Abstract
Throughout development, the nervous system produces patterned spontaneous activity. Research over the last two decades has revealed a core group of mechanisms that mediate spontaneous activity in diverse circuits. Many circuits engage several of these mechanisms sequentially to accommodate developmental changes in connectivity. In addition to shared mechanisms, activity propagates through developing circuits and neuronal pathways (i.e. linked circuits in different brain areas) in stereotypic patterns. Increasing evidence suggests that spontaneous network activity shapes synaptic development in vivo. Variations in activity-dependent plasticity may explain how similar mechanisms and patterns of activity can be employed to establish diverse circuits. Here, I will review common mechanisms and patterns of spontaneous activity in emerging neural networks and discuss recent insights into their contribution to synaptic development.
Keywords: plasticity, synaptogenesis, connectivity, waves, patterned activity, circuit mechanisms
Introduction
Spontaneous activity has been shown to regulate many aspects of neural development including the proliferation of precursors (Liu and others 2005; LoTurco and others 1995; Weissman and others 2004), neuronal survival (Mennerick and Zorumski 2000; Spitzer 2006) and migration (Komuro and Rakic 1996; Manent and others 2005; Yacubova and Komuro 2002; Zheng and Poo 2007), neurotransmitter specification (Borodinsky and others 2004; Marek and others 2010; Spitzer 2012), and axon and dendrite growth and branching (Hua and others 2005; Lohmann and others 2002; Ruthazer and others 2003; Uesaka and others 2006; Wong and Ghosh 2002). Although all these processes can affect how neurons connect to each other, I will focus here on the direct influence of spontaneous activity on synaptic development.
Recent results have revealed considerable diversity in the rules that govern how activity affects synaptic development. Most activity-dependent development is thought to be guided by Hebbian rules, according to which correlated activity of two neurons strengthens connections between them (Hebb 1949). Since its original formulation, variations in the temporal specificity of Hebbian plasticity have been identified. In spike time dependent plasticity (STDP), the order of pre- and postsynaptic spikes within a narrow time window (10–50 ms) determines whether synapses are formed and strengthened or weakened and eliminated (Caporale and Dan 2008). Activity patterns in many developing circuits lack fast correlations and instead encode information relevant to synaptic development in spike bursts lasting 0.5–1 s (Butts and Kanold 2010; Butts and Rokhsar 2001). Slower and time-symmetric burst time dependent plasticity (BTPD) rules, which match these activity patterns, were found to govern synaptic development in subcortical visual areas (Butts and others 2007; Shah and Crair 2008; Zhang and others 2012).
Similar to differences in temporal tuning, variations in the spatial spread of synaptic plasticity have been described. Hebbian plasticity is traditionally viewed as input specific, i.e. regulating synapses along a dendrite independent of each other (Matsuzaki and others 2004), but recent studies revealed that activity-dependent changes in one synapse can shift the plasticity threshold of neighboring connections (Harvey and Svoboda 2007; Harvey and others 2008). Interestingly, in developing hippocampal pyramidal neurons, neighboring synapses on a dendrite were found to be preferentially coactive and the emergence of such synapse clusters shown to depend on spontaneous activity itself (Kleindienst and others 2011). Beyond local interactions, long-range propagation of plasticity has been observed in the visual system of Xenopus tadpoles, where activity-dependent strengthening or weakening of synapses between retinal ganglion cell (RGC) axons and neurons in the tectum lead to matching changes in synaptic strengths on RGC dendrites (Du and others 2009).
Among non-Hebbian forms of plasticity, i.e. those that do not depend on the correlation of pre- and postsynaptic activity, homeostatic plasticity has garnered most attention. At the level of individual neurons, homeostatic plasticity maintains relatively constant action potential firing rates over time (Ibata and others 2008). This is particularly challenging during development when synaptic connectivity is continually changing (Turrigiano and Nelson 2004). To achieve its purpose, homeostatic plasticity can regulate many aspects of neuronal development, including synapse formation and maturation (Pozo and Goda 2010; Turrigiano 2008; Zhang and Linden 2003). At a network level, homeostatic plasticity has been shown to stabilize patterned spontaneous activity across development via flexible transitions between sequentially engaged circuit mechanisms (Blankenship and Feller 2010).
In addition to differences in temporal tuning, spatial specificity and Hebbian vs. non-Hebbian plasticity, effects of activity on synaptic development depend on the neuronal cell type. Time windows for STDP induction are reversed between connections onto excitatory and inhibitory neurons (Caporale and Dan 2008). Likewise, homeostatic plasticity triggered by spike suppression increases and decreases, respectively, the strengths of excitatory and inhibitory synapses (Burrone and others 2002; Hartman and others 2006). Even between excitatory neurons converging onto the same cell, activity can differentially regulate synaptic development (Morgan and others 2011).
Finally, synapses are most receptive to activity-dependent changes during restricted periods of development. Differences in the timing of these critical periods between the dorsolateral geniculate nucleus of the thalamus (dLGN) and superior colliculus (SC) appear to explain how an identical sequence of retinal activity can promote different synaptic organizations in the two main subcortical targets of RGC axons (Chandrasekaran and others 2007; Hooks and Chen 2006; Kerschensteiner and Wong 2008). Together, variations in plasticity may allow developing circuits to employ common mechanisms and patterns of network activity to establish specific wiring patterns.
Mechanisms of spontaneous network activity
Patterned spontaneous activity has been observed in many parts of the developing nervous system including the retina, cochlea, spinal cord, hippocampus, cerebellum, basal ganglia, thalamus and neocortex. In spite of their diverse architectures, these circuits generate and propagate spontaneous activity through a common set of mechanisms (Ben-Ari 2001; Blankenship and Feller 2010; O’Donovan 1999).
Gap junctions
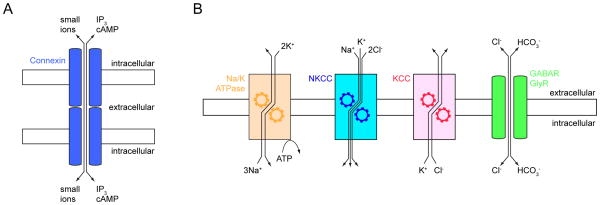
Synchronized Ca2+ oscillations in small groups of newborn neurons and precursors are among the earliest activity patterns in the developing nervous system. In the proliferative ventricular zones of neocortex and the retina, Ca2+ oscillations have been shown to be mediated by gap junctions (Catsicas and others 1998; Owens and Kriegstein 1998). Gap junctions are formed by the association of hexameric connexin channels in the plasma membranes of adjacent cells and allow the passage of inorganic ions (i.e. the flow of current) as well as small signaling molecules (e.g. IP3 and cAMP) between cells to synchronize their activity (Fig. 1A) (Kandler and Katz 1998; Kumar and Gilula 1996; Sohl and others 2005). A recent study revealed that in mouse neocortex excitatory neurons born from the same precursor cell are preferentially coupled by gap junctions (Yu and others 2012). Although these electrical connections are transient, they determine later synaptic connectivity. Thus, in addition to effects on precursor proliferation and neuronal migration (Owens and Kriegstein 1998; Pearson and others 2005; Weissman and others 2004), early gap junctional coupling and the synchronized activity it produces can regulate subsequent excitatory synapse formation. In primary visual cortex (V1), this aligns the orientation tuning of clonally related pyramidal neurons organizing them into functional columns (Li and others 2012; Yu and others 2012; Yuste and others 1992).
Figure 1.
Gap junctions and chloride homeostasis. (A) Aligned connexin hemichannels (blue) in adjacent plasma membranes provide a direct pathway for small ions and signaling molecules between neurons. (B) Na+ and K+ concentration gradients established by the Na+/K+ ATPase (Na/K ATPase, orange) provide the energy for the two transport systems that control neuronal [Cl−]i. Na+-K+-2Cl- cotransporters (NKCCs, blue) import Cl−, whereas K+-Cl− cotransporters (KCCs, red) export Cl−. Cl− flows through GABA and glycine receptors (GABAR and GlyR, green) down its electrochemical gradient. Cl− efflux depolarizes and Cl− influx hyperpolarizes a neuron.
Although most electrical connections are removed as chemical synapses appear (Maxeiner and others 2003; Peinado 2001; Yu and others 2012), gap junctions persist between specific neuron types and can participate in spontaneous network activity during later stages of development. Accordingly, in neocortex, gap junctions were found to be involved not only in synchronous plateau assemblies (SPAs) in newborn mice (postnatal day P0–3) (Allene and others 2008; Dupont and others 2006), but also in spreading waves of activity occurring between P8 and P14 (Siegel and others 2012). Similarly, electrical connections among different sets of neurons contribute to stage I (embryonic day E17-P1) and stage III (P11–14) waves in the rodent retina (Akrouh and Kerschensteiner 2013; Catsicas and others 1998; Syed and others 2004; Wong and others 1998).
Excitatory GABA and glycine
GABA (γ-aminobutyric acid) and glycine activate ligand-gated ion channels permeable to chloride (Cl−) and other anions (e.g. bicarbonate) in different parts of the nervous system (Bormann and others 1987; Kaila and Voipio 1987). The concentration of Cl− in neurons ([Cl−]i) is controlled by two families of transporters (Fig. 1B): Na+-K+-2Cl- cotransporters (NKCCs), which import Cl−, and K+-Cl− cotransporters (KCCs), which export Cl− (Blaesse and others 2009). During ontogeny, expression of KCCs often lags behind that of NKCCs. As a result, many developing neurons have high [Cl−]i (25–40 mM) and depolarize in response to GABA and/or glycine (Blaesse and others 2009; Owens and Kriegstein 2002). Release of GABA and/or glycine typically precedes that of glutamate (Akerman and Cline 2006; Johnson and others 2003), and spontaneous activity patterns in many immature circuits rely on excitatory GABA- and glycinergic transmission (Ben-Ari 2002). For instance, giant depolarizing potentials (GDPs) in the hippocampus and neocortex (P6–8 in rats) and waves of Purkinje cell (PC) activation in the cerebellum (P4–6 in mice) are blocked by antagonist of GABAA receptors (Allene and others 2008; Ben-Ari and others 1989; Garaschuk and others 1998; Watt and others 2009). Similarly, excitatory GABA- and glycinergic transmission increase the frequency of activity bursts in the embryonic rat spinal cord and early stage II retinal waves (Fischer and others 1998; Hanson and Landmesser 2003; Nishimaru and others 1996; Sernagor and others 2003; Wang and others 2007; Zhou 2001).
Later in development, the exact time varies between circuits and species (Blaesse and others 2009), neuronal [Cl−]i falls (~5 mM) and GABA and glycine assume their classical roles as inhibitory neurotransmitters. In different neurons, the decline in [Cl−]i is caused by distinct combinations of increased Cl− extrusion due to changes in gene expression and oligomerization of KCCs (Blaesse and others 2006; Rivera and others 1999) and reduced Cl− import due to lowered expression and functional inactivation of NKCCs (Delpy and others 2008). The transition of GABA- and glycinergic signaling from de- to hyperpolarizing changes spontaneous network activity. In the spinal cord and visual system, local inhibitory circuits desynchronize spontaneous activity in functionally opposite neurons, which, via Hebbian plasticity, may promote the development of parallel neuronal pathways (Akrouh and Kerschensteiner 2013; Kerschensteiner and Wong 2008; O’Donovan and Landmesser 1987). In other circuits, the inhibitory switch of GABA- and glycinergic transmission contributes to the end of patterned spontaneous activity (Ben-Ari 2002; Owens and Kriegstein 2002).
Interestingly, excitatory and inhibitory GABAergic transmission have been shown to differentially regulate glutamatergic and GABAergic synaptogenesis. In immature hippocampal pyramidal neurons, GABA-mediated depolarizations can relieve Mg2+ block of NMDA receptors allowing glutamate to elicit Ca2+ influx and downstream signals that promote the maturation of excitatory synapses (Leinekugel and others 1997; Leinekugel and others 1995). By contrast, the development of inhibitory synapses from parvalbumin-positive basket cells onto pyramidal neurons in visual cortex was found to depend on inhibitory GABAergic transmission (Chattopadhyaya and others 2007). Similarly, postsynaptic interference with GABA signaling by genetic deletion of the GABAA receptor α1 subunit perturbs the formation of synapses between stellate cell terminals and PC dendrites after GABAergic transmission has become inhibitory (Fritschy and others 2006; Patrizi and others 2008). The effects of excitatory and inhibitory GABAergic transmission on glutamatergic and GABAergic synaptogenesis were directly compared in the developing Xenopus tectum. Accelerated expression of KCC in tectal neurons, which causes a premature switch of GABAergic transmission, blocks the insertion of AMPA receptors and with it the maturation of glutamatergic synapses while simultaneously enhancing the formation GABAergic synapses (Akerman and Cline 2006). Initial results indicated that GABA itself may control the expression of KCC and the switch from excitation to inhibition (Ganguly and others 2001). However, subsequent findings pointed to alternative influences on KCC expression and additional control mechanisms of [Cl−]i (Delpy and others 2008; Titz and others 2003). Irrespective of the underlying signals, the timing of the developmental switch from GABAergic excitation to inhibition appears to be critical for the emergence of circuits with balanced excitatory and inhibitory connectivity.
Extrasynaptic neurotransmission
Extrasynaptic chemical communication involves the non-synaptic release of a transmitter or its escape from the synaptic cleft (i.e. spillover). Both processes participate in the generation and propagation of spontaneous activity in neural networks with immature synaptic connections.
Newborn neurons and non-neuronal cells can release transmitters by non-synaptic mechanisms. In the developing cochlea (Fig. 2C), supporting epithelial cells in Kölliker’s organ release ATP via connexin hemichannels (i.e. one half of a gap junction) (Schutz and others 2010; Tritsch and Bergles 2010; Tritsch and others 2007). The resulting waves of extracellular ATP coordinate the action potential firing of inner hair cells (IHCs) (Johnson and others 2012; Johnson and others 2013; Tritsch and Bergles 2010; Tritsch and others 2007). In the developing spinal cord, radial cell progenitors release glycine via volume-sensitive Cl− channels, depolarizing surrounding neurons and increasing the frequency of spontaneous network events (Scain and others 2010). Finally, newborn CA1 pyramidal neurons were shown to release GABA and glutamate in Ca2+- and SNARE-independent manner to support hippocampal GDPs (Demarque and others 2002).
Figure 2.
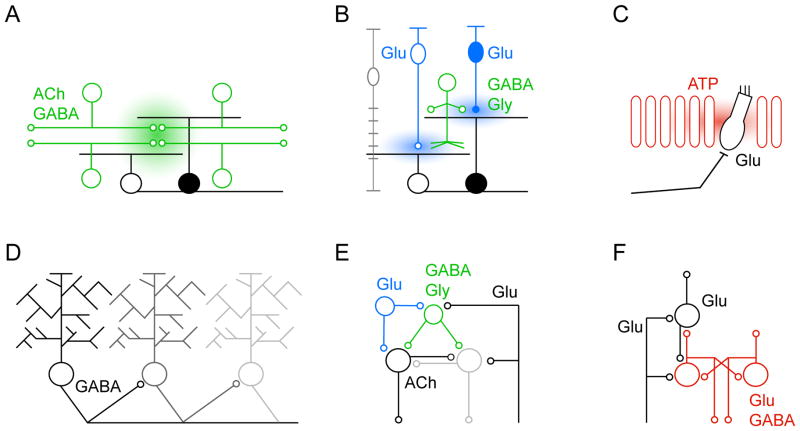
Diverse circuits generate patterned spontaneous activity. (A) Reciprocally connected starburst amacrine cells (SACs, green) initiate and propagate stage II waves in the retina, activating neighboring ON (white) and OFF (black) retinal ganglion cells (RGCs) simultaneously via extrasynaptic diffusion of ACh (and GABA). (B) Glutamate spillover and gap junctions mediate communication between neighboring ON bipolar cells (ON BCs) (open, blue) and laterally spread activity during stage retinal III waves. In addition, ON BCs activate ON RGCs (white) and diffuse ACs (green), which crossover inhibit OFF BCs (closed, blue) and delay their release of glutamate. Sequential glutamate release from ON and OFF BCs desynchronizes the activity of neighboring ON and OFF (black) RGCs during stage III waves. Glutamate uptake by Müller glia (gray) limits vertical spillover and maintains separation between ON and OFF circuits. (C) Support cells (red) in Kölliker’s organ of the developing cochlea generate waves of extracellular ATP, which synchronize the spontaneous spike bursts of nearby inner hair cells (IHCs, white). IHCs in turn release glutamate and activate spiral ganglion neurons. (D) In the developing cerebellum, spontaneous waves of activity travel along chains of PCs. Activity is transmitted in a directional manner established by asymmetric axon collateral. (E) Abundantly connected networks of GABA- and glycinergic interneurons (green, Renshaw cells), glutamatergic interneurons (blue), cholinergic motorneurons and glutamatergic proprioceptive inputs generate spontaneous activity patterns in the developing spinal cord. (F) Subplate neurons (SPNs, red) are a hub of developing thalamocortical circuits. SPNs receive input from thalamus, are reciprocally connected and receive feedback from the developing cortical plate. In addition to feedforward connections particularly with layer 4 neurons (white), SPNs provide feedback to thalamus. The resulting loops can generate activity and amplify activity originating in the sensory periphery (e.g. retina).
As neurons begin to form synapses with each other, transmitters are increasingly released by vesicle fusion at specialized presynaptic active zones (Jahn and Fasshauer 2012). The spread of neurotransmitters from release sites is constrained by uptake transporters and/or degrading enzymes (Abreu-Villaca and others 2011; Bergles and others 1999; Gonzalez-Burgos 2010; Leybaert and Sanderson 2012). In immature circuits, the development of these mechanisms tends to be delayed relative to synaptic release (Demarque and others 2002; Thomas and others 2011). Consequently, neurotransmitters can spill out of the synaptic cleft and act on receptors outside the postsynaptic specialization including on neurons that are not synaptic partners of the releasing cell. Stage II retinal waves exemplify such volume neurotransmission. Stage II waves are generated and propagated by starburst amacrine cells (SACs), retinal interneurons that are recurrently connected by GABA- and cholinergic synapses (Fig. 2A). SACs release acetylcholine (ACh) at synapses in two narrow sublaminae of the retina’s inner plexiform layer (IPL) (Feller and others 1996; Zhou 1998). In spite of this synaptic lamination, cholinergic excitatory postsynaptic currents (EPSCs) activate RGCs with dendrites stratifying outside the SAC’s sublaminae during stage II waves (Wong and others 2000; Zheng and others 2006). Moreover, a cell-based optical sensor detected wave-associated increases in extracellular ACh at the surface of the retina approximately 20 μm from the nearest release site (Ford and others 2012). Thus, it seems that the relatively unconstrained extrasynaptic spread of ACh underlies the uniform recruitment of large RGC populations into stage II waves.
During stage III retinal waves (Fig. 2B), extrasynaptic glutamatergic transmission mediates lateral communication between bipolar cell axons (Akrouh and Kerschensteiner 2013; Blankenship and others 2009; Firl and others 2013). In this case, however, glutamate uptake by excitatory amino acid (EAAT) transporters on Müller glia limits vertical signal transmission and allows for the asynchronous recruitment of circuits in different sublaminae of the IPL (Akrouh and Kerschensteiner 2013).
Outside the retina, glutamate and GABA spillover have been shown to contribute to patterned spontaneous activity in the developing hippocampus, brain stem and neocortex (Allene and others 2008; Cattani and others 2007; Demarque and others 2004; Sharifullina and Nistri 2006).
Much of the influence of excitatory GABA- and glycinergic signals on circuit development, described in the previous section, relies on extrasynaptic spread of these transmitters. In similar fashion, extrasynaptic ACh and glutamate can regulate many aspects of neural maturation including synaptogenesis (Ruediger and Bolz 2007). In the retina and hippocampus, respectively, ACh- and glutamate-evoked Ca2+ transients were shown to stabilize nascent dendritic filopodia, presumably to maintain axo-dendritic contact at sites that mature into synapses (Lohmann and Bonhoeffer 2008; Lohmann and others 2002). Furthermore, increases and decreases in spontaneous glutamate release in the retina were shown to increase and decrease, respectively, the assembly of excitatory postsynaptic specializations (Kerschensteiner and others 2009; Soto and others 2012). Thus, neurotransmitters not only mediate synaptic and extrasynaptic communication, and alter the strength of existing connections, but can also promote the formation of new synapses.
Transient cell types and connections
In addition to electrical connections, extrasynaptic chemical transmission and the “pre-purposing” of inhibitory transmitters systems for excitation, some developing circuits employ transient cells types and/or temporary synaptic connections to generate and propagate spontaneous activity patterns.
As mentioned above, before the onset of hearing, support cells in Kölliker’s organ of the cochlea spontaneously release ATP through connexin hemichannels (Schutz and others 2010; Tritsch and Bergles 2010; Tritsch and others 2007). Purinergic receptors (P2X and P2Y) on and gap junctions between neighboring support cells educe further ATP-release, generating a wave of extracellular ATP (Tritsch and others 2007). This ATP-wave triggers and/or coordinates the firing of immature IHCs (Johnson and others 2011; Johnson and others 2012; Tritsch and Bergles 2010; Tritsch and others 2007). The spread of ATP is limited by ectonucleotidases, a heterogeneous group of plasma membrane-bound enzymes (Leybaert and Sanderson 2012; Mammano 2013). As a result, spontaneous IHC activity is typically synchronized over ~100 μm or ~10 IHCs (Tritsch and Bergles 2010). Spike bursts of developing IHCs activate spiral ganglion neurons and distance-dependent correlations in the activity IHCs may thus inform the tonotopic refinement of downstream auditory pathways (Kandler and others 2009; Lippe 1994; Tritsch and others 2010). Intriguingly, a recent study found that spontaneous activity of IHCs also regulates their own synaptic maturation (Johnson and others 2013). In particular, the developmental linearization of Ca2+-dependent transmitter release appears to depend on the spontaneous firing patterns of IHCs. Spontaneous activity of IHCs declines rapidly around the onset of hearing (Tritsch and Bergles 2010). In addition to changes in the intrinsic excitability of IHCs (Brandt and others 2007; Marcotti and others 2003), the degeneration of Kölliker’s organ by appropriately timed apoptosis of support cells accounts for this transition (Kamiya and others 2001; Knipper and others 1999; Kraus and Aulbach-Kraus 1981).
The developing neocortex of placental mammals contains a transient structure called the subplate (SP), populated by the first neurons generated in the cortical ventricular zone: the subplate neurons (SPNs, Fig. 2F) (Allendoerfer and Shatz 1994; Kanold and Luhmann 2010). Later-born neurons migrate from the ventricular zone past the SP into the cortical plate (CP) and give rise to the canonical six-layered architecture of neocortex (Franco and Muller 2013; Rakic 2006). SPNs are a diverse set of glutamatergic and GABAergic neurons (Hanganu and others 2002; Hoerder-Suabedissen and others 2009; Mrzljak and others 1988; Osheroff and Hatten 2009) and a hub of early thalamocortical circuits. The main input to SPNs comes from thalamic axons, which arrive in the SP as layer 4 neurons are being born, and wait there before growing into the CP (Allendoerfer and Shatz 1994; Friauf and others 1990). Additionally, SPNs are extensively connected to each other via electrical and chemical synapses and receive excitatory feedback from cortex (Dupont and others 2006; Hanganu and others 2001; Kostovic and Rakic 1980; Kostovic and Rakic 1990; Viswanathan and others 2012). The primary output from SPNs is to layer 4, with auxiliary projections within the SP and back to thalamus (De Carlos and O’Leary 1992; Friauf and others 1990; Hanganu and others 2001; McConnell and others 1989). Excitatory feedforward connections from thalamus to SPNs and on to layer 4 cells relay spontaneous activity patterns generated in the sensory periphery (e.g. retina) to developing cortical circuits (Ackman and others 2012; Hanganu and others 2006; Siegel and others 2012). Recurrent excitation among SPNs and feedback loops with thalamus and the CP amplify peripheral patterns and can produce spontaneous activity bursts independent of the sensory periphery (Dupont and others 2006; Kilb and others 2011; Siegel and others 2012; Viswanathan and others 2012; Voigt and others 2001). Likely due to their role in spontaneous network activity, SPNs are critical for the maturation of thalamocortical connections and the development of cortical circuits. Accordingly, selective SP ablation has been shown to prevent the developmental increase of AMPA receptor expression in layer 4 and blocks the strengthening of thalamocortical connections in visual and somatosensory cortices, decoupling cortical and thalamic activity (Kanold and others 2003; Tolner and others 2012). In visual cortex, SPN ablation also inhibits KCC expression and the maturation of GABAA receptors, resulting in persistently depolarizing GABAergic responses (Kanold and Shatz 2006). At a higher organizational level, ocular dominance columns and whisker barrels fail to emerge in visual and somatosensory cortices, respectively, in the absence of SPNs (Kanold and others 2003; Tolner and others 2012). As direct connections from thalamus to layer 4 CP neurons mature, SPN neurons undergo programmed cell death. In mice 80% of SPNs are gone by P21 (Allendoerfer and Shatz 1994). The demise of SPNs coincides with the transition of cortical networks towards sensory information processing.
Another way for developing circuits to produce spontaneous activity for a limited time is to elaborate transient connections between permanent cells. The retinal networks underlying stage II waves illustrate this strategy (Fig. 2A). As mentioned earlier, stage II waves originate in SACs, dual transmitter neurons that corelease GABA and ACh (Lee and others 2010; O’Malley and others 1992; Yonehara and others 2011). During development, SACs are recurrently connected via excitatory GABA- and cholinergic synapses, which form the substrate of wave propagation (Ford and others 2012; Zheng and others 2004). In addition to changes in the intrinsic excitability of SACs, the period of stage II waves is ended by the disassembly of cholinergic synapses between SACs via downregulation of nicotinic ACh receptors (nAChRs) and the inhibitory switch of recurrent GABAergic connections as KCC expression increases (Vu and others 2000; Zhang and others 2006; Zheng and others 2006; Zheng and others 2004).
Similarly transient synapses are found in the developing spinal cord (Fig. 2E), where spontaneous activity relies on abundant connectivity between glutamatergic, GABAergic (i.e. Renshaw cells) and glycinergic interneurons, cholinergic motor neurons and glutamatergic proprioceptive sensory neurons (Hanson and Landmesser 2003; O’Donovan and others 1998). During postnatal development proprioceptive inputs onto Renshaw cells are weakened and connections between motor neurons eliminated (Mentis and others 2006; Nishimaru and others 2005). Together with the inhibitory switch of GABA- and glycinergic transmission this suppresses spontaneous network activity in mature circuits (Blankenship and Feller 2010; O’Donovan and others 1998).
Recently, developing PCs were shown to fire spontaneous bursts of action potentials that travel as waves from the apex of a cerebellar lobule to its base (Watt and others 2009). Monosynaptic GABAergic connections between PCs formed by axon collaterals propagate these waves (Fig. 2D). PC-PC connections are prevalent during the first postnatal week, but mostly eliminated by the third postnatal week coinciding with the disappearance of spontaneous waves of activity in cerebellar circuits (van Welie and others 2011; Watt and others 2009).
Initiation and Refractoriness
Spontaneous network activity typically occurs in bursts separated by periods of quiescence. To understand this temporal pattern, we need to know the mechanisms that initiate network events as well as those that end them and account for the silent intervals between bursts (i.e. refractory periods).
Two sets of mechanisms contribute to varying degrees to the initiation of different network events. First, interactions of intrinsic conductances can cause individual neurons to generate spontaneous bursts of action potentials and function as pacemakers for the networks in which they are embedded. Second, synaptic and non-synaptic interactions between neurons can build up excitation leading to bursts of activity.
Spontaneous activity in the developing cerebellum exemplifies pacemaker-driven network activity. Resurgent Na+ currents and rapidly activating and deactivating K+ conductances, mediated largely by Nav1.6 and Kv3.3, respectively, generate action potentials in PCs in the absence of synaptic input (Akemann and Knopfel 2006; Grieco and Raman 2004; Raman and Bean 1997). This intrinsically generated activity propagates along chains of developing PCs via excitatory GABAergic synapses (Watt and others 2009).
By contrast, hippocampal GDPs are generated by a combination of intrinsic conductances and network interactions. GDPs most frequently initiate in the CA3 region of the developing hippocampus (Ben-Ari 2001) where persistent Na+ currents and tonic excitatory GABAA receptor-mediated inputs elicit spontaneous bursts of action potentials in pyramidal neurons (Ben-Ari and others 1989; Sipila and others 2005). Activity then propagates via glutamatergic collaterals among pyramidal neurons and connections with interneurons through hippocampal circuits (Ben-Ari and others 1989; Bolea and others 1999; Garaschuk and others 1998). In addition to synaptic communication, extrasynaptic transmission of GABA and glutamate contributes to GDPs (Demarque and others 2002). GDPs are terminated by inhibitory effects of presynaptic GABAB receptors on release of GABA and glutamate, and the activation of Ca2+-dependent K+ channels, which mediate afterhyperpolarizations (AHPs) in pyramidal neurons (McLean and others 1996; Sipila and others 2006). Pyramidal neuron AHPs largely account for the 2–3 s refractory period of GDPs (Sipila and others 2006).
A similar mix of intrinsic and network mechanisms underlies the generation of stage II waves in the retina. As mentioned before, developing SACs form recurrently connected circuits in which each cell receives excitatory GABA- and cholinergic input from 10–30 others (Zheng and others 2006; Zheng and others 2004). SACs fire spontaneous bursts of Ca2+ spikes even when they are pharmacologically uncoupled from their neighbors (Ford and others 2012; Zheng and others 2006). Each spike burst is followed by a slow AHP (sAHP) (Ford and others 2012; Zheng and others 2006), recently shown to be mediated by activation of the 2-pore K+ channel TREK1 via Ca2+-dependent phosphodiesterases (Ford and others 2013). In addition to coalescing SAC activity into waves, synaptic inputs prolong sAHPs by increasing Ca2+ influx and determine the lengths of refractory periods between waves, which are an order of magnitude longer (~30 s) than those of GDPs (Ford and others 2012; Hennig and others 2009; Zheng and others 2006).
No pacemakers have been identified in the circuits that produce stage III waves in the retina or those that give rise to spontaneous activity in the developing spinal cord. A specific class of bipolar cells (ON BCs) drives network activity during stage III waves (Akrouh and Kerschensteiner 2013). ON BCs receive wave-associated excitatory input via extrasynaptic glutamatergic transmission and gap junctions (Akrouh and Kerschensteiner 2013; Blankenship and others 2009; Firl and others 2013). In contrast to stage II waves, blockade of either coupling mechanism prevents spontaneous depolarizations of ON BCs, suggesting that their activity emerges from network interactions rather than intrinsic properties (Akrouh and Kerschensteiner 2013; Firl and others 2013).
Episodic activity in the developing avian spinal cord has been explained by the conjunction of two network properties (O’Donovan and others 1998). First, diverse transmitter systems (GABA, glycine, glutamate and ACh) mediate recurrent excitatory communication among immature spinal neurons that can culminate in spreading bursts of activity (Hanson and Landmesser 2003; Wenner and O’Donovan 2001). Second, at the end of each network event, excitability is depressed due in part to a negative shift in the reversal potential of Cl− (ECl) caused by its efflux during activity bursts (Chub and O’Donovan 2001; Marchetti and others 2005; Tabak and others 2001). This reduces the excitatory drive provided by GABA and glycine. Network excitability recovers slowly by NKCC-mediated Cl− import during prolonged refractory periods of spinal cord activity (Marchetti and others 2005).
Network transitions and homeostasis
A remarkable feature of developing circuits is their ability to reliably generate spontaneous activity while constantly changing neuronal connections. This homeostasis of network activity is mediated in part by gradual transitions between different circuit mechanisms that generate it. In spinal cord, hippocampus, neocortex and the retina, specific sequences of mechanisms that support spontaneous activity across development have been described (Allene and others 2008; Crepel and others 2007; Hanson and Landmesser 2003; Wong and others 2000; Zhou and Zhao 2000). The notion that transitions between circuit mechanisms evolved to stabilize network activity is supported by their role in homeostatic responses to a number of experimental perturbations.
Homeostasis of spontaneous network activity is particularly well characterized in the developing retina. When stage II retinal waves are disrupted by genetic deletion of choline acetyltransferase (ChAT−/− mice) or the β2 subunit of nAChRs (β2−/− mice), gap junctions (i.e. stage I mechanisms) continue to mediate spontaneous activity up to ~P8 (Stacy and others 2005; Stafford and others 2009; Sun and others 2008), when precocious stage III waves appear in β2−/− mice (Bansal and others 2000). In mice lacking the vesicular transporter required for glutamate release from bipolar cells (VGluT1−/− mice), cholinergic activity (i.e. stage II mechanisms) persists until eye opening (~P14), taking over the developmental shift normally covered by stage III waves (Blankenship and others 2009). Interestingly, acute pharmacological perturbations of stage II waves reactivate gap-junctional wave mechanisms, similar to what is observed in β2−/− and ChAT−/− mice (Stacy and others 2005). This suggests that the respective connections are suppressed, possibly by neuromodulatory influences, rather than disassembled (Kirkby and Feller 2013; Stacy and others 2005), presumably to enhance the robustness spontaneous network activity.
Similar cases of plasticity have been observed in the hippocampus and spinal cord (Chub and O’Donovan 1998; Crepel and others 2007; Sipila and others 2009), indicating that homeostatic control of spontaneous network activity may be a conserved feature of developing circuits.
Patterns of spontaneous network activity
Spontaneous activity in developing circuits commonly occurs in bursts separated by periods of quiescence. The spatiotemporal patterns of activity during bursts share characteristics between different circuits, due in part to conserved underlying mechanisms. Furthermore, activity can propagate stereotypically between circuits linked in neuronal pathways.
Lateral propagation
Spontaneous network activity spreads from a point of origin through the generating circuit (i.e. lateral propagation). The extent of activity propagation and the fraction of cells recruited separate network events into two broad categories: synchronized assemblies and spreading waves (Fig. 3).
Figure 3.
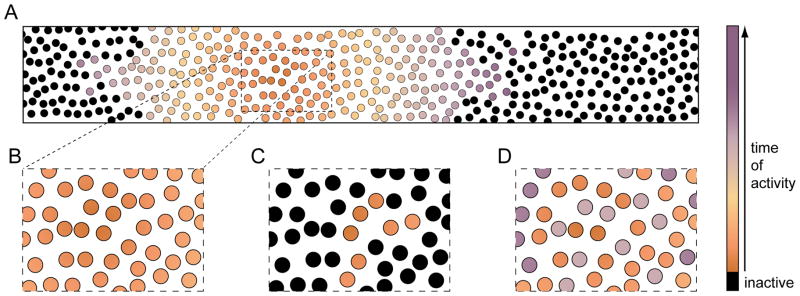
Characteristic patterns of neuronal activation during different spontaneous network events. (A) The large scale propagation of activity during spreading waves is illustrated by color-coding the time of cellular recruitment. (B) An excerpt from a smaller region shows homogenous and synchronous activation of nearby neurons. (C) By contrast, restricted clusters of neurons are activated during synchronized assemblies. (D) In some instances (e.g. stage III retinal waves), different types of neighboring neurons are recruited sequentially into spreading waves (i.e. precise asynchrony).
Synchronized assemblies engage sparse groups of local neurons (2–50 cells) via gap junctions (Fig. 3C) (Allene and others 2008; Catsicas and others 1998; Crepel and others 2007; Owens and Kriegstein 1998; Yuste and others 1992). Hippocampal and cortical SPAs exemplify activity in this category. Around birth, a subset of neurons in both circuits exhibit spontaneous prolonged (~10 s) bursts of Ca2+ spikes that spread to nearby electrically coupled neurons (Allene and others 2008; Crepel and others 2007). In combination with Hebbian plasticity rules, these activity patterns seemed ideally suited to promote the emergence of specific local circuits among intermingled cells (Peinado and others 1993). A link between early coactivation and later connectivity was recently demonstrated in visual cortex, where pyramidal neurons that are electrically coupled before P5 are subsequently more likely to form glutamatergic synapses with each other than nearby alternative partners, giving rise to functional cortical columns (Li and others 2012; Yu and others 2012). The bias in synaptogenesis is suppressed by genetic interference with early gap junctions (Yu and others 2012).
A couple of days after the onset of SPAs, hippocampal and neocortical circuits exhibit spreading waves of activity in the form of GDPs and early network oscillations (ENOs) (Allene and others 2008; Ben-Ari and others 1989; Crepel and others 2007; Garaschuk and others 1998; Garaschuk and others 2000). Unlike SPAs, these activity patterns rely on GABAergic (hippocampal and neocortical GDPs, hippocampal ENOs) and glutamatergic (neocortical ENOs) neurotransmission (Allene and others 2008; Crepel and others 2007; Garaschuk and others 1998; Garaschuk and others 2000). Spreading waves tend to propagate further and recruit cells more uniformly than synchronized assemblies (Fig. 3A, B). For example, neocortical ENOs, which most frequently initiate posteriorly, propagate several millimeters along the longitudinal axis of cortex, crossing boundaries between anatomically distinct subregions (Adelsberger and others 2005; Garaschuk and others 2000). Along their paths, ENOs recruit ~85% of neurons (Garaschuk and others 2000). Given the high participation rate of nearby cells in ENOs and patterns like it (Allene and others 2008; Crepel and others 2007; Firl and others 2013; Garaschuk and others 2000; Wong and others 1995), spreading waves are thought to influence the development of local circuits mostly by determining the level of synaptic activity. In support of this notion, genetic manipulations that enhance or block spontaneous glutamate release from retinal BCs during stage III waves, have been shown to increase and decrease, respectively, the rate of synapse formation in local retinal circuits (Kerschensteiner and others 2009; Soto and others 2012). Over longer distances, activity correlations between neurons gradually decline in spreading waves. This pattern is thought to sharpen the topographic organization of projections between subsequent stages of sensory pathways, in which spontaneous activity propagates not only laterally but also forward.
Like in hippocampus and neocortex, synchronized assemblies precede spreading waves in many developing circuits. A recent study in zebrafish spinal cord showed that transient optogenetic silencing of neurons participating in synchronized assemblies prevents the emergence of spreading waves (Warp and others 2012). Whether spontaneous activity is generally required for the assembly of small groups of synchronized neurons into larger networks remains to be tested.
Forward propagation
At maturity, sensory signals elicited in the periphery (e.g. cochlea, retina) traverse a series of circuits on their way to cortex (i.e. forward propagation). This raises the question whether spontaneous activity patterns generated by the developing cochlea and retina are relayed to cortex as well and, if so, how they shape the synaptic refinement of auditory and visual pathways.
In the cochlea, the preferred sound frequency of sensory neurons (IHCs) varies systematically from apex (high) to base (low) (Hudspeth 2008). This tonotopic organization is repeated throughout the auditory system and emerges sequentially in subsequent circuits (Kandler and others 2009). In the auditory brainstem, synaptic refinement coincides with the period of spontaneous activity in the cochlea, where, before the onset of hearing (P12 in mice), nearby IHCs are synchronously active (Johnson and others 2012; Tritsch and Bergles 2010; Tritsch and others 2007). The spontaneous firing patterns of IHCs were recently reported to vary between cells in the apex (bursting) and base (sustained) of the cochlea (Johnson and others 2011). Both distance-dependent correlations and positions-specific firing patterns of IHCs could in principle guide the tonotopic refinement of downstream circuits. However, evidence for forward propagation of spontaneous activity in the auditory system, so far, is limited. Spike bursts of developing IHCs have been shown to activate spiral ganglion neurons (SGNs), which project to the cochlear nucleus (CN) (Jones and others 2007; Lippe 1994; Tritsch and Bergles 2010). But, whether spontaneous activity reaches secondary brain stem nuclei and possibly spreads further toward inferior colliculus, thalamus and auditory cortex, is unknown. Likewise, whether spontaneous activity patterns generated in the cochlea instruct tonotopic refinement of these circuits remains to be tested (Kandler and others 2009).
By contrast, waves of activity generated in the retina, have been shown to propagate through subcortical way stations to V1 in vivo (Ackman and others 2012; Hanganu and others 2006; Siegel and others 2012). Most RGC axons target the dLGN SC, where, guided by molecular cues, they initially form imprecise retinotopic maps (Clandinin and Feldheim 2009; Huberman and others 2008). During the period of stage II waves, which correlate retinal activity over 300–600 μm (Feller and others 1997; Stafford and others 2009), RGC axons are refined, adding branches in narrow termination zones and pruning ectopic processes (Dhande and others 2011). In β2−/− mice, in which stage II waves are replaced by less reliable activity patterns with different spatiotemporal properties (Stafford and others 2009; Sun and others 2008; Xu and others 2011), topographic refinement of RGC axons is disrupted (Chandrasekaran and others 2005; Dhande and others 2011; Grubb and others 2003; McLaughlin and others 2003; Mrsic-Flogel and others 2005). Interestingly, transgenic expression of β2 nAChRs in RGCs of β2−/− mice (β2TG mice), which restores activity correlations between nearby cells to normal levels, was shown to rescue retinotopic mapping defects, suggesting that patterns of spontaneous activity can instruct the topographic refinement of connectivity patterns in developing circuits (Xu and others 2011).
Retinotopic organization is maintained in the projections from dLGN to V1. Similar to retinofugal connections, geniculocortical maps are established prior to vision (Lund and Mustari 1977). When stage II waves are perturbed genetically or pharmacologically, topographic maps from dLGN to V1 are degraded in a manner reminiscent of defects in subcortical retinotopic maps (Cang and others 2008; Cang and others 2005). In addition to RGC axons, SC receives visual input from V1 (Wang and Burkhalter 2013). The respective maps are retinotopically aligned (Rhoades and Chalupa 1978). In mice that ectopically express EphA3 in approximately half of their RGCs (EphA3ki/ki), retinocollicular maps are duplicated but a single map is maintained in V1 (Triplett and others 2009). In EphA3ki/ki mice, corticollicular projections bifurcate to align with both retinotopic maps in SC. This alignment is prevented by disruption of stage II retinal waves (Triplett and others 2009). Together, these findings suggest that forward propagation of retinal waves serves to establish repeated and aligned retinotopic maps in the visual system.
In most mammals, the central part of the visual field is seen by both eyes. To support binocular vision, RGC axons from the nasal retina project to subcortical targets on the same side, whereas RGC axons from the temporal retina cross at the optic chiasm (Huberman and others 2008). Ipsi- and contralateral projections occupy separate territories in dLGN and SC. In both structures, eye-specific segregation emerges from initially overlapping projections during the period of stage II waves (Demas and others 2006; Godement and others 1984). These waves have long been suggested to promote eye-specific segregation because they encompass large portions of one retina and generally occur during silence in the other (Butts and Rokhsar 2001; Meister and others 1991; Wong and others 1993). Pharmacological and genetic manipulations that interfere with stage II waves, indeed, were shown to disrupt the separation of RGC axons from opposite eyes in dLGN and SC (Chandrasekaran and others 2005; Penn and others 1998; Rossi and others 2001; Shatz and Stryker 1988). However, because most experimental perturbations affected the levels of activity as well as its patterns, the question whether retinal waves play an instructive or permissive role in eye-specific segregation remained contentious (Chalupa 2009; Feller 2009). In β2TG mice, activity levels of RGCs are normal, but lateral wave propagation is truncated (Xu and others 2011). This both decreases, on average, the correlation of activity among RGCs of the same eye, and, as waves are generated more frequently in each retina, increases the likelihood that they coincide in both eyes. The persistent overlap of ipsi- and contralateral projections in dLGN and SC of β2TG mice argues that activity patterns of stage II waves instruct eye-specific segregation (Xu and others 2011). The effect of the binocular timing of activity on eye-specific segregation has since been examined systematically using optogenetics (Zhang and others 2012). In mice expressing channelrhodopsin-2 (ChR2) in ~45% of RGCs, repeated synchronous (±100 ms) stimulation of cells in both eyes for 2–3 days between P5 – P8, prevented eye-specific segregation in dLGN and SC, further supporting the notion that the asynchronous firing of RGCs in both eyes during stage II waves guides this process via BTDP (Zhang and others 2012).
In many carnivores and primates, axons of dLGN neurons are organized into stripes in V1 that separate input from the two eyes and give rise to ocular dominance columns (ODCs) (Hubel and Wiesel 1962; Wiesel and others 1974). Although ocular dominance in V1 neurons can famously be shifted by early visual experience (Wiesel and Hubel 1963), ODCs emerge prior to vision (Crair and others 2001; Des Rosiers and others 1978; Horton and Hocking 1996; Rakic 1976). In addition to molecular cues, spontaneous activity in V1, driven in part by forward propagation of retinal waves, may guide ODC development (Espinosa and Stryker 2012). This is supported by the observation that binocular injections of TTX prevent the refinement of geniculocortical axon arbors and the maintenance of ODCs in cats (Antonini and Stryker 1993; Stryker and Harris 1986). Similarly, subplate ablation, which decouples cortical activity patterns from thalamic input, disrupts the formation of ODCs (Kanold and others 2003). Although mice lack ODCs, they have a binocular region in V1. In β2−/− mice, this binocular region is expanded, providing additional evidence that retinal waves may shape ocular dominance (Rossi and others 2001).
Feedback loops
Feedback connections between circuits are a common feature of sensory and other neuronal pathways, and can regulate the gain and timing of signal transmission (Briggs and Usrey 2008). During development, excitatory feedback from the subplate and layer 6 of V1 to dLGN can enhance transmission of retinal waves and generate correlated activity independent of retinal input (Kilb and others 2011; Weliky and Katz 1999). Accordingly, even after removal of both eyes, spontaneous activity persists in the developing dLGN, but is abolished by additional disruption of corticothalamic feedback (Weliky and Katz 1999). Feedback connections may contribute similarly to spontaneous activity in somatosensory and auditory pathways (Kilb and others 2011). Interestingly, cortical innervation of dLGN occurs earlier than normal in enucleated mice, suggesting that its timing is controlled in part by retinal input (Seabrook and others 2013).
Cortex and thalamus are also part of a loop through the basal ganglia (BG) (Alexander and others 1986). In this loop, cortical input to striatum (i.e. the largest component of the BG) enters two parallel pathways (direct vs. indirect). The output of both pathways converges in the thalamus from where projections go back to cortex (Alexander and Crutcher 1990; Gerfen 1992; Smith and others 1998). Whereas the direct pathway through the BG is part of a positive feedback loop, activation of the indirect pathway suppresses cortical input to striatum (i.e. negative feedback) (Ferguson and others 2011; Kozorovitskiy and others 2012). Using a combination of transgenic mice and viral gene delivery, a recent study demonstrated that selective silencing of the direct or indirect pathway decreases or increases, respectively, the formation excitatory synapses in striatum in vivo (Kozorovitskiy and others 2012). These findings imply that during development spontaneous activity propagates through multi-stage loops involving cortex, BG and thalamus and highlight the importance of balance between direct and indirect pathways for appropriate synaptic development.
Precise asynchrony
During development, spontaneous activity patterns in the spinal cord undergo a remarkable transition in their left-right coordination. Whereas early activity (before birth in rodents) synchronizes central pattern generators (CPGs) on both sides, later bursts of activity (after birth in rodents) occur antiphasically in left and right CPGs (Nakayama and others 2002). Commissural interneurons, which release GABA during development and glycine at maturity, coordinate the bilateral recruitment of CPGs (Grillner 2006). While [Cl−]i in developing spinal neurons is high, commissural interneurons on each side excite CPGs on the other, synchronizing activity bilaterally. As [Cl−]i is lowered with maturation, GABA and glycine release from commissural interneurons inhibits contralateral CPGs, generating alternating spontaneous activity patterns, which are critical for locomotion (Hanson and Landmesser 2003; Nakayama and others 2002).
Another example of precise asynchrony (Fig. 3D) is found in the activity of circuits with opposite visual function in stage III retinal waves. During vision, photoreceptor signals are transmitted to BCs, which, by expressing metabotropic or ionotropic glutamate receptors on their dendrites, depolarize (ON BCs) or hyperpolarize (OFF BCs), respectively, to light. BCs form glutamatergic synapses with a diverse class of interneurons (i.e. amacrine cells, ACs) and RGCs, the output neurons of the eye. Most RGCs form synapses with either ON or OFF BCs and inherit their response sign. ON and OFF pathways remain separate in dLGN, but converge in V1, where input from retinotopically offset clusters of ON and OFF dLGN neurons, among other mechanisms (Niell 2013), gives rise to orientation selective responses.
Axons from ON and OFF RGCs segregate in dLGN prior to vision and neurons in V1 are orientation selective at eye opening. This raises the question whether retinal waves contain patterns that could instruct ON/OFF segregation and the development of orientation selectivity. Simultaneous recordings from ensembles of RGCs in mice, revealed that during stage III waves neighboring ON and OFF RGCs fire non-overlapping bursts of action potentials in a fixed order: ON before OFF (Kerschensteiner and Wong 2008). By contrast, the activity of same sign neuron pairs (ON-ON, OFF-OFF) is synchronized. Whereas in vision the dichotomy of ON and OFF signals arises at the synapse between photoreceptors and bipolar cells, circuits in the inner retina generate asynchronous activity of ON and OFF RGCs during waves (Akrouh and Kerschensteiner 2013; Blankenship and others 2009; Firl and others 2013). In the respective circuits (Fig. 2B), ON BCs communicate laterally via gap junctions and extrasynaptic glutamatergic transmission to generate and propagate stage III waves (Akrouh and Kerschensteiner 2013). In addition, they activate diffuse ACs, which inhibit and delay glutamate release from OFF BCs. This presynaptic crossover inhibition locally desynchronize the activity of ON and OFF RGCs within spreading waves (Akrouh and Kerschensteiner 2013; Kerschensteiner and Wong 2008).
The notion that asynchronous spontaneous activity of ON and OFF RGCs may instruct the segregation of their axons in dLGN is supported by the following observations. First, in dLGNs of β2−/− mice, which show precocious stage III waves, ON and OFF responsive neurons cluster rather than intermingle, suggesting excessive segregation of retinal inputs (Grubb and others 2003). Second, pharmacological blockade of retinal activity or its transmission to dLGN neurons during the period of stage III waves, prevents ON/OFF segregation (Cramer and Sur 1997; Dubin and others 1986; Hahm and others 1991). Third, artificial neuronal networks with BTPD rules similar to those observed in dLGN undergo reliable ON/OFF segregation in response to stage III wave patterns (Gjorgjieva and others 2009; Kerschensteiner and Wong 2008).
The local asynchrony of stage III waves seems to match predictions for activity patterns that could drive the emergence of orientation selective responses in V1 via Hebbian plasticity (Miller 1994), and cortical activity blockade prevents the maturation of orientation selectivity (Chapman and Stryker 1993). However, both for ON/OFF segregation in dLGN and orientation selectivity in V1 an instructive role for stage III waves in their development remains to be experimentally tested.
Conclusions and Outlook
The developing nervous system internally generates patterned activity, which regulates the formation and refinement of synaptic circuits. Research over the past two decades has identified a set of common mechanisms that mediate spontaneous activity in emerging circuits. In addition, recurring patterns of local recruitment and activity propagation between circuits have been described. The effects of activity on synaptic development are governed by Hebbian and non-Hebbian plasticity rules with distinct spatial, temporal and cell-type specific properties. In turn, synaptic maturation mediates transitions between different mechanisms and patterns of spontaneous activity.
In spite of recent insights, much remains to be learned about the complex interactions between spontaneous activity and synaptic development. Technological advances that enable cell-type specific, temporally and spatially precise control of neuronal activity in vivo allow specific hypothesis to be tested in intact systems (Fenno and others 2011; Packer and others 2013; Warp and others 2012; Zhang and others 2012). Furthermore, the development of sensitive genetically encoded Ca2+- and voltage indicators and neurotransmitter sensors combined with innovative imaging approaches permit direct observation of neuronal activity at unprecedented scale in vivo (Ahrens and others 2013; Chen and others 2013; Grienberger and Konnerth 2012; Holekamp and others 2008; Marvin and others 2013; Peterka and others 2011). Application of these new tools will advance our understanding of spontaneous network activity and synaptic development and promises exciting years ahead.
Acknowledgments
I thank Dr. F. Soto for critical reading of the manuscript
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Eye Institute R01 (EY021855), the Sloan Foundation, Whitehall Foundation and Edward Mallinckrodt Jr. Foundation
Footnotes
Declaration of Conflicting Interests: The author declares no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abreu-Villaca Y, Filgueiras CC, Manhaes AC. Developmental aspects of the cholinergic system. Behav Brain Res. 2011;221(2):367–78. doi: 10.1016/j.bbr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490(7419):219–25. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8(8):988–90. doi: 10.1038/nn1502. [DOI] [PubMed] [Google Scholar]
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10(5):413–20. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- Akemann W, Knopfel T. Interaction of Kv3 potassium channels and resurgent sodium current influences the rate of spontaneous firing of Purkinje neurons. J Neurosci. 2006;26(17):4602–12. doi: 10.1523/JNEUROSCI.5204-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci. 2006;26(19):5117–30. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akrouh A, Kerschensteiner D. Intersecting circuits generate precisely patterned retinal waves. Neuron. 2013;79(2):322–34. doi: 10.1016/j.neuron.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, et al. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 2008;28(48):12851–63. doi: 10.1523/JNEUROSCI.3733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Development of individual geniculocortical arbors in cat striate cortex and effects of binocular impulse blockade. J Neurosci. 1993;13(8):3549–73. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20(20):7672–81. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24(6):353–60. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–25. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9(3):293–8. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61(6):820–38. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26(41):10407–19. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, et al. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62(2):230–41. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol. 1999;81(5):2095–102. doi: 10.1152/jn.1999.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–86. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429(6991):523–30. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Brandt N, Kuhn S, Munkner S, Braig C, Winter H, Blin N, et al. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci. 2007;27(12):3174–86. doi: 10.1523/JNEUROSCI.3965-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18(4):403–7. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420(6914):414–8. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO. The applicability of spike time dependent plasticity to development. Front Synaptic Neurosci. 2010;2:30. doi: 10.3389/fnsyn.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based “Hebbian” learning rule at retinogeniculate synapses links retinal waves to activity-dependent refinement. PLoS Biol. 2007;5(3):e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Rokhsar DS. The information content of spontaneous retinal waves. J Neurosci. 2001;21(3):961–73. doi: 10.1523/JNEUROSCI.21-03-00961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron. 2008;57(4):511–23. doi: 10.1016/j.neuron.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Renteria RC, Kaneko M, Liu X, Copenhagen DR, Stryker MP. Development of precise maps in visual cortex requires patterned spontaneous activity in the retina. Neuron. 2005;48(5):797–809. doi: 10.1016/j.neuron.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. doi: 10.1146/annurev.neuro.31.060407.125639. [DOI] [PubMed] [Google Scholar]
- Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol. 1998;8(5):283–6. doi: 10.1016/s0960-9822(98)70110-1. [DOI] [PubMed] [Google Scholar]
- Cattani AA, Bonfardin VD, Represa A, Ben-Ari Y, Aniksztejn L. Generation of slow network oscillations in the developing rat hippocampus after blockade of glutamate uptake. J Neurophysiol. 2007;98(4):2324–36. doi: 10.1152/jn.00378.2007. [DOI] [PubMed] [Google Scholar]
- Chalupa LM. Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:25. doi: 10.1186/1749-8104-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005;25(29):6929–38. doi: 10.1523/JNEUROSCI.1470-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27(7):1746–55. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993;13(12):5251–62. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, O’Donovan MJ. Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J Neurosci. 1998;18(1):294–306. doi: 10.1523/JNEUROSCI.18-01-00294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, O’Donovan MJ. Post-episode depression of GABAergic transmission in spinal neurons of the chick embryo. J Neurophysiol. 2001;85(5):2166–76. doi: 10.1152/jn.2001.85.5.2166. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Feldheim DA. Making a visual map: mechanisms and molecules. Curr Opin Neurobiol. 2009;19(2):174–80. doi: 10.1016/j.conb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Horton JC, Antonini A, Stryker MP. Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age. J Comp Neurol. 2001;430(2):235–49. doi: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KS, Sur M. Blockade of afferent impulse activity disrupts on/off sublamination in the ferret lateral geniculate nucleus. Brain Res Dev Brain Res. 1997;98(2):287–90. doi: 10.1016/s0165-3806(96)00188-5. [DOI] [PubMed] [Google Scholar]
- Crepel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 2007;54(1):105–20. doi: 10.1016/j.neuron.2007.03.007. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, O’Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12(4):1194–211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy A, Allain AE, Meyrand P, Branchereau P. NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J Physiol. 2008;586(4):1059–75. doi: 10.1113/jphysiol.2007.146993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36(6):1051–61. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben-Ari Y, et al. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci. 2004;24(13):3289–94. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas J, Sagdullaev BT, Green E, Jaubert-Miazza L, McCall MA, Gregg RG, et al. Failure to maintain eye-specific segregation in nob, a mutant with abnormally patterned retinal activity. Neuron. 2006;50(2):247–59. doi: 10.1016/j.neuron.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Des Rosiers MH, Sakurada O, Jehle J, Shinohara M, Kennedy C, Sokoloff L. Functional plasticity in the immature striate cortex of the monkey shown by the [14C]deoxyglucose method. Science. 1978;200(4340):447–9. doi: 10.1126/science.417397. [DOI] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, et al. Development of single retinofugal axon arbors in normal and beta2 knock-out mice. J Neurosci. 2011;31(9):3384–99. doi: 10.1523/JNEUROSCI.4899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du JL, Wei HP, Wang ZR, Wong ST, Poo MM. Long-range retrograde spread of LTP and LTD from optic tectum to retina. Proc Natl Acad Sci U S A. 2009;106(45):18890–6. doi: 10.1073/pnas.0910659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin MW, Stark LA, Archer SM. A role for action-potential activity in the development of neuronal connections in the kitten retinogeniculate pathway. J Neurosci. 1986;6(4):1021–36. doi: 10.1523/JNEUROSCI.06-04-01021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439(7072):79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–49. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Butts DA, Aaron HL, Rokhsar DS, Shatz CJ. Dynamic processes shape spatiotemporal properties of retinal waves. Neuron. 1997;19(2):293–306. doi: 10.1016/s0896-6273(00)80940-x. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272(5265):1182–7. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–4. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firl A, Sack GS, Newman ZL, Tani H, Feller MB. Extrasynaptic glutamate and inhibitory neurotransmission modulate ganglion cell participation during glutamatergic retinal waves. J Neurophysiol. 2013;109(7):1969–78. doi: 10.1152/jn.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998;18(10):3767–78. doi: 10.1523/JNEUROSCI.18-10-03767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Arroyo DA, Kay JN, Lloyd EE, Bryan RM, Jr, Sanes JR, et al. A role for TREK1 in generating the slow afterhyperpolarization in developing starburst amacrine cells. J Neurophysiol. 2013;109(9):2250–9. doi: 10.1152/jn.01085.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KJ, Felix AL, Feller MB. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci. 2012;32(3):850–63. doi: 10.1523/JNEUROSCI.5309-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron. 2013;77(1):19–34. doi: 10.1016/j.neuron.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990;10(8):2601–13. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Panzanelli P, Kralic JE, Vogt KE, Sassoe-Pognetto M. Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAA receptors containing the alpha1 subunit in Purkinje cells. J Neurosci. 2006;26(12):3245–55. doi: 10.1523/JNEUROSCI.5118-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507 ( Pt 1):219–36. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Linn J, Eilers J, Konnerth A. Large-scale oscillatory calcium waves in the immature cortex. Nat Neurosci. 2000;3(5):452–9. doi: 10.1038/74823. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gjorgjieva J, Toyoizumi T, Eglen SJ. Burst-time-dependent plasticity robustly guides ON/OFF segregation in the lateral geniculate nucleus. PLoS Comput Biol. 2009;5(12):e1000618. doi: 10.1371/journal.pcbi.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230(4):552–75. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G. GABA transporter GAT1: a crucial determinant of GABAB receptor activation in cortical circuits? Adv Pharmacol. 2010;58:175–204. doi: 10.1016/S1054-3589(10)58008-6. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Raman IM. Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin. J Neurosci. 2004;24(1):35–42. doi: 10.1523/JNEUROSCI.3807-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73(5):862–85. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52(5):751–66. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40(6):1161–72. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Hahm JO, Langdon RB, Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991;351(6327):568–70. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Ben-Ari Y, Khazipov R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J Neurosci. 2006;26(25):6728–36. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex. 2001;11(5):400–10. doi: 10.1093/cercor/11.5.400. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 2002;22(16):7165–76. doi: 10.1523/JNEUROSCI.22-16-07165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci. 2003;23(2):587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9(5):642–9. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450(7173):1195–200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321(5885):136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Hennig MH, Adams C, Willshaw D, Sernagor E. Early-stage waves in the retinal network emerge close to a critical state transition between local and global functional connectivity. J Neurosci. 2009;29(4):1077–86. doi: 10.1523/JNEUROSCI.4880-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakic S, et al. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19(8):1738–50. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Holekamp TF, Turaga D, Holy TE. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron. 2008;57(5):661–72. doi: 10.1016/j.neuron.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52(2):281–91. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hocking DR. An adult-like pattern of ocular dominance columns in striate cortex of newborn monkeys prior to visual experience. J Neurosci. 1996;16(5):1791–807. doi: 10.1523/JNEUROSCI.16-05-01791.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434(7036):1022–6. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–54. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron. 2008;59(4):530–45. doi: 10.1016/j.neuron.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57(6):819–26. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–7. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Tian N, Caywood MS, Reimer RJ, Edwards RH, Copenhagen DR. Vesicular neurotransmitter transporter expression in developing postnatal rodent retina: GABA and glycine precede glutamate. J Neurosci. 2003;23(2):518–29. doi: 10.1523/JNEUROSCI.23-02-00518.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, et al. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci. 2011;14(6):711–7. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kennedy HJ, Holley MC, Fettiplace R, Marcotti W. The resting transducer current drives spontaneous activity in prehearing mammalian cochlear inner hair cells. J Neurosci. 2012;32(31):10479–83. doi: 10.1523/JNEUROSCI.0803-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Kuhn S, Franz C, Ingham N, Furness DN, Knipper M, et al. Presynaptic maturation in auditory hair cells requires a critical period of sensory-independent spiking activity. Proc Natl Acad Sci U S A. 2013;110(21):8720–5. doi: 10.1073/pnas.1219578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B. Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol. 2007;98(4):1898–908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330(6144):163–5. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain Res. 2001;901(1–2):296–302. doi: 10.1016/s0006-8993(01)02300-9. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12(6):711–7. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Katz LC. Coordination of neuronal activity in developing visual cortex by gap junction-mediated biochemical communication. J Neurosci. 1998;18(4):1419–27. doi: 10.1523/JNEUROSCI.18-04-01419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301(5632):521–5. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51(5):627–38. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460(7258):1016–20. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Wong RO. A precisely timed asynchronous pattern of ON and OFF retinal ganglion cell activity during propagation of retinal waves. Neuron. 2008;58(6):851–8. doi: 10.1016/j.neuron.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W, Kirischuk S, Luhmann HJ. Electrical activity patterns and the functional maturation of the neocortex. Eur J Neurosci. 2011;34(10):1677–86. doi: 10.1111/j.1460-9568.2011.07878.x. [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Feller MB. Intrinsically photosensitive ganglion cells contribute to plasticity in retinal wave circuits. Proc Natl Acad Sci U S A. 2013;110(29):12090–5. doi: 10.1073/pnas.1222150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011;72(6):1012–24. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Knipper M, Gestwa L, Ten Cate WJ, Lautermann J, Brugger H, Maier H, et al. Distinct thyroid hormone-dependent expression of TrKB and p75NGFR in nonneuronal cells during the critical TH-dependent period of the cochlea. J Neurobiol. 1999;38(3):338–56. [PubMed] [Google Scholar]
- Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17(2):275–85. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9(2):219–42. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–70. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485(7400):646–50. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus HJ, Aulbach-Kraus K. Morphological changes in the cochlea of the mouse after the onset of hearing. Hear Res. 1981;4(1):89–102. doi: 10.1016/0378-5955(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84(3):381–8. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68(6):1159–72. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18(2):243–55. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487 ( Pt 2):319–29. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Sanderson MJ. Intercellular Ca(2+) waves: mechanisms and function. Physiol Rev. 2012;92(3):1359–92. doi: 10.1152/physrev.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lu H, Cheng PL, Ge S, Xu H, Shi SH, et al. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature. 2012;486(7401):118–21. doi: 10.1038/nature11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14(3 Pt 2):1486–95. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8(9):1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59(2):253–60. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Myhr KL, Wong RO. Transmitter-evoked local calcium release stabilizes developing dendrites. Nature. 2002;418(6894):177–81. doi: 10.1038/nature00850. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15(6):1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. J Comp Neurol. 1977;173(2):289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Mammano F. ATP-dependent intercellular Ca2+ signaling in the developing cochlea: facts, fantasies and perspectives. Semin Cell Dev Biol. 2013;24(1):31–9. doi: 10.1016/j.semcdb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Manent JB, Demarque M, Jorquera I, Pellegrino C, Ben-Ari Y, Aniksztejn L, et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J Neurosci. 2005;25(19):4755–65. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Tabak J, Chub N, O’Donovan MJ, Rinzel J. Modeling spontaneous activity in the developing spinal cord using activity-dependent variations of intracellular chloride. J Neurosci. 2005;25(14):3601–12. doi: 10.1523/JNEUROSCI.4290-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548(Pt 2):383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KW, Kurtz LM, Spitzer NC. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat Neurosci. 2010;13(8):944–50. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10(2):162–70. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119(3):689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245(4921):978–82. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Torborg CL, Feller MB, O’Leary DD. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron. 2003;40(6):1147–60. doi: 10.1016/s0896-6273(03)00790-6. [DOI] [PubMed] [Google Scholar]
- McLean HA, Caillard O, Khazipov R, Ben-Ari Y, Gaiarsa JL. Spontaneous release of GABA activates GABAB receptors and controls network activity in the neonatal rat hippocampus. J Neurophysiol. 1996;76(2):1036–46. doi: 10.1152/jn.1996.76.2.1036. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252(5008):939–43. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Neural activity and survival in the developing nervous system. Mol Neurobiol. 2000;22(1–3):41–54. doi: 10.1385/MN:22:1-3:041. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26(51):13297–310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. A model for the development of simple cell receptive fields and the ordered arrangement of orientation columns through activity-dependent competition between ON- and OFF-center inputs. J Neurosci. 1994;14(1):409–41. doi: 10.1523/JNEUROSCI.14-01-00409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Soto F, Wong RO, Kerschensteiner D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron. 2011;71(6):1014–21. doi: 10.1016/j.neuron.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Creutzfeldt C, Cloez-Tayarani I, Changeux JP, Bonhoeffer T, et al. Altered map of visual space in the superior colliculus of mice lacking early retinal waves. J Neurosci. 2005;25(29):6921–8. doi: 10.1523/JNEUROSCI.1555-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, Van Eden CG. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271(3):355–86. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Kudo N. Basis of changes in left-right coordination of rhythmic motor activity during development in the rat spinal cord. J Neurosci. 2002;22(23):10388–98. doi: 10.1523/JNEUROSCI.22-23-10388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM. Vision: more than expected in the early visual system. Curr Biol. 2013;23(16):R681–4. doi: 10.1016/j.cub.2013.07.049. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Iizuka M, Ozaki S, Kudo N. Spontaneous motoneuronal activity mediated by glycine and GABA in the spinal cord of rat fetuses in vitro. J Physiol. 1996;497 (Pt 1):131–43. doi: 10.1113/jphysiol.1996.sp021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci U S A. 2005;102(14):5245–9. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9(1):94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ, Chub N, Wenner P. Mechanisms of spontaneous activity in developing spinal networks. J Neurobiol. 1998;37(1):131–45. doi: 10.1002/(sici)1097-4695(199810)37:1<131::aid-neu10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ, Landmesser L. The development of hindlimb motor activity studied in the isolated spinal cord of the chick embryo. J Neurosci. 1987;7(10):3256–64. doi: 10.1523/JNEUROSCI.07-10-03256.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12(4):1394–408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff H, Hatten ME. Gene expression profiling of preplate neurons destined for the subplate: genes involved in transcription, axon extension, neurotransmitter regulation, steroid hormone signaling, and neuronal survival. Cereb Cortex. 2009;19(Suppl 1):i126–34. doi: 10.1093/cercor/bhp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Patterns of intracellular calcium fluctuation in precursor cells of the neocortical ventricular zone. J Neurosci. 1998;18(14):5374–88. doi: 10.1523/JNEUROSCI.18-14-05374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–27. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16(7):805–15. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrizi A, Scelfo B, Viltono L, Briatore F, Fukaya M, Watanabe M, et al. Synapse formation and clustering of neuroligin-2 in the absence of GABAA receptors. Proc Natl Acad Sci U S A. 2008;105(35):13151–6. doi: 10.1073/pnas.0802390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Luneborg NL, Becker DL, Mobbs P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 2005;25(46):10803–14. doi: 10.1523/JNEUROSCI.2312-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A. Immature neocortical neurons exist as extensive syncitial networks linked by dendrodendritic electrical connections. J Neurophysiol. 2001;85(2):620–9. doi: 10.1152/jn.2001.85.2.620. [DOI] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 1993;3(5):488–98. doi: 10.1093/cercor/3.5.488. [DOI] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279(5359):2108–12. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Peterka DS, Takahashi H, Yuste R. Imaging voltage in neurons. Neuron. 2011;69(1):9–21. doi: 10.1016/j.neuron.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–51. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261(5560):467–71. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006;16(Suppl 1):i3–17. doi: 10.1093/cercor/bhk036. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17(12):4517–26. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Functional properties of the corticotectal projection in the golden hamster. J Comp Neurol. 1978;180(3):617–34. doi: 10.1002/cne.901800312. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001;98(11):6453–8. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruediger T, Bolz J. Neurotransmitters and the development of neuronal circuits. Adv Exp Med Biol. 2007;621:104–15. doi: 10.1007/978-0-387-76715-4_8. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301(5629):66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Scain AL, Le Corronc H, Allain AE, Muller E, Rigo JM, Meyrand P, et al. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci. 2010;30(1):390–403. doi: 10.1523/JNEUROSCI.2115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz M, Scimemi P, Majumder P, De Siati RD, Crispino G, Rodriguez L, et al. The human deafness-associated connexin 30 T5M mutation causes mild hearing loss and reduces biochemical coupling among cochlear non-sensory cells in knock-in mice. Hum Mol Genet. 2010;19(24):4759–73. doi: 10.1093/hmg/ddq402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, El-Danaf RN, Krahe TE, Fox MA, Guido W. Retinal input regulates the timing of corticogeniculate innervation. J Neurosci. 2013;33(24):10085–97. doi: 10.1523/JNEUROSCI.5271-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci. 2003;23(20):7621–9. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah RD, Crair MC. Retinocollicular synapse maturation and plasticity are regulated by correlated retinal waves. J Neurosci. 2008;28(1):292–303. doi: 10.1523/JNEUROSCI.4276-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifullina E, Nistri A. Glutamate uptake block triggers deadly rhythmic bursting of neonatal rat hypoglossal motoneurons. J Physiol. 2006;572(Pt 2):407–23. doi: 10.1113/jphysiol.2005.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242(4875):87–9. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Siegel F, Heimel JA, Peters J, Lohmann C. Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Curr Biol. 2012;22(3):253–8. doi: 10.1016/j.cub.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25(22):5280–9. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci. 2006;23(9):2330–8. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Yamada J, Afzalov R, Voipio J, Blaesse P, et al. Compensatory enhancement of intrinsic spiking upon NKCC1 disruption in neonatal hippocampus. J Neurosci. 2009;29(21):6982–8. doi: 10.1523/JNEUROSCI.0443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–87. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Sohl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6(3):191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Soto F, Ma X, Cecil JL, Vo BQ, Culican SM, Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci. 2012;32(16):5426–39. doi: 10.1523/JNEUROSCI.0194-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444(7120):707–12. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13(2):94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy RC, Demas J, Burgess RW, Sanes JR, Wong RO. Disruption and recovery of patterned retinal activity in the absence of acetylcholine. J Neurosci. 2005;25(41):9347–57. doi: 10.1523/JNEUROSCI.1800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford BK, Sher A, Litke AM, Feldheim DA. Spatial-temporal patterns of retinal waves underlying activity-dependent refinement of retinofugal projections. Neuron. 2009;64(2):200–12. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6(8):2117–33. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Warland DK, Ballesteros JM, van der List D, Chalupa LM. Retinal waves in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 2008;105(36):13638–43. doi: 10.1073/pnas.0807178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed MM, Lee S, Zheng J, Zhou ZJ. Stage-dependent dynamics and modulation of spontaneous waves in the developing rabbit retina. J Physiol. 2004;560(Pt 2):533–49. doi: 10.1113/jphysiol.2004.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak J, Rinzel J, O’Donovan MJ. The role of activity-dependent network depression in the expression and self-regulation of spontaneous activity in the developing spinal cord. J Neurosci. 2001;21(22):8966–78. doi: 10.1523/JNEUROSCI.21-22-08966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Tian H, Diamond JS. The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. J Neurosci. 2011;31(12):4743–54. doi: 10.1523/JNEUROSCI.5953-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titz S, Hans M, Kelsch W, Lewen A, Swandulla D, Misgeld U. Hyperpolarizing inhibition develops without trophic support by GABA in cultured rat midbrain neurons. J Physiol. 2003;550(Pt 3):719–30. doi: 10.1113/jphysiol.2003.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci. 2012;32(2):692–702. doi: 10.1523/JNEUROSCI.1538-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, et al. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139(1):175–85. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30(4):1539–50. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13(9):1050–2. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450(7166):50–5. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–35. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5(2):97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Uesaka N, Ruthazer ES, Yamamoto N. The role of neural activity in cortical axon branching. Neuroscientist. 2006;12(2):102–6. doi: 10.1177/1073858405281673. [DOI] [PubMed] [Google Scholar]
- van Welie I, Smith IT, Watt AJ. The metamorphosis of the developing cerebellar microcircuit. Curr Opin Neurobiol. 2011;21(2):245–53. doi: 10.1016/j.conb.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 2012;32(5):1589–601. doi: 10.1523/JNEUROSCI.4748-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Opitz T, de Lima AD. Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J Neurosci. 2001;21(22):8895–905. doi: 10.1523/JNEUROSCI.21-22-08895.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J Neurosci. 2000;20(4):1414–23. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Blankenship AG, Anishchenko A, Elstrott J, Fikhman M, Nakanishi S, et al. GABA(A) receptor-mediated signaling alters the structure of spontaneous activity in the developing retina. J Neurosci. 2007;27(34):9130–40. doi: 10.1523/JNEUROSCI.1293-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Burkhalter A. Stream-related preferences of inputs to the superior colliculus from areas of dorsal and ventral streams of mouse visual cortex. J Neurosci. 2013;33(4):1696–705. doi: 10.1523/JNEUROSCI.3067-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warp E, Agarwal G, Wyart C, Friedmann D, Oldfield CS, Conner A, et al. Emergence of patterned activity in the developing zebrafish spinal cord. Curr Biol. 2012;22(2):93–102. doi: 10.1016/j.cub.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12(4):463–73. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43(5):647–61. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Weliky M, Katz LC. Correlational structure of spontaneous neuronal activity in the developing lateral geniculate nucleus in vivo. Science. 1999;285(5427):599–604. doi: 10.1126/science.285.5427.599. [DOI] [PubMed] [Google Scholar]
- Wenner P, O’Donovan MJ. Mechanisms that initiate spontaneous network activity in the developing chick spinal cord. J Neurophysiol. 2001;86(3):1481–98. doi: 10.1152/jn.2001.86.3.1481. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–17. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79(2):273–9. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wong RO, Chernjavsky A, Smith SJ, Shatz CJ. Early functional neural networks in the developing retina. Nature. 1995;374(6524):716–8. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3(10):803–12. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11(5):923–38. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong WT, Myhr KL, Miller ED, Wong RO. Developmental changes in the neurotransmitter regulation of correlated spontaneous retinal activity. J Neurosci. 2000;20(1):351–60. doi: 10.1523/JNEUROSCI.20-01-00351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Sanes JR, Wong RO. Developmentally regulated spontaneous activity in the embryonic chick retina. J Neurosci. 1998;18(21):8839–52. doi: 10.1523/JNEUROSCI.18-21-08839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron. 2011;70(6):1115–27. doi: 10.1016/j.neuron.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacubova E, Komuro H. Stage-specific control of neuronal migration by somatostatin. Nature. 2002;415(6867):77–81. doi: 10.1038/415077a. [DOI] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469(7330):407–10. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- Yu YC, He S, Chen S, Fu Y, Brown KN, Yao XH, et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 2012;486(7401):113–7. doi: 10.1038/nature10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Peinado A, Katz LC. Neuronal domains in developing neocortex. Science. 1992;257(5070):665–9. doi: 10.1126/science.1496379. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ackman JB, Xu HP, Crair MC. Visual map development depends on the temporal pattern of binocular activity in mice. Nat Neurosci. 2012;15(2):298–307. doi: 10.1038/nn.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: membrane insertion and differences between cell types. J Comp Neurol. 2006;499(1):132–43. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci. 2006;9(3):363–71. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44(5):851–64. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ. Direct participation of starburst amacrine cells in spontaneous rhythmic activities in the developing mammalian retina. J Neurosci. 1998;18(11):4155–65. doi: 10.1523/JNEUROSCI.18-11-04155.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ. A critical role of the strychnine-sensitive glycinergic system in spontaneous retinal waves of the developing rabbit. J Neurosci. 2001;21(14):5158–68. doi: 10.1523/JNEUROSCI.21-14-05158.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. J Neurosci. 2000;20(17):6570–7. doi: 10.1523/JNEUROSCI.20-17-06570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]