Abstract
Objective
Diazoxide (DZ) is a pharmacological opener of ATP-sensitive K+ channels and has been used for mimicking ischemic preconditioning and shows protection against ischemic damage. Here, we investigated whether Diazoxide supplementation to University of Wisconsin (UW) solution has cellular protection during islet isolation and improves in vivo islet transplant outcomes in rodent ischemia model.
Research Design and Methods
C57/B6 mice pancreata were flushed with UW or UW+DZ solution and cold preserved for 6 or 10 hrs prior to islet isolation. Islet yield, in vitro and in vivo function, mitochondrial morphology, and apoptosis were evaluated.
Results
Significantly higher islet yields were observed in the UW+DZ group than in the UW group (237.5 ± 25.6 vs. 108.7 ± 49.3, p < 0.01). The islets from the UW+DZ group displayed a significantly higher glucose-induced insulin secretion (0.97 ng/ml ± 0.15 vs. 0.758 ng/ml ± 0.21, p = 0.009) and insulin content (6095.6 ng/islet ± 1394.5 vs. 4209.2 ng/islet ± 815.1, p = 0.002). The DZ-treated islets had well-preserved mitochondrial morphology with superior responses of mitochondrial potentials and calcium influx responded to glucose. Higher living cells and less late apoptotic cells were observed in the UW+DZ group (p < 0.05). Additionally, the transplanted islets from the UW+DZ group had a significantly higher cure rate and improved glucose tolerance.
Conclusion
This study is the first to report mitoprotective effects of DZ for pancreas preservation and islet isolation. It remains to be tested whether these findings can be replicated in human islet isolation and transplantation.
Keywords: Diazoxide, Cold Ischemia, Islet Isolation, Islet Transplantation, Pancreas Preservation, Mitoprotection
INTRODUCTION
Islet transplantation has emerged as an alternative treatment for Type 1 diabetes to restore euglycaemia [35,38], but remains characterized by variable outcomes. Factors that affect transplant outcomes are largely dependent on the islet isolation procedure and donor variables, such as ischemia time. While donor factors, for the most part, are not subject to change, improvements in organ preservation and the islet manufacturing process offer opportunities to enhance transplant outcomes.
Ischemia and/or ischemia-reperfusion (IR) injuries have been shown to significantly decrease the quantity and quality of isolated islets [15,23,32]. Current preservation solutions, such as the University of Wisconsin solution (UW) and Histidine-Tryptophan-Ketoglutarate (HTK), are designed to protect organs from those injuries. However, these solutions do not prevent the deleterious effects of ischemia, per se [7,10,33]. Other attempts have been made to reduce ischemia-related injuries through the oxygenation of preservation solutions, both by physical and chemical means [5,6,13,28,45], with controversial results. In 2006, our group demonstrated that Polyheme (polymerized human hemoglobin) improves islet isolation outcomes through the preservation of mitochondrial integrity [4]. Another study has shown that adding nicotinamide supplements to the isolation medium ameliorates the injuries caused by mitochondrial oxidative stress [18]. While the specific underlying mechanisms responsible for these observed benefits have not been identified, it is evident that isolation outcomes can be improved through the preserving β-cell mitochondrial integrity and reducing oxidative stress.
Numerous studies have shown the involvement of mitochondrial ATP-sensitive potassium (mitoKATP) channels in ischemic preconditioning (IPC) [22,36,40]. While the role of mitoKATP channels is not as well defined as sarcKATP channels, a second subtype of KATP channels that regulate metabolism and membrane excitability and are specifically critical in β-cells for insulin secretion regulation [24,26,27]. Recent evidences have suggested the involvement of mitoKATP in mitochondrial membrane potentials maintenance, ion homeostasis, and free radical species (ROS) regulation [2,3,44]. Diazoxide (DZ), a mitoKATP channel opener, has been successfully used to mimic IPC and displays cytoprotective effects in various ischemia models [9,11,17,37]. Specific to pancreatic tissue, however, the impact of DZ has not yet been examined.
In this present study, our objective was to determine whether mitochondrial integrity preservation through the use of DZ could prevent β-cell ischemia-related injury and, consequently, improve islet function in vitro and in vivo. This study investigated the effects of DZ supplementation of common organ preservation solution and islet isolation process on islet isolation and transplant outcomes in a rodent ischemia model.
MATERIALS AND METHODS
Animals
Male C57/B6 mice (Charles River Laboratory, 8–10 weeks of age) were used for this study. All animals were housed at the Biologic Resources Laboratory (BRL) at the University of Illinois at Chicago (UIC) and procedures involving animals were performed in accordance with the guidelines of the National Institute of Health and the Animal Care Committee (ACC) at UIC.
Pancreatic cold ischemic model
The mice were divided into five groups: 1.) Control group (non-ischemic control); 2.) UW group (cold ischemic control): animal pancreata preserved with UW solution; 3.) UW+ DZ group: animal pancreata preserved with UW solution supplemented with 150 μM DZ (Sigma, St. Louis, MO), 4.) UW+DZ+5-HD group: animal pancreata preserved with UW supplemented with both 150 μM DZ and 100 μM 5-hydroxydecanoate (5-HD; Sigma, St. Louis, MO), a specific blocker of mitoKATP channels, 5.) UW+5-HD group. All animals were anesthetized by isoflurane inhalation using a vaporizer and masks (Viking Medical, Medford Lakes, NJ). The mouse abdomen was opened along the midline, and small clips were placed on the lower abdominal aorta and the thoracic aorta. Either a 25 G or 27 G catheter was inserted into the lower abdominal aorta, just above the small clips, and the pancreata were flushed with 8 ml of UW solution (Duramed Pharmaceuticals, Pomana, NY), with or without supplemental drugs (DZ and/or 5-HD), at a speed of 1 mL/min controlled by a syringe pump (Harvat Apparatus, model 22). The pancreata from all four ischemic groups were then preserved at 4 °C for either 6 or 10 hrs.
Islet isolation
Following cold ischemic preservation, islet isolations were performed as previously described [20]. Briefly, 0.224 mg/mL Collagenase P (Roche, Applied Science, IN) was dissolved in HBSS (Mediatech Inc. VA), with or without drugs (DZ and/or 5-HD) and injected via the bile duct for pancreatic distention. After excision, the pancreata were digested in 15 mL conical tubes at 37 °C for 11 min, gently shaken, and washed twice with HBSS supplemented with or without drugs (DZ and/or 5-HD). Discontinuous Ficoll density gradients (Mediatech Inc., VA) were used for islet purification. Islets were then cultured in RPMI 1640 containing 10 % FBS at 37 °C for overnight prior to experimental procedures.
Islet yield and static glucose incubation
Islets bigger than 50 μm were manually counted for the islet yield. Static glucose incubation was used to compare glucose-stimulated insulin secretion. Briefly, 5 islets of approximately the same size were handpicked from each group, placed in Krebs-Ringer buffer (KRB) containing 2 mM glucose and 20 mM Hepes (5 replicas), and incubated for 30 min. The islets were then stimulated with 14 mM glucose for 60 min at 37 °C and 5 % CO2. Following stimulation, 200 μL of supernatant was collected and frozen at −20 °C for insulin assessment by ELISA (Mercodia, Uppsala, Sweden), according to manufacture protocol. All samples were tested in duplicate.
Assessment of mitochondrial membrane potentials and morphology
Rhodamine 123 (Rh123, Sigma, St. Louis, MO) was used to measure mitochondrial membrane potentials, as previously described [1,25]. Briefly, 25–30 isolated islets from each group were incubated in KRB solution with 2 mM glucose and 10 ng/mL of Rh123 for 30 min at 37 °C. The islets were then placed into a temperature-controlled microfluidic perifusion chamber, which was mounted on an inverted epifluorescence microscope (TE-2000U, Nikon). The islets were perifused by a continuous flow of KRB buffer at 37 °C (pH 7.4) and then stimulated with 14 mM glucose. Rh123 fluorescence was excited at 490 nm, and the emission was measured at 530 nm. Images were collected with a charged coupled camera (Roper Cascade CCD, Photometrics, and Tucson, AZ). Data was normalized to the average basal fluorescence intensity that had been recorded during a 5-minute period prior to the glucose stimulation. Changes in fluorescence were measured for 15 min. To examine the mitochondrial morphology, each group of islets was incubated with Rh123 in KRB solution for 15 min at a concentration of 10 ng/mL. The islets were then visualized using a Carl Zeiss LSM 510 confocal microscopy equipped with 60 X water immersion objective. The excitation line at 488 nm from the argon-krypton laser and the emission line were detected through a long-pass 505 nm filter. The intensity and the distribution of fluorescence were used to characterize morphology and integrity.
Intracellular Ca2+ measurement
Intracellular Ca2+ measurements were performed as previously described [1,25]. Briefly, 25–30 islets were incubated with 5 μM Fura-2/AM (a calcium indicator, Invitrogen) for 30 min in KRB containing 2 mM glucose. The islets were then placed into a temperature-controlled microfluidic perfusion chamber mounted on an inverted epifluorescence microscope (TE-2000U, Nikon) and perifused by a continuous flow of KRB at 37°C (pH 7.4). A 14 mM glucose solution was then administered to the islets for 10 min. After a 15-minute rinse with 2 mM glucose KRB, 30 mM potassium chloride (KCI) was then administered to the islets. Multiple islets were simultaneously observed with 10–20 x objectives. Dual-wavelength Fura-2 was excited ratiometrically at 340 and 380 nm; the fluorescence changes were expressed as F340/F380. Metafluor/Metamorph (Universal Imaging Corporation) was used for imaging acquisition and analysis and the images were collected with a high-speed, high-resolution charge-coupled device (Roper Cascade CCD). The percent changes of intracellular Ca2+ between both groups were calculated by subtracting the maximum increase after glucose and KCI stimulations from the basal (2 mM glucose) Ca2+ level for each group.
Assessment of Apoptosis by Flow Cytometry
The isolated islets were dissociated using Accutase (Sigma, St. Louis, MO) for 10 min at 37 °C and then stained with Annexin V and PI, using the TACS Annexin V-FITC staining kit (R&D Systems, Minneapolis, MN). Briefly, dissociated islets were washed with PBS and then incubated with Annexin V-FITC and PI solution for 15 min at room temperature in the dark. After another wash, the cells were resuspended in binding buffer and analyzed by flow cytometery (CyAn ADP Analyzer, Beckman Coulter, Fullerton, CA). Annexin− and PI − labeled cells were classified as living cells. Annexin+ and PI − labeled cells were classified as early apoptotic cells and Annexin+ and PI + labeled cells were classified as late apoptotic or necrotic cells. Four to five independent experiments were performed and the data is presented as mean ± SD.
Islet transplantation and in vivo evaluation
The isolated islets were assessed for in vivo function through allogenic transplantation under the kidney capsule of isogeneic diabetic C57/B6 mice. Induction of diabetes was achieved by intraperitoneal (IP) injection of Streptozotocin (Sigma, St. Louis, MO) at a dosage of 200 mg/kg of body weight. Mice with blood glucose levels higher than 20 mM or 360 mg/dL were considered recipients for islet transplantation. After overnight incubation, 350 islets were transplanted under the left kidney capsule in each mouse under isoflurane anesthetization, as previously described [4]. Blood glucose levels were monitored every day during the 1st week and every other day thereafter. Transplantation was considered successful if the blood glucose levels were lower than 210 mg/dL for two consecutive days. On day 14, an intraperitoneal glucose tolerance test (IPGTT, glucose 2 g/kg of body weight) was performed to assess graft function. All animals were fasted overnight, and blood glucose levels were measured at several time points (0, 15, 30, 60, 90, and 120 min). Area Under the Curve (AUC) was used to compare each group. Six weeks post-transplantation, recipients underwent a graft-bearing nephrectomy. Returning to hyperglycemia was interpreted as indirect evidence of islet graft function rather than spontaneous recovery of the native pancreas.
Statistical methods
Statistical analysis was carried out by Student’s t-test and Pearson chi-square test. For multiple comparisons between each group, one-way ANOVA was used and then confirmed with bonferroni post-test. P values <0.05 were regarded as statistically significant.
RESULTS
DZ-supplemented UW solution increases islet yield
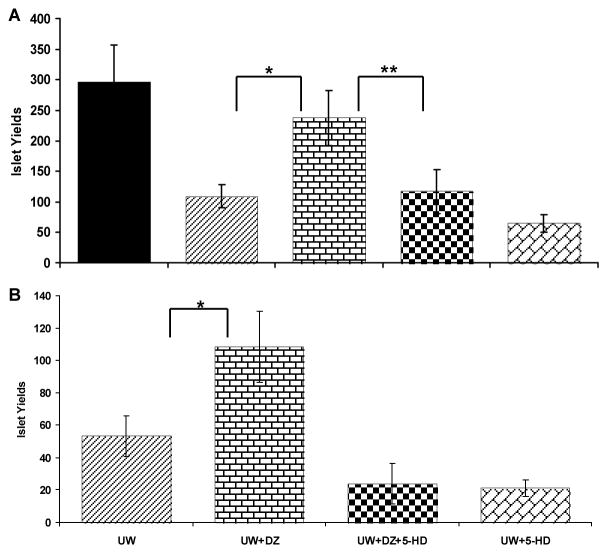
In the 6-hour cold ischemia (CI) model, the UW+DZ group had a significantly higher islet yield compared to the UW group (237.5 ± 25.6 vs. 108.7 ± 49.3 islets/mouse, respectively; p < 0.01; Fig. 1A). The difference between the UW+DZ and the control group was not significant (p = 0.156). To test whether the protective effect of DZ was specifically due to the opening of the mitoKATP channels, 100 μM of 5-HD (a specific mitoKATP closer) was added to the UW+DZ solution during pancreas preservation and islet isolation. Pancreata from the UW+DZ+5-HD group had significant lower islet yields compared to without 5-HD (p < 0.01), but similar to the UW group (117 ± 33.1 vs. 108.7 ± 49.3 islets/mouse, respectively; p > 0.05; Fig. 1A,). The UW+5-HD as negative control of DZ also had low islet yield, even lower than the UW group (65.4± 12.3).
Figure 1. Isolated islet yield in the rodent pancreatic CI preservation model.
A: Islet yields from the non-ischemia control (n = 3) and four 6-hour CI groups: the UW (n = 7), the UW + DZ (n = 8), the UW + DZ + 5-HD (n = 7), and UW+5-HD (n=5). * p < 0.01. B: Islet yields from two 10-hour CI groups: the UW (n=3), the UW + DZ (n=4), the UW+DZ+5-HD (n=5), and the UW+5-HD (n=4). * p = 0.02.
In the 10-hour CI model, both the UW and the UW+DZ groups had much lower islet yields than in the 6-hour CI model. Yet, the UW+DZ group still had a significantly higher islet yield than the UW group (108.2 ± 22.1 vs.53.2 ± 12.3 islets/mouse, respectively; p = 0.02; Fig. 1B). Similar to 6-hour model, the both UW+DZ+5-HD and UW+5-HD had very lower yields (24 ± 12.4 and 21 ± 5.1, respectively).
DZ-supplemented UW solution improves glucose-stimulated insulin secretion and total insulin content
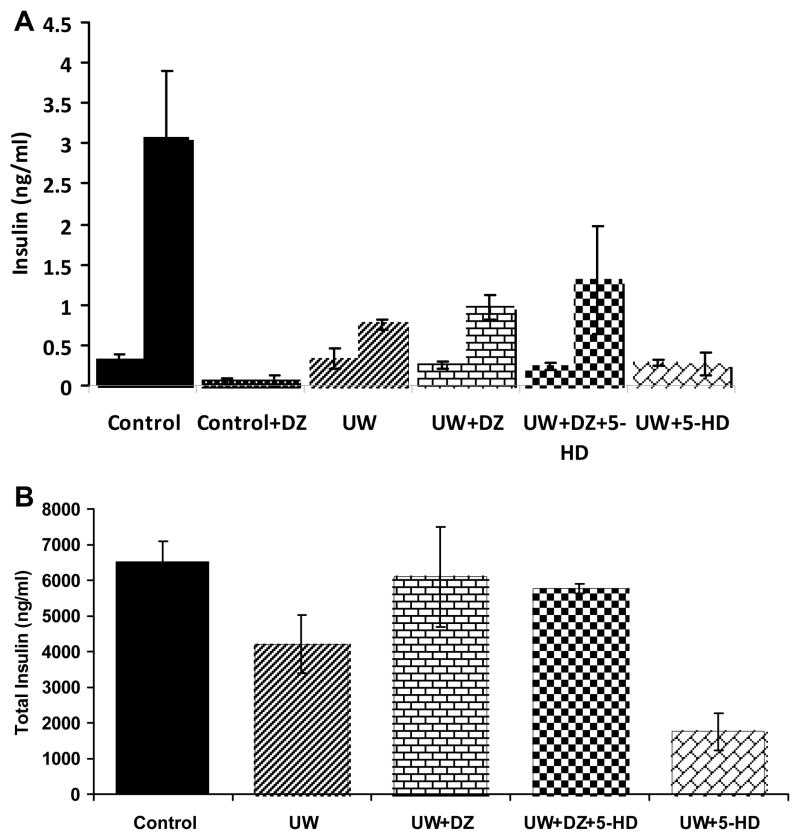
Insulin secretions were performed at both basal (2 mM) and stimulated (14 mM) glucose concentrations. For comparison between the UW+DZ group and the UW group, basal secretions were similar (0.264 ng/ml ± 0.05 vs. 0.332 ng/ml ± 0.12, p = 0.29); however the UW+DZ group had a higher insulin secretion when stimulated with 14 mM glucose with 0.97 ng/ml ± 0.15 vs. the UW group of 0.758 ng/ml ± 0.21 (p = 0.009) as shown in Figure. 2A. The addition of 5-HD in UW+DZ did not have a negative impact on the 14 mM glucose-stimulated insulin secretion, even secreted more insulin (1.30 ng/ml ± 0.67) and not significantly different from the UW+DZ group (p = 0.55). For the UW+5-DH group, the stimulated insulin level was significantly lower than both the UW and UW+5-HD groups (0.262 ng/ml ± 0.14). With addition of 150 μM of DZ to control islets during the static incubation, both basal and glucose stimulated insulin secretions were almost completely inhibited (Fig. 2A).
Figure 2. Glucose-stimulated insulin secretion and total insulin content of islets.
A: 2 mM and 14 mM glucose-stimulated insulin secretion (mean ± SD) for non-ischemia control islets (n = 3), four 6-hour CI groups: the UW, the UW + DZ, the UW + DZ + 5-HD, and the UW+5-HD (n of each group = 5). *p = 0.009, **p = 0.031. B: Total insulin content measurement for the UW control islets (n = 7), the UW + DZ (n = 7), UW+DZ+5-HD (n =7), and UW+5=DH (n =5). * p = 0.002.
Additionally, the islets from the UW+DZ group had significantly higher insulin contents (6095.6 ng/islet ± 1394.5) than the islets from the UW group (4209.2 ng/islet ± 815.1) with p = 0.002. Similar to insulin secretion data, the UW+DZ+5-HD had comparable total insulin content (5765.1 ng/islet ± 132.2) with the UW+DZ group. The UW+5-HD group had significantly lower insulin content (1761.2 ng/islet ± 513.6, Fig. 2B).
DZ-supplemented UW solution preserves mitochondrial integrity
Observation of mitochondrial morphology, using Rh123, indicated that the mitochondrial structure in the UW+DZ group had less mitochondrial swelling, fragmentation, and aggregation than the UW group (Fig. 3). Additionally, the islets from the UW+DZ group had significantly improved mitochondrial function, as measured by glucose-stimulated mitochondrial potentials changes when stimulated with 14 mM glucose, than islets from the UW group (28.73 % ± 9.87 vs. 4.59 % ± 1.23 for the UW + DZ group, respectively; p < 0.05; Fig. 4A and B).
Figure 3. The integrity of mitochondrial morphology.
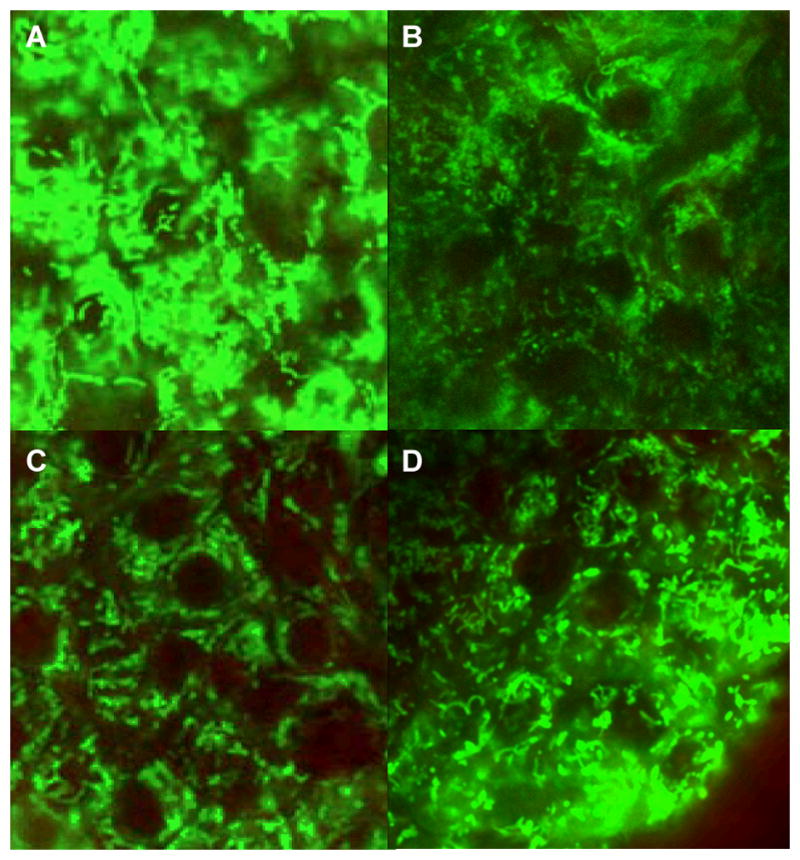
Two representative confocal images of Rh 123-stained islets from the UW group (A,B) and the UW + DZ group (C,D). Contrast has been balanced to reveal the details of mitochondrial morphology. N = 3–4 for each experimental condition, 10–20 islets/group. Scale bar = 5 nm.
Figure 4. Alteration in mitochondrial membrane potential changes induced by glucose.
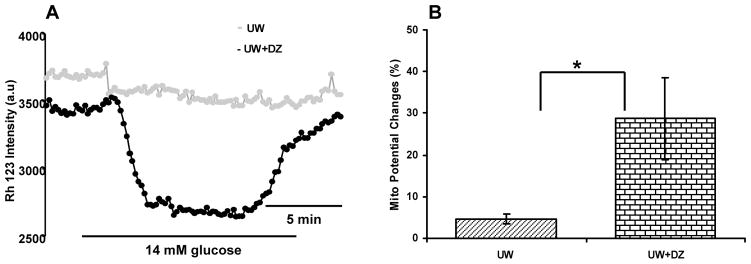
Islets were loaded with Rh123 and the intensity of Rh123 fluorescence was monitored in response to 14 mM glucose stimulation. A: A representative figure of observed mitochondrial potentials changes. B: A summary of mitochondrial potentials changes, represented as percentage over basal level of the UW + DZ and the UW treated islets. Data represent mean ± SD of 4–5 experiments (islets n = 15–20) performed. *p < 0.05.
DZ-supplemented UW solution improves the β-cell calcium signaling in response to glucose
To determine if preserved mitochondrial morphology and function using DZ was associated with alterations in calcium signaling, the calcium influx responses were examined, using the fluorescence imaging of Fura-2. The islets from UW+DZ group showed improved responsiveness to glucose and potassium challenges (Fig. 5A). The Ca2+glu index of the islets from the UW+ DZ group was 122.3 % ± 20.87 vs. 56.1 % ± 13.45 for islets from the UW group; p < 0.05. The Ca2+KCI index of islets from the UW+ DZ was 132.1 % ± 22.2 vs. 98.8 % ± 10.9 for islets from the UW group; p < 0.05 (Fig. 5B).
Figure 5. Intracellular calcium concentration responses to glucose and KCI.
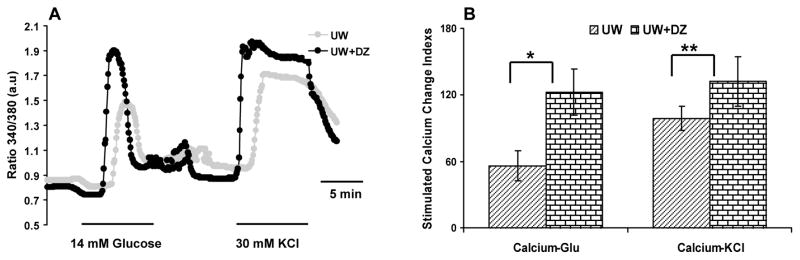
A: Representative record of islet responses from the UW + DZ and the UW groups. Islets were loaded with 5 μM Fura 2-AM and then stimulated with 14 mM glucose and 30 mM KCI. Calcium changes are reflected by the ratio changes of 340/380 nm. The responses of 15–30 islets tested in both groups are recorded. Fig. 5B shows the delta [Ca2+]glu and [Ca2+]KCI of the UW + DZ and the UW groups. * and ** p < 0.05.
DZ-supplemented UW solution decreases apoptosis
To determine the impact of DZ on the levels of apoptosis and necrosis, the islets isolated from 6-hour CI preservation were dissociated. Cell viability and survival were then analyzed by flow cytometry using Annexin V for apoptosis and PI for necrosis. Non-ischemia control islets were also assessed with Annexin V and PI; while most islet cells were healthy (80.04 % ± 11.45) a small amount of early apoptosis (9.11 % ± 3.92) and necrotic/late apoptotic cells (10.87 % ± 7.73) was observed, mostly due to process of islet dissociation. Within the CI groups, a significantly greater amount of living cells were observed in the UW+DZ group than in the UW group (66.45 % ± 5.58 vs. 17.12 % ± 12.28, respectively; p < 0.001; Table 1). Islets in the UW+DZ group had the same amount of early apoptosis as that of the non-ischemia group (8.05% ± 5.98 vs. 9.11% ± 3.92), while the UW group tended to have increased early apoptotic cells present although this number was not significantly different from the UW+ DZ group (8.05 % ± 5.98 vs. 19.91 % ± 10.90, p = 0.24). However, there was a significantly smaller amount of necrotic/late apoptotic cells that were observed in the UW+DZ group when compared to the UW group (25.49 % ± 4.90 vs. 62.85 % ± 37.30; p = 0.038). The UW+5-HD without DZ served as a negative control and had higher percentage of early apoptotic cell (28.99% ± 0.91) and necrotic/late apoptotic cells (50.02% ±1.01).
Table 1. Annexin V flow cytometric analysis.
Annexin V and PI staining for the percentage of live, apoptotic, and necrotic cells present in the dissociated cell population of ischemia groups and non-ischemia control group. P values were determined between UW (n=3) and UW+DZ (n=3) groups. Data from three separate experiments are presented, each with 5 independent samples (mean % ± SD).
| Control | UW | UW+DZ | UW+5-HD | P | |
|---|---|---|---|---|---|
| Alive Cells (Annexin−/PI−) | 80.04±11.45 | 17.12±12.28 | 66.48±5.58 | 10.97±1.20 | < 0.001 |
| Early Apoptotic Cell (Annexin+/PI−) | 9.11±3.92 | 19.91±10.90 | 8.05±5.96 | 28.99±0.91 | = 0.241 |
| Necrotic/Late Apoptotic Cells (Annexin+/PI+) | 10.87±7.73 | 62.85±37.30 | 25.49±4.90 | 50.02±1.01 | = 0.038 |
DZ-supplemented UW solution improves islet in vivo graft function
The percentage of mice cured at week 2 was significantly higher for the UW+DZ group (n=7) than for the UW group (n=7) (85.7 % vs. 42.5 %, respectively; p < 0.05; Fig. 6A). Mice transplanted with islets from the UW+DZ group also displayed a significantly lower number of days required to reach normoglycemia than the mice transplanted with islets from the UW group (7.2 days ± 3.3 vs. 12.4 days ± 2.5 respectively; p < 0.05; Fig. 6B). The 28-day glycemic profile of mice transplanted with islets from both groups is depicted in Figure 7A. In order to verify in vivo graft function in responses to glucose challenge, IPGTTs were conducted at day 14. Mice in the UW+DZ group had a significant decrease of glycemic AUC, as compared to mice in the UW group (13825.7 mg/dL ± 2901.9 vs. 24351.4 mg/dL ± 6261.4, respectively; p < 0.01; Fig. 7B and C).
Figure 6. Cure rate and days to reach normoglycemia after islet transplantation into diabetic mice.
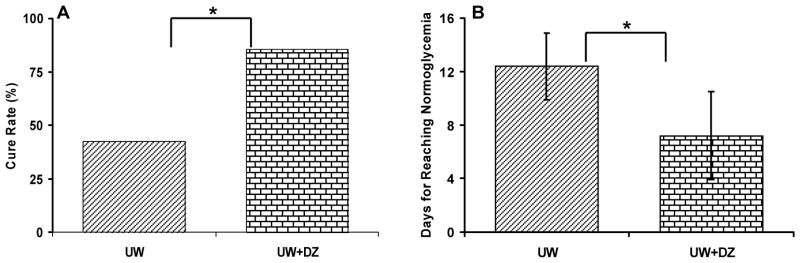
A: The number of mice reaching normoglycemia at day 14 after 350 islets were transplanted from the UW + DZ (n = 6 of 7) and the UW control (n = 3 of 7) mice. * p < 0.05. B: The number of days to reach normoglycemia in cured mice (UW + DZ: 7.2 days ± 3.3 vs. UW: 12.4 days ± 2.5, * p < 0.05).
Figure 7. In vivo graft function.
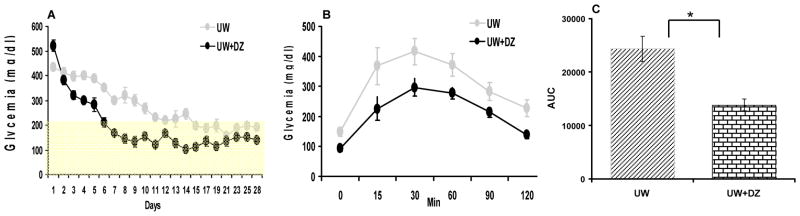
A: The 28-day glycemic profiles (mean ± SEM) for the UW group (n=7) and the UW + DZ group (n=7). B: The 120-minute glycemic profiles of IPGTTs (mean ± SEM). C: AUCs of IPGTT were calculated for both groups (mg/dL, mean ± SEM), * p < 0.05).
DISCUSSION
This is the first report to show that DZ supplementation during pancreas preservation and during the islet isolation process improves islet yield and function through preserving mitochondrial integrity. Considerable evidence supports the beneficial effects of DZ in IR [2,16,39,40,43,46]. While the cytoprotection mechanism of DZ has not been completely elucidated, post-ischemic mitochondrial protection has been shown to occur though sustaining mitochondrial membrane potentials, reducing calcium influx, preventing opening and release of cytochrome C, and sparing ATP during the period of sustained ischemia [16,17,36].
DZ supplementation led to a significantly increased post-isolation islet yield in both the 6 and 10-hour ischemia models. The effect of DZ on islet yield could be abolished by the addition of 5-HD, which is a specific mitoKATP channels closer. This indicates that the higher islet yield with DZ supplementation was achieved by keeping mitoKATP channels open during the prolonged ischemia.
Our results also demonstrated that the islets in the UW+DZ group had a significantly enhanced glucose-stimulated insulin secretion and total insulin content, compared to the islets in the UW group. Interestingly, the effect of DZ on the insulin secretion was not reversed by the addition of 5-HD. This finding suggests that the effect of DZ on sarcKATP channels may also contribute to this observed preservation in islet function. The effect of DZ on insulin secretion has been extensively studied in pancreatic β-cells and demonstrated to specifically function through sarcKATP channel modulation. In rat islets transplanted into a diabetic environment, DZ causes prolonged improvement of β-cell function, accompanied with increased insulin content and pre-proinsulin mRNA levels [14]. DZ also preserves insulin stores and pulsatile secretion by inducing β-cell rest [34,41] and protects rat islets against the toxic effect of streptozotocin [8,19]. We speculated that in addition to preventing ischemia or ischemia-reperfusion related mitochondrial injury, the attenuation of ischemic-induced calcium overload via sarcKATP channels opening and resting β-cell membrane potential could prevent insulin granule dumping during pancreas preservation and the islet isolation process; this was supported in part in this study, as the DZ-treated islets had significantly higher total insulin content. Intracellular calcium overload has also been long considered as a precursor to IR-related injury and cell death. In the pancreatic IPC, DZ may decrease intracellular calcium overload either by sparing ATP production to support ATP-dependent calcium pumps or by stabilizing membrane potentials through activating sarcKATP channels and preventing voltage-dependent calcium influx. However, the affinities of DZ to mitoKATP and sarcKATP channels in pancreatic β-cells are not known. In cardiomyocytes, the affinity of DZ to the mitoKATP channel (0.4 μM) has been shown to be roughly 2000-fold higher than the affinity for the sarcKATP channel (855 μM) [12,31], but it is not know whether this applies similarly to the situation in pancreatic β-cells. In future study, it has to be determined underlying mitoprotective mechanism, especially related to which stages: either ischemia or reperfusion or both.
The islets from the UW+DZ group displayed better-preserved mitochondria morphology than islets from the UW group, with a more peri-nuclear distribution, less swelling, and less mitochondrial fragmentation. These results were also in accordance with previous studies in cardiomyocyte, which have demonstrated that DZ treatment preserves mitochondrial morphology [30]. Additionally, the islets from the UW + DZ group had superior calcium responses to glucose and potassium challenge, as measured by in vitro calcium influx assays. Because glucose-stimulated calcium influx depends on mitochondrial integrity and energetic status, this measurement can be used as an indirect assay for mitochondrial integrity and β-cell function. The results of our calcium influx assays suggested that DZ-induced mitochondria preservation consequently preserves calcium influx and insulin secretion.
While the percentage of early apoptotic cell populations was similar between the UW and UW+DZ groups, the UW+DZ group had significantly smaller population of necrotic/late apoptotic cells and significantly larger population of living cells when compared to the UW group. These findings suggest that DZ may reduce IR-related injury by suppressing or stopping early apoptotic cells into late apoptotic status. As previously discussed, DZ protects mitochondria during ischemia. Ischemia-reperfusion studies in the heart and brain have also shown that DZ reduces ROS formation during reperfusion [21,29]. In the presence of ROS, the calcium overload in damaged mitochondria can facilitate the opening of the mitochondrial permeability transition pore (mPTP). The opening of the mPTP further compromises cellular energetics and leads to increased cell necrosis and apoptosis. Ischemia and reperfusion injures cells by a variety of mechanisms, and DZ seems to act at multiple levels [30,42].
Our in vivo data indicated that the islets isolated from the UW + DZ group had a higher percentage cure rate and reduced the number of days needed to reach normoglycemia when compared to the UW group. IPGTT at day 14 showed that the islets treated with the UW + DZ had superior responses to glucose challenge, confirming the in vitro findings of the improved islet function with DZ treatment.
Overall, significant beneficial effects were observed with the addition of DZ to the pancreas preservation and islet isolation processes via a direct involvement of mitoprotection. While the specific mechanism of cellular and mitochondrial protection with DZ is still under investigation, our results suggested a combination of different cytoprotective mechanisms involved, as consistent with findings in other tissues studies. In conclusion, this study established a new approach for the application of mitoKATP channel openers to improve islet isolation and transplant outcomes. In the future it will be necessary to further understand underlying mechanism for the mitoprotection and test this promising approach to pancreas preservation and the islet isolation process in humans.
Acknowledgments
We thank Dr. Chen ML for her excellent technical assistance. This study was supported by a start up grant by the College of Medicine at the University of Illinois at Chicago (JO), The Chicago Diabetes Project, a research grant by the Gift of Hope Foundation in Illinois (JO and YW) and R01 DK091526 (JO).
References
- 1.Adewola AF, Lee D, Harvat T, Mohammed J, Eddington DT, Oberholzer J, Wang Y. Microfluidic perifusion and imaging device for multi-parametric islet function assessment. Biomed Microdevices. 2011;12:409–417. doi: 10.1007/s10544-010-9398-1. [DOI] [PubMed] [Google Scholar]
- 2.Akao M, Ohler A, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- 3.Ardehali H, O’Rourke B. Mitochondrial K(ATP) channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila JG, Wang Y, Barbaro B, Gangemi A, Qi M, Kuechle J, Doubleday N, Doubleday M, Churchill T, Salehi P, Shapiro J, Philipson LH, Benedetti E, Lakey JR, Oberholzer J. Improved outcomes in islet isolation and transplantation by the use of a novel hemoglobin-based O2 carrier. Am J Transplant. 2006;6:2861–2870. doi: 10.1111/j.1600-6143.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 5.Brandhorst H, Asif S, Andersson K, Theisinger B, Andersson HH, Felldin M, Foss A, Salmela K, Tibell A, Tufveson G, Korsgren O, Brandhorst D. A new oxygen carrier for improved long-term storage of human pancreata before islet isolation. Transplantation. 89:155–160. doi: 10.1097/TP.0b013e3181c9266c. [DOI] [PubMed] [Google Scholar]
- 6.Caballero-Corbalan J, Eich T, Lundgren T, Foss A, Felldin M, Kallen R, Salmela K, Tibell A, Tufveson G, Korsgren O, Brandhorst D. No beneficial effect of two-layer storage compared with UW-storage on human islet isolation and transplantation. Transplantation. 2007;84:864–869. doi: 10.1097/01.tp.0000284584.60600.ab. [DOI] [PubMed] [Google Scholar]
- 7.Canelo R, Hakim NS, Ringe B. Experience with hystidine tryptophan ketoglutarate versus University Wisconsin preservation solutions in transplantation. Int Surg. 2003;88:145–151. [PubMed] [Google Scholar]
- 8.Culbert S, Sharp R, Rogers M, Felts P, Burr IM. Diazoxide modification of streptozotocin-induced diabetes in rats. Diabetes. 1974;23:282–286. doi: 10.2337/diab.23.4.282. [DOI] [PubMed] [Google Scholar]
- 9.Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal-vascular function after cerebral ischemia in newborn pigs. Stroke. 1999;30:2713–2718. doi: 10.1161/01.str.30.12.2713. discussion 2718–2719. [DOI] [PubMed] [Google Scholar]
- 10.Fridell JA, Agarwal A, Milgrom ML, Goggins WC, Murdock P, Pescovitz MD. Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution for organ preservation in clinical pancreas transplantation. Transplantation. 2004;77:1304–1306. doi: 10.1097/01.tp.0000122222.93740.b2. [DOI] [PubMed] [Google Scholar]
- 11.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 12.Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 13.Hering BJ, Matsumoto I, Sawada T, Nakano M, Sakai T, Kandaswamy R, Sutherland DE. Impact of two-layer pancreas preservation on islet isolation and transplantation. Transplantation. 2002;74:1813–1816. doi: 10.1097/00007890-200212270-00033. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu S, Hoog A, Moller C, Grill V. Treatment with diazoxide causes prolonged improvement of beta-cell function in rat islets transplanted to a diabetic environment. Metabolism. 2000;49:657–661. doi: 10.1016/s0026-0495(00)80044-x. [DOI] [PubMed] [Google Scholar]
- 15.Hogan AR, Doni M, Molano RD, Ribeiro MM, Szeto A, Cobianchi L, Zahr-Akrawi E, Molina J, Fornoni A, Mendez AJ, Ricordi C, Pastori RL, Pileggi A. Beneficial Effects of Ischemic Preconditioning on Pancreas Cold Preservation. Cell Transplant. doi: 10.3727/096368911X623853. [DOI] [PubMed] [Google Scholar]
- 16.Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol. 1999;519(Pt 2):347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 18.Ichii H, Wang X, Messinger S, Alvarez A, Fraker C, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R, Ricordi C. Improved human islet isolation using nicotinamide. Am J Transplant. 2006;6:2060–2068. doi: 10.1111/j.1600-6143.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 19.Kullin M, Li Z, Hansen JB, Bjork E, Sandler S, Karlsson FA. K(ATP) channel openers protect rat islets against the toxic effect of streptozotocin. Diabetes. 2000;49:1131–1136. doi: 10.2337/diabetes.49.7.1131. [DOI] [PubMed] [Google Scholar]
- 20.Lacy PE, Walker MM, Fink CJ. Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin. Diabetes. 1972;21:987–998. doi: 10.2337/diab.21.10.987. [DOI] [PubMed] [Google Scholar]
- 21.Liang HW, Xia Q, Bruce IC. Reactive oxygen species mediate the neuroprotection conferred by a mitochondrial ATP-sensitive potassium channel opener during ischemia in the rat hippocampal slice. Brain Res. 2005;1042:169–175. doi: 10.1016/j.brainres.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Light PE, Kanji HD, Fox JE, French RJ. Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. FASEB J. 2001;15:2586–2594. doi: 10.1096/fj.01-0188com. [DOI] [PubMed] [Google Scholar]
- 23.Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144:179–187. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohammed JS, Wang Y, Harvat TA, Oberholzer J, Eddington DT. Microfluidic device for multimodal characterization of pancreatic islets. Lab Chip. 2009;9:97–106. doi: 10.1039/b809590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols CG, Koster JC. Diabetes and insulin secretion: whither KATP? Am J Physiol Endocrinol Metab. 2002;283:E403–412. doi: 10.1152/ajpendo.00168.2002. [DOI] [PubMed] [Google Scholar]
- 27.Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JPt, Gonzalez G, Aguilar-Bryan L, Permutt MA, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi H, Ueda M, Nakai Y, Iwanaga Y, Okitsu T, Nagata H, Yonekawa Y, Kobayashi N, Nakamura T, Wada H, Matsumoto S. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am J Transplant. 2006;6:496–504. doi: 10.1111/j.1600-6143.2006.01223.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozcan C, Bienengraeber M, Dzeja PP, Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H531–539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 30.Ozcan C, Terzic A, Bienengraeber M. Effective pharmacotherapy against oxidative injury: alternative utility of an ATP-sensitive potassium channel opener. J Cardiovasc Pharmacol. 2007;50:411–418. doi: 10.1097/FJC.0b013e31812378df. [DOI] [PubMed] [Google Scholar]
- 31.Paucek P, Yarov-Yarovoy V, Sun X, Garlid KD. Inhibition of the mitochondrial KATP channel by long-chain acyl-CoA esters and activation by guanine nucleotides. J Biol Chem. 1996;271:32084–32088. doi: 10.1074/jbc.271.50.32084. [DOI] [PubMed] [Google Scholar]
- 32.Pileggi A, Ribeiro MM, Hogan AR, Molano RD, Embury JE, Ichii H, Cobianchi L, Fornoni A, Ricordi C, Pastori RL. Effects of pancreas cold ischemia on islet function and quality. Transplant Proc. 2009;41:1808–1809. doi: 10.1016/j.transproceed.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 33.Potdar S, Malek S, Eghtesad B, Shapiro R, Basu A, Patel K, Broznick B, Fung J. Initial experience using histidine-tryptophan-ketoglutarate solution in clinical pancreas transplantation. Clin Transplant. 2004;18:661–665. doi: 10.1111/j.1399-0012.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- 34.Ritzel RA, Hansen JB, Veldhuis JD, Butler PC. Induction of beta-cell rest by a Kir6.2/SUR1-selective K(ATP)-channel opener preserves beta-cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab. 2004;89:795–805. doi: 10.1210/jc.2003-031120. [DOI] [PubMed] [Google Scholar]
- 35.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Sasaki N, O’Rourke B, Marban E. Adenosine primes the opening of mitochondrial ATP-sensitive potassium channels: a key step in ischemic preconditioning? Circulation. 2000;102:800–805. doi: 10.1161/01.cir.102.7.800. [DOI] [PubMed] [Google Scholar]
- 37.Sato T, Sasaki N, Seharaseyon J, O’Rourke B, Marban E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal K(ATP) channels in ischemic cardioprotection. Circulation. 2000;101:2418–2423. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu S, Nagayama T, Jin KL, Zhu L, Loeffert JE, Watkins SC, Graham SH, Simon RP. bcl-2 Antisense treatment prevents induction of tolerance to focal ischemia in the rat brain. J Cereb Blood Flow Metab. 2001;21:233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Simerabet M, Robin E, Aristi I, Adamczyk S, Tavernier B, Vallet B, Bordet R, Lebuffe G. Preconditioning by an in situ administration of hydrogen peroxide: involvement of reactive oxygen species and mitochondrial ATP-dependent potassium channel in a cerebral ischemia-reperfusion model. Brain Res. 2008;1240:177–184. doi: 10.1016/j.brainres.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 41.Song SH, Rhodes CJ, Veldhuis JD, Butler PC. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology. 2003;144:3399–3405. doi: 10.1210/en.2003-0056. [DOI] [PubMed] [Google Scholar]
- 42.Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol. 2005;288:H1900–1908. doi: 10.1152/ajpheart.01244.2003. [DOI] [PubMed] [Google Scholar]
- 43.Szewczyk A, Wojcik G, Nalecz MJ. Potassium channel opener, RP 66471, induces membrane depolarization of rat liver mitochondria. Biochem Biophys Res Commun. 1995;207:126–132. doi: 10.1006/bbrc.1995.1162. [DOI] [PubMed] [Google Scholar]
- 44.Teshima Y, Akao M, Li RA, Chong TH, Baumgartner WA, Johnston MV, Marban E. Mitochondrial ATP-sensitive potassium channel activation protects cerebellar granule neurons from apoptosis induced by oxidative stress. Stroke. 2003;34:1796–1802. doi: 10.1161/01.STR.0000077017.60947.AE. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimura T, Kuroda Y, Kin T, Avila JG, Rajotte RV, Korbutt GS, Ryan EA, Shapiro AM, Lakey JR. Human islet transplantation from pancreases with prolonged cold ischemia using additional preservation by the two-layer (UW solution/perfluorochemical) cold-storage method. Transplantation. 2002;74:1687–1691. doi: 10.1097/00007890-200212270-00007. [DOI] [PubMed] [Google Scholar]
- 46.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]