τ2). As fluid associated with inflammation increases within the tissue and the tissue loses its structural stability following muscle damage, the fluid would become less constrained by the surrounding connective tissues. At the commencement of the hold phase, the dissipated fluid would flow more freely, thus decreasing τ1. However, at longer hold times, the amount of time to reach equilibrium would be mostly unaffected due to loss of structural stability and thus increased fluid flow. Testing this hypothesis is outside the scope of the present study and would need to be investigated in future studies.
It should be noted that we observed large variation in the slow relaxation time constant (τ2). The great variability of the τ2 values may be attributed to several reasons. First, skeletal muscle is an anisotropic biomaterial, whose viscoelastic properties highly depend on the angle between the loading direction and the longitudinal axis of the muscle fibers (van Loocke et al. 2008, 2009). Although we tried to position the muscle so that loading was applied perpendicular to the fiber orientation for the stress-relaxation tests, it was impossible to ensure the angle was consistent amongst all measurements for all animals in the study. Secondly, skeletal muscle has a highly complex structure and composition. Previous reports (van Loocke et al., 2009) showed that multiple time constants ranging from 0.6–300s are needed to fit the viscoelastic behavior of the tissue, which indicates that there are multiple viscous elements in the muscle contributing to the time-dependent behavior (Lieber et al., 2011). Different numbers of these viscous elements may have contributed to the inter-animal variation. Finally, the variability of the viscoelastic properties may also be due to the presence of non-muscular tissues, such as skin, subcutaneous tissues, and tendons. When these viscous elements are incorporated into the subject loading area, great inter-group variation is expected amongst different animals due to differing soft tissue compositions and interactions. Despite the effects of EEX on the relaxation coefficients, neither immediate nor delayed MLL had a significant effect on the RI of the relaxation parameters over the four days of MLL ( , τ1, and τ2). In addition, we observed no consistent within-day trends due to daily MLL for any of these parameters. There we also no statistical differences due to daily MLL, suggesting that MLL had little effect in altering the relaxation behavior of the muscle-tendon complex. This finding is somewhat surprising, but we propose two different hypotheses to explain why we did not observe any differences in the relaxation parameters. First, we hypothesize that there may be some interaction between the skin, connective tissues, fat, and underlying muscle. According to previous reports by other groups, the fast and slow relaxation time constants for skin and subcutaneous tissue range from 0.09–1.38 sec and 5.286–31.559 sec, respectively (Wu et al., 2006). Although our experimental design and viscoelastic model do not allow us to take into account these various tissue properties, the in vivo testing allows for repeated measures of mechanical properties on the same tissue following EEX and after each bout of massage. Secondly, the viscoelastic properties are determined by the structure and subcellular components of the tissue. Although our previous work demonstrated that functional recovery (active muscle properties) could be improved in 5–7 days (Haas et al., 2012a), the same time course may not be sufficient enough to repair the various structural components that contribute to the viscous response (Lieber et al., 2011) in order to detect differences in the recovery of the relaxation response.
Our findings should be noted in the context of technical limitations of this study. The animals were anesthetized during all procedures and the effects of anesthesia on the measurement of passive mechanical properties and their relevance to the in vivo human condition is difficult to ascertain. We also did not directly measure the properties of the muscle tissue itself but instead measured non-invasively through the skin. Although our mechanical testing was carried out on the muscle’s mid-belly, we cannot eliminate the effects of the tendon and other connective tissues on the passive mechanical responses. In order to minimize variability, we performed the stress-relaxation testing in approximately the same spot on each animal, thus allowing us to compare differences in viscoelastic response due to exercise and massage-like intervention.
Conclusions
When analyzed over a four day period, neither immediate nor delayed MLL produced a significant effect of recovery of AG0 (instantaneous elastic response) compared to the exercised no-MLL control group. However, both MLL strategies did produce up to a 50% decrease in AG0, while the control group AG0 remained unchanged — indicating MLL had some beneficial effect in reducing stiffness compared to natural recovery. Furthermore, reduction in daily AG0 values (pre- to post-massage) was observed in both immediate and delayed MLL groups over all four days of massage. The significance of these within-day changes would need to be confirmed in humans and whether or not this leads to clinical improvement such as pain reduction or improvement in function. In contrast, the relaxation parameters showed no difference in recovery over four days of MLL application, and the daily effects of MLL on these parameters were inconclusive. Taken together, these data suggest that MLL appears to affect muscle instantaneous elastic response (stiffness) more than the viscous response, and these effects may be more pronounced within-day rather than having a sustained effect over multiple days.
Acknowledgments
Research reported in this publication was supported by the National Center for Complementary and Alternative Medicine of the National Institutes of Health under Award Number R01AT004922 and Department of Biomedical Engineering at The Ohio State University.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramowitch SD, Woo SLY. An improved method to analyze the stress relaxation of ligaments following a finite ramp time based on the quasi-linear viscoelastic theory. J of Biomechanics. 2004;34:92–97. doi: 10.1115/1.1645528. [DOI] [PubMed] [Google Scholar]
- Bosboom EM, Hesselink MK, Oomens CW, Bouten CV, Drost MR, Baaijens FP. Passive transverse mechanical properties of skeletal muscle under in vivo compression. J of Biomechanics. 2001;34:1365–1368. doi: 10.1016/s0021-9290(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Medicine and Science in Sports and Exercise. 2008;40:1289–1296. doi: 10.1249/MSS.0b013e31816c4e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chleboun, et al. Intermittent pneumatic compression effect on eccentric exercise-induced swelling, stiffness, and strength loss. Archives of Physical Medicine and Rehabilitation. 1995;76:744–749. doi: 10.1016/s0003-9993(95)80529-x. [DOI] [PubMed] [Google Scholar]
- Chleboun GS, Howell JN, Conaster RR, Giesey JJ. Relationship between muscle swelling and stiffness after eccentric exercise. Medicine and Science in Sports and Exercise. 1998;30:529–535. doi: 10.1097/00005768-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Farr T, Nottle C, Nosaka K, Sacco P. The effects of therapeutic massage on delayed onset muscle soreness and muscle function following downhill walking. J of Sci and Med in Sport. 2002;5:297–306. doi: 10.1016/s1440-2440(02)80018-4. [DOI] [PubMed] [Google Scholar]
- Fung YC. Stress strain history relations of soft tissues in simple elongation. In: Fung YC, Perrone N, Anliker M, editors. Biomechanics: Its Foundations and Objectives. Prentice Hall; NJ: 1972. pp. 181–207. [Google Scholar]
- Green MA, Sinkus R, Gandevia SC, Herbert RD, Bilston LE. Measuring changes in muscle stiffness after eccentric exercise using elastography. NMR Biomed. 2012;25:852–858. doi: 10.1002/nbm.1801. [DOI] [PubMed] [Google Scholar]
- Haas C, Butterfield TA, Abshire S, Zhao Y, Zhang X, Jarjoura D, Best TM. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med Sci Sports Exerc. 2012a doi: 10.1249/MSS.0b013e31827fdf18. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Butterfield TA, Zhao Y, Zhang X, Jarjoura D, Best TM. Dose-dependency of massage-like compressive loading on recovery of active muscle properties following eccentric exercise: rabbit study with clinical relevance. British J of Sports Med. 2013;47:83–88. doi: 10.1136/bjsports-2012-091211. [DOI] [PubMed] [Google Scholar]
- Haas C, Best TM, Wang Q, Butterfield TA, Zhao Y. In vivo passive mechanical properties of skeletal muscle improve with massage-like loading following eccentric exercise. J of Biomechanics. 2012b;45:2630–2636. doi: 10.1016/j.jbiomech.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert JE, Sforzo GA, Swensen T. The effects of massage on delayed onset muscle soreness. Br J Sports Med. 2003;37:72–75. doi: 10.1136/bjsm.37.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Howell JN, Chleboun G, Conaster R. Muscle stiffness, strength loss, swelling and soreness following eccentric exercise-induced human injury. J of Physiology. 1993;464:183–196. doi: 10.1113/jphysiol.1993.sp019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Clarkson PM. Skeletal muscle stiffness and pain following eccentric exercise of elbow flexors. Pain. 1987;30:233–242. doi: 10.1016/0304-3959(87)91079-7. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil. 2002;81:S70–S79. doi: 10.1097/00002060-200211001-00008. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Meyer GA, McCulloch AD. A nonlinear model of passive muscle viscosity. J of Biomechanical Engineering. 2011;133:091007. doi: 10.1115/1.4004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MP, Connolly DAJ, Eston RG, Gleim GW. Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Medicine. 1999;27:157–170. doi: 10.2165/00007256-199927030-00002. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Leonard TR. An implantable electrical interface for in vivo studies of the neuromuscular system. J of Neuroscience Methods. 1996;70:27–32. doi: 10.1016/S0165-0270(96)00099-4. [DOI] [PubMed] [Google Scholar]
- Page P. Pathophysiology of acute exercise-induced muscular injury: Clinical implications. J of Athletic Training. 1995;30:29–34. [PMC free article] [PubMed] [Google Scholar]
- Palevski A, Glaich I, Portnoy S, Linder-Ganz E, Gefen A. Stress relaxation of porcine gluteus muscle subjected to sudden transverse deformation as related to pressure sore modeling. J of Biomechanical Engineering. 2006;128:782–787. doi: 10.1115/1.2264395. [DOI] [PubMed] [Google Scholar]
- Pousson M, Van Hoecke J, Goubel F. Changes in elastic characteristics of human muscle induced by eccentric exercise. J of Biomechanics. 1990;23:343–348. doi: 10.1016/0021-9290(90)90062-8. [DOI] [PubMed] [Google Scholar]
- Smith, et al. The effects of athletic massage on delayed onset muscle soreness, creatine kinase, and neutrophil count: A preliminary report. J of Orthapaedic & Sports Physical Therapy. 1994;19:93–99. doi: 10.2519/jospt.1994.19.2.93. [DOI] [PubMed] [Google Scholar]
- Van Loocke M, Lyons CG, Simms CK. A validated model of passive muscle in compression. J of Biomechanics. 2006;39:2999–3009. doi: 10.1016/j.jbiomech.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Van Loocke M, Lyons CG, Simms CK. Viscoelastic properties of passive skeletal muscle in compression: Stress relaxation behaviour and constitutive modeling. J of Biomechanics. 2008;41:1555–1566. doi: 10.1016/j.jbiomech.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Van Loocke M, Simms CK, Lyons CG. Viscoelastic properties of passive skeletal muscle in compression—Cyclic behavior. J of Biomechanics. 2009;42:1038–1048. doi: 10.1016/j.jbiomech.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zeng H, Best TM, Haas C, Heffner NT, Agarwal S, et al. A mechatronic system for quantitative application and assessment of massage-like actions in small animals. Ann Biomed Eng. 2013 doi: 10.1007/s10439-013-0886-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35:235–256. doi: 10.2165/00007256-200535030-00004. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Weerakkody NS, Gregory JE, Morgan DL, Proske U. Changes in passive tension of muscle in humans and animals after eccentric exercise. J of Physiology. 2001;533:593–604. doi: 10.1111/j.1469-7793.2001.0593a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JZ, Cutlip RG, Welcome D, Dong RG. Estimation of the viscous properties of skin and subcutaneous tissue in uniaxial stress relaxation tests. Bio-Medical Materials and Engineering. 2006;16:53–66. [PubMed] [Google Scholar]
- Zainuddin Z, Newton M, Sacco P, Nosaka K. Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J of Athletic Training. 2005;40:174–180. [PMC free article] [PubMed] [Google Scholar]