Abstract
Hydrogels have been extensively used for regenerative medicine strategies given their tailorable mechanical and chemical properties. Gene delivery represents a promising strategy by which to enhance the bioactivity of the hydrogels, though the efficiency and localization of gene transfer have been challenging. Here, we functionalized porous poly(ethylene glycol) hydrogels with heparin-chitosan nanoparticles to retain the vectors locally and enhance lentivirus delivery while minimizing changes to hydrogel architecture and mechanical properties. The immobilization of nanoparticles, as compared to homogeneous heparin and/or chitosan, is essential to lentivirus immobilization and retention of activity. Using this gene-delivering platform, we over-expressed the angiogenic factors sonic hedgehog (Shh) and vascular endothelial growth factor (Vegf) to promote blood vessel recruitment to the implant site. Shh enhanced endothelial recruitment and blood vessel formation around the hydrogel compared to both Vegf-delivering and control hydrogels. The nanoparticle-modified porous hydrogels for delivering gene therapy vectors can provide a platform for numerous regenerative medicine applications.
Keywords: hydrogel, vascularization, lentivirus
Introduction
Swift, robust vascularization of the implant is required in regenerative medicine in order to support the survival and function of endogenous or transplanted cells [1-4]. Hydrogels have traditionally supported cell infiltration by providing sites for cell adhesion, and by their degradation can create space for cells to enter. More recently, cell infiltration has been facilitated through the creation of porous hydrogels, as cells can readily infiltrate through the pores without the requirement of degrading the hydrogel [5]. Furthermore, cell infiltration has been enhanced through the application of drug delivery technology, which has promoted processes such as vascular ingrowth [6-10]. Many technologies have delivered factors for time scales of days to weeks; however, delivering for long time scales [5] or delivering a combination of factors [11-14], which may be necessary for some applications, has been challenging.
Gene delivery provides the opportunity to obtain sustained expression for time scales consistent with the time required for tissue regeneration, as transduction of endogenous cells permits the expression of transgenes for months after delivery [15, 16]. Microporous scaffolds have been extensively investigated for gene delivery for numerous applications [16-18]. Microporous poly(lactide-co-glycolide) (PLG) scaffolds have been used to provide sustained release, and upon modification, to reversibly associate with the vector and enhance vector retention [15, 16, 19]. However, in our laboratory, gene delivery from hydrogels has been less effective in vivo relative to delivery from PLG scaffolds. The inclusion of micropores within the hydrogel led to enhanced transduction through increased cell infiltration [5]. Furthermore, the inclusion of inorganic nanoparticles that reversibly associate with the vector have increased the vector half-life and maintained greater concentrations within the material [15]. While these strategies have enhanced transgene expression, the opportunity remains to further enhance gene delivery from hydrogels.
In this report, our objective was to investigate the incorporation and properties of heparin and chitosan, separately and in combination, to immobilize lentivirus onto porous, poly(ethylene glycol) (PEG) hydrogels in order to promote localized, sustained over-expression of regenerative factors. We recently reported that surface modification of PLG scaffolds with heparin and chitosan to reversibly associate with viral vectors retained the vectors locally, enhanced the vector half-life, and increased transgene expression [16]. We hypothesized that the manner in which heparin and chitosan were included (i.e., a component of the bulk hydrogel, or as nanoparticles) would influence the interaction with lentivirus and subsequent gene transfer. Vector binding and activity were investigated in vitro, along with the extent and duration of transgene expression in vivo. Lentivirus encoding for sonic hedgehog (Shh) and vascular endothelial growth factor (Vegf) were delivered, both of which have been implicated in angiogenesis [11-14], and their ability to enhance host integration and vascularization of the hydrogel were assessed for their future use in regenerative medicine applications.
Materials and Methods
Formation and Characterization of Hydrogels
Fabrication: PEG-acrylate (4 arm, 20,000 Da; Laysan biomaterials; 10% w/v) was dissolved into PBS with photoinitiator (Irgacure 2959, Ciba; 0.5% w/v). To form porous hydrogels, the solution was frozen for 16 hours at −20 °C and then exposed while frozen to UV light (365 nm, 50 mW/cm2; 2 min). To form non-porous hydrogels, UV exposure was employed to crosslink the liquid-phase PEG solution. The formed hydrogel was then immersed in PBS or distilled water until use.
Mesh Size and Swelling Ratio: PEG hydrogels were formed as before (n = 4 per condition), weighed, and placed in distilled water. After 24 hours, the hydrogels were weighed, frozen in liquid nitrogen, lyophilized for 24 hours, and weighed again. Swelling ratio and mesh size was approximated using a modified Flory-Rehner model [20-22].
Cysteine Addition of Heparin and Chitosan
Heparin (180 USP/mg, ~16,000 Da [22]) and chitosan (8183 Da) were stirred for 30 min in varying ratios of cysteines in 1 M 2-(N-morpholino)ethanesulfonic acid buffer (1 mg/mL polysaccharide) in the presence of 9 mg/mL N-ethyl-N′-(3-(dimethylamino)propyl)carbodiimide (EDC; CreoSalus Inc., Louisville, KY) and 6 mg/mL N-hydroxysuccinimide (NHS; Research Organics, Cleveland, OH). Unbound cysteines were removed using 10,000 and 3,000 molecular weight cutoff Slide-A-Lyzer Dialysis Cassettes (Pierce), respectively. Incorporation of cysteines on the polysaccharides was assessed by measuring the absorbance of the modified polysaccharides in Ellman's (Pierce) solution at 412 nm [23], and the extinction of absorbance in toluidine blue (Fisher Scientific) and orange II assay solutions at 610 nm and 480 nm [16] as described previously. For hydrogel incorporation, filtered solutions were flash frozen in nitrogen, lyophilized, and stored with dessicants until use. All materials are from Sigma unless otherwise indicated.
Heparin-chitosan Nanoparticles
Heparin and chitosan solutions (0.9 mg/mL ea. in 2% v/v acetic acid) were dissolved, mixed in varying ratios, and stirred for 2 hours. Aggregates were removed using a 0.22 μm filter. Size and charge density of nanoparticles were assessed using the Zetasizer Nano ZSP (Malvern Instruments). For hydrogel incorporation, filtered solutions were flash frozen in nitrogen, lyophilized, and stored with dessicants until use.
Incorporation and retention of nanoparticles were assessed using the toluidine blue assay [24]. Solutions of unbound nanoparticles were assayed by immersing the supernatant for 1 hour in toluidine blue (Fisher Scientific). Assay solutions were made according to previously established methods [16]. Incorporation was defined as the percentage of nanoparticles remaining after two 5 min washes, consistent with prior reports [16, 19]. Retention was defined as the percentage remaining of incorporated nanoparticles. Release rate is defined as the difference in nanoparticle retention at the specified time period divided by that time period (in days).
Lentivirus Incorporation and in vitro Expression
To make cell-compatible porous PEG hydrogels, precursor solution (1% w/v PEG, 0.5% w/v photoinitiator in PBS) was conjugated to GCYKNRGCYKNRCGRGD (5 mM, fabricated at the Northwestern University peptide synthesis core) by Michael-type addition at 37 °C for 10 min to promote cell-adhesi on and incorporate plasmin-degradable sites, frozen at −20 °C overnight, and e xposed to UV light (365 nm, 50 mW/cm2) for 2 min. Hydrogels were washed in PBS (twice, 5 min ea.) and partially dried in air to facilitate wetting of the surface by lentivirus (1×107-1×108 particles encoding for firefly luciferase) pipetted onto the gel. To evaluate in vitro expression lentivirus-incorporated hydrogels were seeded with 100,000 human embryonic kidney cells and imaged as done previously [16]. In brief, the hydrogels were incubated with 50 mM of D-luciferin (Molecular Therapeutics, Inc., MI) for 4 hours and imaged with two 10-second intervals for bioluminescence (integrated photon flux, p/s), which was assessed using the In Vivo Imaging System (IVIS; Caliper, Hopkinton, MA, USA) [16].
Subcutaneous Implantation
Animals were treated according the Animal Care and use Committee guidelines at Northwestern University. Surgery was performed as previously described (n = 4 per hydrogel design and time-point) [5, 25]. Male CD1 mice (30g, Charles River) were anesthetized using isoflurane (2% v/v). An incision was made in the upper and lower back to allow for implantation of lentivirus-incorporated hydrogels that were stored at -80 °C until use. The site was secured in place by s uturing the skin together and stapling the skin. Postoperative care consisted buprenorphine (0.1 mg/kg) administered immediately after surgery. To quantify gene expression, animals implanted with hydrogels delivering firefly luciferase-encoding lentivirus were injected intraperitoneally with 150 mg/kg body weight of D-Luciferin and imaged using the IVIS at 5 minute intervals until expression peaked as previously reported [16].
Immunohistochemistry: Hematoxylin and Eosin
Hydrogels were implanted for 8 weeks. Hydrogels extracted from mice were fixed using 4% w/v paraformaldehyde (Sigma-Aldrich), embedded in sucrose O.C.T. and frozen as done previously [5], and sectioned transversally in 18 μm thick slices and collected serially. To detect overall cell presence these sections were stained with eosin and counter-stained with Mayer's hematoxylin (Surgipath Medical Industries). Images were captured at 5× magnification for light microscopy (Leica Microsystems, Wetzlar, Germany).
Immunofluorescence: Vascularization
Upon retrieval of the hydrogels, mice were injected by tail vein with biotinylated lectin (175 μL at 1 mg/mL). To assess vascularization, CD31 (Abcam, ab56299; 1:400 dilution) was used with AlexaFluor 555 goat anti-rabbit IgG (1:500 dilution) and fluorescein anti-biotin IgG (1:200 dilution) secondaries to label infiltrating endothelial cells and functional blood vessels, respectively. Images were captured at 10× magnification for fluorescence microscopy (Leica Microsystems, Wetzlar, Germany) and the outer 20 μm perimeter of the hydrogel was assessed for lectin and CD31 presence.
Statistics
One- and two-way ANOVA with Bonferroni post-hoc analysis were used to assess statistical differences.
Results
Heparin- and Chitosan-functionalized PEG Hydrogels
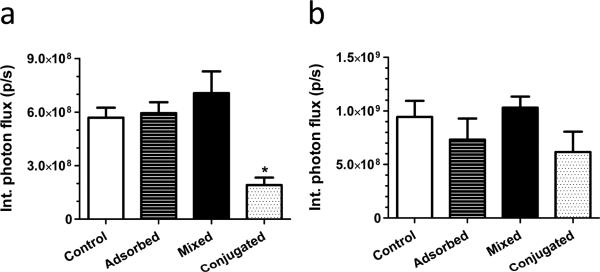
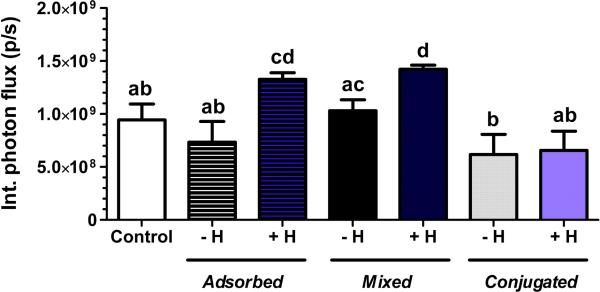
Heparin and chitosan were investigated as potential lentivirus immobilization agents that minimally influenced the bulk properties of porous PEG hydrogels. Initially, the method of incorporation of these polysaccharides was assessed for their ability to promote gene transfer in vitro (Figure 1): adsorption of unmodified polysaccharides after hydrogel formation, mixing unmodified polysaccharides before formation, or conjugating cysteine-modified polysaccharides using Michael-type addition before formation. Cysteines were added to the polysaccharides in varying proportions using EDC/NHS chemistry to facilitate their incorporation into PEG (Supp. 1a). The extent of cysteine conjugation increased linearly for heparin (6.4-fold) and chitosan (4.5-fold) as the ratio of cysteine:polysaccharide ranged from 250 to 1000. Hydrogels formed with at a ratio of cysteine:polysaccharide equal to 1000 swelled to a final volume that was larger than unmodified hydrogels (Supp. 1d,e), yet a significant difference was not observed at ratios for cysteine:polysaccharide equal to 500 (Supp. 1b,c). Expression levels were not significantly different between control hydrogels, which did not contain polysaccharides, and hydrogels that incorporated polysaccharides by adsorption or mixing. Expression levels for hydrogels that incorporated polysaccharides by conjugation onto the hydrogel were reduced relative to other conditions, with the greatest reductions for conjugation of heparin (Figure 1). In the case of heparin, extensive cysteine modification to increase heparin loading decreased transgene expression (Supp. 2). We next investigated incorporating both heparin and chitosan into PEG hydrogels, which yielded the highest gene expression when mixing or adsorbing the unmodified polysaccharides and lower expression when conjugated onto the hydrogels using cysteines (Figure 2), consistent with hydrogels in which heparin and chitosan were incorporated alone.
Figure 1.
Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 2 days of cell culture on porous PEG hydrogels containing 25 μg of (a) heparin or (b) chitosan. Polysaccharides were incorporated either by adsorption of unmodified polysaccharides after hydrogel formation, mixing of unmodified polysaccharides into the polymer solution before formation (mixed), or conjugating cysteine-modified polysaccharides using Michael-type addition before formation (conjugated). Control hydrogels do not contain polysaccharides. * indicates statistically significant difference to the other groups (p < 0.05).
Figure 2.
Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 2 days of cell culture on porous PEG hydrogels containing 25 μg of chitosan with or without 25 μg of heparin. Polysaccharides were incorporated either by adsorption of unmodified polysaccharides after hydrogel formation (adsorbed), mixing of unmodified polysaccharides into the polymer solution before formation (mixed), or conjugating cysteine-modified polysaccharides using Michael-type addition before formation (conjugated). -H indicates hydrogels with only chitosan. +H indicates hydrogels with both chitosan and unmodified heparin. Control hydrogels do not contain polysaccharides. Different letter designations indicate statistically significant differences (p < 0.05).
Nanoparticle-modified Hydrogels
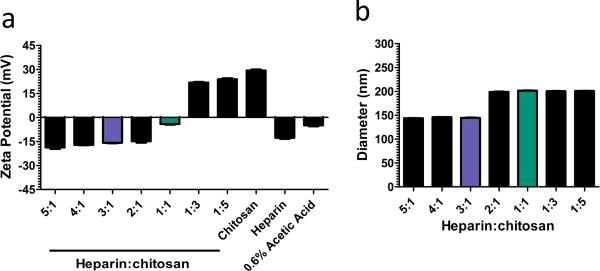
Heparin-chitosan nanoparticles (HCNPs) were subsequently investigated as a means to immobilize lentivirus within porous PEG hydrogels, with initial studies investigating the fabrication and incorporation of HCNPs into PEG hydrogels. Nanoparticles were fabricated using multiple ratios of heparin and chitosan solutions ranging from 5:1 to 1:5. For this range, the charge densities of HCNPs varied from -19 mV to +23 mV (Figure 3a), and after filtration, the nanoparticle diameters were between 143 and 202 nm (Figure 3b). HCNPs fabricated with 3:1 (-17 mV) and 1:1 (-4 mV) ratios of heparin-to-chitosan (25 μg to 250 μg) were incorporated within porous PEG hydrogels and their retention was assessed (Table 1). Neither particle amount nor charge affected incorporation and retention. On average, 95.6% of nanoparticles (by weight) were incorporated into the PEG hydrogels after washing. A majority of the incorporated HCNPs (89.2%) was retained through 14 days, with most of the release occurring within the initial 24 hours.
Figure 3.
(a) Zeta potential of heparin-chitosan nanoparticles (HCNPs) formulated at multiple ratios of heparin to chitosan (0.9 mg/mL total polysaccharide content). Chitosan (0.9 mg/mL. CHI), heparin (0.9 mg/mL, HEP) and plain acetic acid (0.6% AA) solutions served as a reference. (b) Mean diameter of HCNPs formulated at multiple ratios after filtering with 0.22 μm mesh.
Table 1.
Incorporation and retention of HCNPs (25 μg - 250 μg total polysaccharide content) in PEG hydrogels. Incorporation was defined as % remaining nanoparticles after two 5 min washes. Retention was defined as % remaining of incorporated nanoparticles. Release rate is defined as the difference in nanoparticle retention at the specified time period divided by that time period (in hours).
| HCNP | INCORP. | RETENTION | RELEASE RATE (per hour) | HCNP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Mixture | 0.2 hr | 3 hr | 24 hr | 336 hr | 0.2-3 hr | 3-24 hr | 24-336 hr | Mixture | Initial |
| 25 μg | 3:1 | 94.1% | 93.4 % | 89.7% | 88.0% | 2.4% | 0.2% | 0.01% | 3:1 | 25 μg |
| 1:1 | 96.3% | 97.2% | 89.0% | 87.9% | 1.0% | 0.4% | 0.00% | 1:1 | ||
| 50 μg | 3:1 | 95.6% | 93.2% | 89.2% | 88.7% | 2.4% | 0.2% | 0.00% | 3:1 | 50 μg |
| 1:1 | 96.5% | 96.6% | 90.3% | 89.1% | 1.2% | 0.3% | 0.00% | 1:1 | ||
| 250 μg | 3:1 | 92.9% | 95.0% | 92.7% | 91.3% | 1.8% | 0.1% | 0.00% | 3:1 | 250 μg |
| 1:1 | 97.9% | 97.6% | 91.8% | 89.6% | 0.9% | 0.3% | 0.01% | 1:1 | ||
We subsequently investigated the effect of nanoparticle incorporation on hydrogel pore structure and properties (Supp. 3). HCNPs were visualized with toluidine blue dye, which turns purple in the presence of heparin. For loadings of 25 μg and 50 μg of HCNPs, the purple staining was observed throughout the gel around unstained regions that were likely pores within the hydrogel. At 250 μg of HCNPs, staining was sporadic likely reflecting a loss of the porous architecture. Swelling ratios of hydrogels with 0 μg to 50 μg HCNPs were similar. In contrast, hydrogels with 250 μg of HCNPs had swell ratios nearing non-porous PEG hydrogels. Cells seeded onto the hydrogels had a consistent density across the surface and appeared to be unaffected by nanoparticle content up to 50 μg (Supp. 4). Based on these observations, hydrogels loaded with 50 μg of HCNPs were used for the remaining studies.
Lentivirus Immobilization and Expression using Heparin-chitosan Nanoparticles
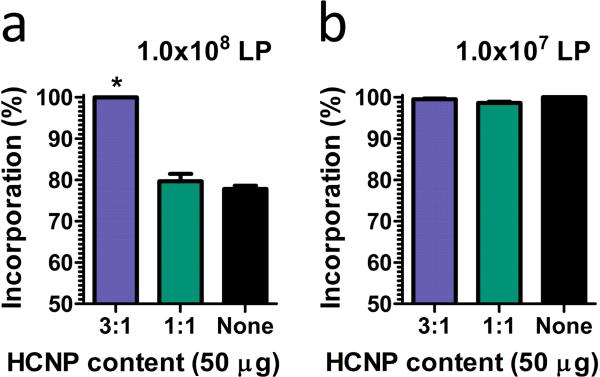
Two formulations of HCNPs, 3:1 and 1:1 heparin-to-chitosan ratios, were assessed for their ability to interact with lentivirus particles (Figure 4). At a loading of 107 lentiviral particles, the loading efficiency surpassed 98% for all hydrogel formulations. However, at a loading of 108 lentiviral particles, only the heparin-rich (3:1) HCNP-loaded hydrogels were able to capture greater than 98% of the lentivirus particles, whereas the control hydrogels or hydrogels loaded with the other HCNP formulation captured less than 80% (p < 0.05). This observation is consistent with a prior report, which revealed enhanced incorporation of the lentivirus due to enhanced binding and retention in the presence of chitosan and heparin [16]. Subsequent studies used a loading of 107 lentiviral particles in order to compare transduction at similar particle loadings.
Figure 4.
Incorporation efficiency of porous PEG hydrogels loaded with (a) 1 × 108 and (b) 1 × 107 lentiviral particles (LP). * indicates statistically significant difference of negatively-charged (3:1) HCNP-functionalized hydrogels to neutrally-charged (1:1) HCNP-functionalized hydrogels and unmodified hydrogels (p < 0.05).
The dynamics of lentivirus-mediated gene expression were subsequently characterized for 5 days of in vitro cell culture (Figure 5). After 1 day of culture, transgene expression by cells on unmodified hydrogels was similar to transgene expression for cells on HCNP-functionalized hydrogels. By day 3 and 5 of culture, gene expression had increased relative to day 1 for both conditions. Expression by cells on HCNP-functionalized hydrogel was significantly increased compared to unmodified hydrogel (p < 0.05). We subsequently investigated whether differences in gene expression were due to differences in cell proliferation. Calcein-AM and Ethidium homodimer staining (30 min incubation at 37 °C, 2 μM each) at 1, 3, and 5 days revealed similar cell densities on unmodified and HCNP-functionalized hydrogel at all time-points (Figure 5b-g).
Figure 5.
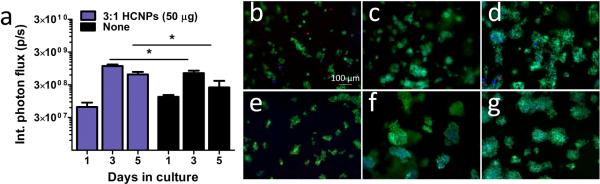
(a) Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) from day 1 to day 5 on (3:1) HCNP-functionalized and unmodified porous PEG hydrogels. * indicates statistically significant difference (p < 0.05). Cell content, as assessed by a live/dead assay, of cells seeded onto (b-d) unmodified or (e-g) HCNP-functionalized hydrogels. Viable cells on (b,e) day 1, (c,f) day 3, and (d,g) day 5.
The retention of active virus on the hydrogel was assessed by characterizing the expression by that successfully adhered onto the hydrogel (termed ‘seeded’) and cells within the well (termed ‘non-seeded’) after 3 days of culture (Supp. 5). Seeded cells on hydrogels functionalized with HCNPs had significantly enhanced gene expression relative to hydrogels without HCNPs. In contrast, ‘non-seeded’ cells from HCNP-functionalized hydrogels had lower gene expression compared to unmodified hydrogels. The total expression, i.e. the sum of expressions from both ‘non-seeded’ and ‘seeded’ cells, was similar in HCNP-functionalized and unmodified hydrogels. These observations suggest that the shift in gene expression—from ‘non-seeded’ cells that were exposed to the first 4 hours of released lentivirus to ‘seeded’ cells—when hydrogels are functionalized with HCNPs is due to the retention of lentivirus on the hydrogel. Figures 4 and 5, when considered with Supp. 5, reveal changes in gene expression profiles in vitro that result from enhanced retention and transduction of lentivirus, and not greater cell growth, in HCNP-functionalized hydrogels, which is consistent with a prior report in which these polysaccharides were shown to immobilize the lentivirus [16]. As cells adhere to the hydrogel, the vector is present at the hydrogel to which cells are adhered, which concentrates the vector within the cell microenvironment [26]. This high concentration within the cell environment leads to cell binding, internalization, and ultimately expression of the transgene.
In vivo Gene Expression and Vascularization
We subsequently assessed the extent and duration of transgene expression in vivo (Figure 6). For the initial 2 weeks, unmodified hydrogels had greater levels of expression than HCNP-loaded hydrogels. However, by four weeks, the HCNPs loaded hydrogels had greater expression than unmodified hydrogels. Unmodified hydrogels had expression that increased through 2 weeks, and subsequently declined over the subsequent 5 weeks. Interestingly the HCNP-loaded hydrogels produced more consistent expression throughout the 8 weeks of the study.
Figure 6.
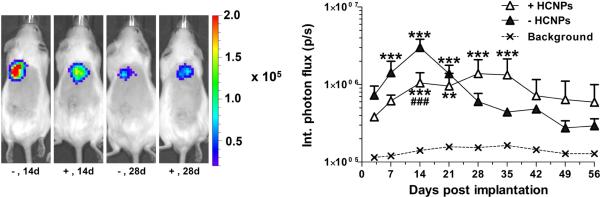
Gene expression (integrated photon flux) after subcutaneous implantation of lentivirus-delivering (1 × 107 particles) porous PEG hydrogels in the upper back for 8 weeks. * indicates statistically significant difference (p < 0.05) to background levels. # indicates statistically significant difference (p < 0.05) of (3:1) HCNP-functionalized hydrogels to unmodified hydrogels. Doubling and tripling of symbols indicates differences significant at the p < 0.01 and p < 0.001 levels, respectively.
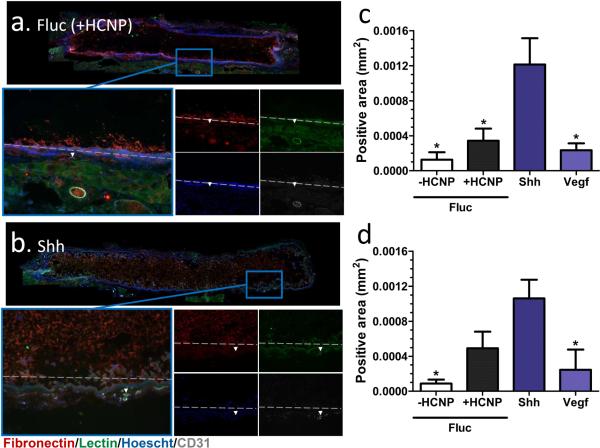
Hydrogels with nanoparticles were functionalized with lentivirus particles encoding for sonic hedgehog (Shh), vascular endothelial growth factor (Vegf), or firefly luciferase (Fluc, as a control) and implanted subcutaneously for 8 weeks (Figure 7). Delivery of Shh or Vegf increased cell infiltration into the pores of the hydrogel. Furthermore, Shh increased the presence of endothelial (CD31+) and blood vessels (lectin+) around the hydrogel, with the area of positive staining increased by approximately 3-fold relative to the Fluc control. However, Vegf did not significantly affect endothelial cell or blood vessel presence compared to controls, possibly due to the formation of immature vessels that regressed by 8 weeks [27-29].
Figure 7.
Vascularization after subcutaneous implantation of lentivirus-delivering (1 × 107 particles) porous PEG hydrogels in the upper back for 8 weeks. Hoechst+ cell (blue) and fibronectin+ (white) infiltration of the hydrogel, lectin+ blood vessel (green) and CD31+ endothelial (white) recruitment to the implantation site in the presence of (a) firefly luciferase (Fluc, control) or (b) sonic hedgehog (Shh). Quantification of (c) CD31+ and (d) lectin+ area surrounding the hydrogel after delivery of Fluc, Shh or vascular endothelial growth factor (Vegf). * indicates statistically significant difference to Shh-delivering hydrogels (p < 0.05).
Discussion
In this report, we demonstrated that the incorporation of HCNPs could significantly enhance the binding and retention of lentiviruses leading to prolonged transgene expression in vivo. These studies highlight that these components are more effective when formulated as nanoparticles for incorporation into the gel as opposed to incorporation of the components individually. Both polysaccharides were modified with varying amounts of cysteines in order to enhance their ability to bind to and crosslink PEG monomers using Michael-type addition. When incorporated in to the PEG hydrogel separately, neither polysaccharide significantly enhanced expression. For heparin, transgene expression was actually significantly inhibited at the highest extent of cysteine modification, which likely results from limited access of the cell to the virus for incorporation in this manner. Previously, heparin-chitosan complexes were revealed to enhance gene expression of poly(lactide-co-glycolide) hydrogels in vitro and in vivo [16]. Herein, heparin and chitosan were mixed at varying ratios to create nanoparticles (< 220 μm in diameter) of multiple charge densities. The incorporation and retention of nanoparticles onto PEG hydrogels was not dependent on their charge. Nanoparticles in quantities of 50 μg or less could be incorporated into the hydrogels with minimal change to mechanical properties such as porosity and swelling ratio. Lentivirus incorporation onto PEG hydrogels, however, was significantly improved using negatively charged nanoparticles (3:1 ratio of heparin-to-chitosan, -17 mV).
Transgene expression from our PEG hydrogels was increased both in vitro and in vivo in the presence of HCNPs, consistent with the observations that interactions between the vector and the biomaterial can modulate and localize transgene expression [5, 16, 25, 30]. In vitro gene expression was higher in PEG hydrogels with negatively-charged heparin-chitosan nanoparticles than unmodified hydrogels. Additionally, the amount of virus released from the hydrogel was reduced, as evidenced by a substantial reduction in the transduction of cells that were in the well but not on the hydrogel. In contrast, the unmodified hydrogel had greater expression at early time points and had greater transduction of cells not on the hydrogel. The transgene expression profile observed in vitro was similar to that observed in vivo. At a subcutaneous site, unmodified hydrogels, which achieved greater expression at earlier time-points relative to modified hydrogels, had expression decline after 4 weeks, which is consistent with previous observations [30] and suggests the transduction of inflammatory cells that can be transiently present after biomaterial implantation. In contrast, nanoparticlefunctionalized hydrogels had a gradual increase in expression that plateaued after 4 weeks. These studies reveal that retention of lentivirus on PEG hydrogels using nanoparticles resulted in more sustained gene expression at the hydrogel in vitro and in vivo.
Nanoparticle-functionalized PEG hydrogels implanted subcutaneously for 8 weeks demonstrated enhanced vascular growth with expression of Shh. Shh has been shown both in vitro [11, 12] and in vivo [13, 14] to induce the expression of vascular endothelial growth factor, as well as factors known to promote the maturation and stabilization of blood vessels. Shh enhanced cell infiltration into the hydrogels. Shh recruited CD31+ endothelial cells to the implant site, which contributed to an increase in lectin+ functional blood vessels, consistent with previous reports [11-14]. In contrast, after 8 weeks with induced expression of Vegf, endothelial cells were not observed to be present in greater numbers at the implantation site compared to controls. Furthermore, the number of lectin+ blood vessels was also similar to control conditions. The inability of Vegf to promote blood vessel formation using our gene-delivering system may be due to the requirement of co-factors to mature and stabilize blood vessels promoted using Vegf [11-14]. More generally, the induced overexpression of an angiogenic factor can lead to abnormal vessel formation, that may regress with time [31]. Taken together, this report reveals the potential of Shh as a pro-angiogenic factor for use in regenerative medicine.
Conclusions
We assessed a novel gene delivery platform for porous PEG hydrogels that can promote and localize lentivirus-mediated transgene expression. Chitosan and heparin promoted lentivirus-mediated gene delivery only when used in combination. Interestingly, the method of incorporating these polysaccharides into the hydrogel dictated their ability to promote transgene expression. Heparin and chitosan incorporated as individual or combined components through blending or conjugation to the gel did not significantly enhance gene delivery. However, their formulation as nanoparticles led to more effective lentivirus association with the hydrogel and sustained transgene expression in vitro and in vivo. Shh expression promoted vascularization of the implantation site at 8 weeks post implantation, which in combination with other factors, can be used to promote regeneration in a variety of applications.
Supplementary Material
Supp. 1. (a) Functionalization of heparin and chitosan with cysteine using EDC/NHS chemistry. Visualization of polysaccharide incorporation to porous PEG hydrogels when 25 μg of (c) heparin (1:500 ratio of polysaccharide-to-cysteine) or (d) chitosan (1:1000 ratio of polysaccharide-to-cysteine) is added using the Toluidine blue and Orange II assays respectively. Toluidine blue is (c) purple when heparin is present and (b) blue for controls. Orange II is a (e) dark orange when chitosan is present and (d) yellow-orange for controls.
Supp. 2. Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 2 days of cell culture on porous PEG hydrogels containing 25 μg of heparin after functionalization with cysteine using EDC/NHS chemistry. Different letter designations indicate statistically significant differences (p < 0.05).
Supp. 3. Visualization of HCNPs in porous PEG hydrogels loaded with (a) 0 μg, (b) 25 μg, (c) 50 μg, and (d) 250 μg of HCNPs (3:1). Change in color from (a) blue to (b-d) purple using the Toluidine Blue assay reveals the presence of HCNPs. (e) Swell ratio of these hydrogels compared to a non-porous PEG hydrogel.
Supp. 4. Visualization of the seeding efficiency of porous PEG hydrogels with (a) 0 μg, (b) 25 μg, (c) 50 μg, and (d) 250 μg of HCNP content (3:1) stained using Calcein-AM and Ethidium homodimer-1 counterstained with Hoechst.
Supp. 5. Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 3 days of cell culture in seeded, non-seeded, and total (seeded + non-seeded) cells. * indicates statistically significant difference (p < 0.05).
Acknowledgements
This work was supported by the NIH (RO1 EB005678, R21 EB006520, RO1 EB003806, RO1 CA173745). Imaging work was performed at the Northwestern University Center for Advanced Molecular Imaging generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Laporte L, des Rieux A, Tuinstra HM, Zelivyanskaya ML, De Clerck NM, Postnov AA, et al. Vascular endothelial growth factor and fibroblast growth factor 2 delivery from spinal cord bridges to enhance angiogenesis following injury. Journal of Biomedical Materials Research Part A. 2011;98A:372–82. doi: 10.1002/jbm.a.33112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Min J-Y, Rana JS, Ke Q, Cai J, Chen Y, et al. VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC-differentiated cells. Journal of Applied Physiology. 2002;93:1140–51. doi: 10.1152/japplphysiol.00307.2002. [DOI] [PubMed] [Google Scholar]
- 3.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, et al. Influence of VEGF on the Viability of Encapsulated Pancreatic Rat Islets After Transplantation in Diabetic Mice. Cell Transplantation. 2003;12:627–35. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 4.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated Vascular Endothelial Growth Factor Production in Islets Improves Islet Graft Vascularization. Diabetes. 2004;53:963–70. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 5.Shepard JA, Virani FR, Goodman AG, Gossett TD, Shin S, Shea LD. Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials. 2012;33:7412–21. doi: 10.1016/j.biomaterials.2012.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman I, Cohen S. The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization. Biomaterials. 2009;30:2122–31. doi: 10.1016/j.biomaterials.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 7.Freeman I, Kedem A, Cohen S. The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins. Biomaterials. 2008;29:3260–8. doi: 10.1016/j.biomaterials.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Wu BM, Dunn JCY. The enhancement of VEGF-mediated angiogenesis by polycaprolactone scaffolds with surface cross-linked heparin. Biomaterials. 2011;32:2059–69. doi: 10.1016/j.biomaterials.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.des Rieux A, Ucakar B, Mupendwa BPK, Colau D, Feron O, Carmeliet P, et al. 3D systems delivering VEGF to promote angiogenesis for tissue engineering. Journal of Controlled Release. 2011;150:272–8. doi: 10.1016/j.jconrel.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Silva E, Yuen W, Mooney D. Spatio–temporal VEGF and PDGF Delivery Patterns Blood Vessel Formation and Maturation. Pharmaceutical Research. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 11.Dohle E, Fuchs S, Kolbe M, Hofmann A, Schmidt H, Kirkpatrick CJ. Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur Cell Mater. 2011;21:144–56. doi: 10.22203/ecm.v021a12. [DOI] [PubMed] [Google Scholar]
- 12.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic Hedgehog Induces Capillary Morphogenesis by Endothelial Cells through Phosphoinositide 3-Kinase. Journal of Biological Chemistry. 2003;278:8244–9. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 13.Coultas L, Nieuwenhuis E, Anderson GA, Cabezas J, Nagy A, Henkelman RM, et al. Hedgehog regulates distinct vascular patterning events through VEGF-dependent and -independent mechanisms. Blood. 2010;116:653–60. doi: 10.1182/blood-2009-12-256644. [DOI] [PubMed] [Google Scholar]
- 14.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 15.Boehler RM, Shin S, Fast AG, Gower RM, Shea LD. A PLG/HAp composite scaffold for lentivirus delivery. Biomaterials. 2013;34:5431–8. doi: 10.1016/j.biomaterials.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas AM, Shea LD. Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. J Control Release. 2013;170:421–9. doi: 10.1016/j.jconrel.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin S, Salvay DM, Shea LD. Lentivirus delivery by adsorption to tissue engineering scaffolds. Journal of Biomedical Materials Research Part A. 2010;93A:1252–9. doi: 10.1002/jbm.a.32619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuinstra HM, Aviles MO, Shin S, Holland SJ, Zelivyanskaya ML, Fast AG, et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials. 2012;33:1618–26. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin S, Tuinstra HM, Salvay DM, Shea LD. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials. 2010;31:4353–9. doi: 10.1016/j.biomaterials.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–57. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 22.Peppas NA, Merrill EW. Poly(vinyl alcohol) hydrogels: Reinforcement of radiation-crosslinked networks by crystallization. Journal of Polymer Science: Polymer Chemistry Edition. 1976;14:441–57. [Google Scholar]
- 23.Shepard JA, Wesson PJ, Wang CE, Stevans AC, Holland SJ, Shikanov A, et al. Gene therapy vectors with enhanced transfection based on hydrogels modified with affinity peptides. Biomaterials. 2011;32:5092–9. doi: 10.1016/j.biomaterials.2011.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith PK, Mallia AK, Hermanson GT. Colorimetric method for the assay of heparin content in immobilized heparin preparations. Analytical Biochemistry. 1980;109:466–73. doi: 10.1016/0003-2697(80)90679-x. [DOI] [PubMed] [Google Scholar]
- 25.Kidd ME, Shin S, Shea LD. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. Journal of Controlled Release. 2012;157:80–5. doi: 10.1016/j.jconrel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Laporte L, Shea LD. Matrices and scaffolds for DNA delivery in tissue engineering. Advanced Drug Delivery Reviews. 2007;59:292–307. doi: 10.1016/j.addr.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;104:3198–204. doi: 10.1182/blood-2004-04-1485. [DOI] [PubMed] [Google Scholar]
- 28.Hao X, Silva EA, Månsson-Broberg A, Grinnemo K-H, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovascular Research. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotech. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 30.Shin S, Shea LD. Lentivirus Immobilization to Nanoparticles for Enhanced and Localized Delivery From Hydrogels. Mol Ther. 2010;18:700–6. doi: 10.1038/mt.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies N, Dobner S, Bezuidenhout D, Schmidt C, Beck M, Zisch AH, et al. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials. 2008;29:3531–8. doi: 10.1016/j.biomaterials.2008.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. 1. (a) Functionalization of heparin and chitosan with cysteine using EDC/NHS chemistry. Visualization of polysaccharide incorporation to porous PEG hydrogels when 25 μg of (c) heparin (1:500 ratio of polysaccharide-to-cysteine) or (d) chitosan (1:1000 ratio of polysaccharide-to-cysteine) is added using the Toluidine blue and Orange II assays respectively. Toluidine blue is (c) purple when heparin is present and (b) blue for controls. Orange II is a (e) dark orange when chitosan is present and (d) yellow-orange for controls.
Supp. 2. Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 2 days of cell culture on porous PEG hydrogels containing 25 μg of heparin after functionalization with cysteine using EDC/NHS chemistry. Different letter designations indicate statistically significant differences (p < 0.05).
Supp. 3. Visualization of HCNPs in porous PEG hydrogels loaded with (a) 0 μg, (b) 25 μg, (c) 50 μg, and (d) 250 μg of HCNPs (3:1). Change in color from (a) blue to (b-d) purple using the Toluidine Blue assay reveals the presence of HCNPs. (e) Swell ratio of these hydrogels compared to a non-porous PEG hydrogel.
Supp. 4. Visualization of the seeding efficiency of porous PEG hydrogels with (a) 0 μg, (b) 25 μg, (c) 50 μg, and (d) 250 μg of HCNP content (3:1) stained using Calcein-AM and Ethidium homodimer-1 counterstained with Hoechst.
Supp. 5. Gene expression (integrated photon flux) of HEK 293T cells transduced with luciferase-encoding lentivirus (1 × 107 particles) after 3 days of cell culture in seeded, non-seeded, and total (seeded + non-seeded) cells. * indicates statistically significant difference (p < 0.05).