Abstract
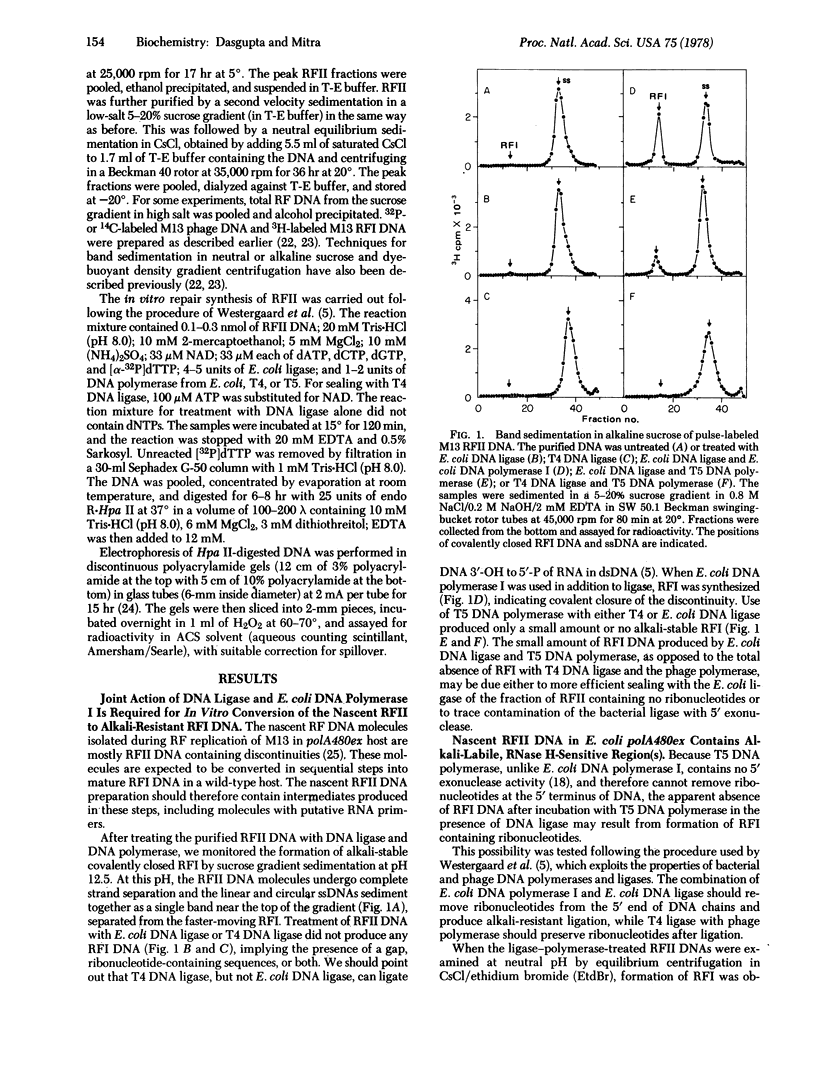
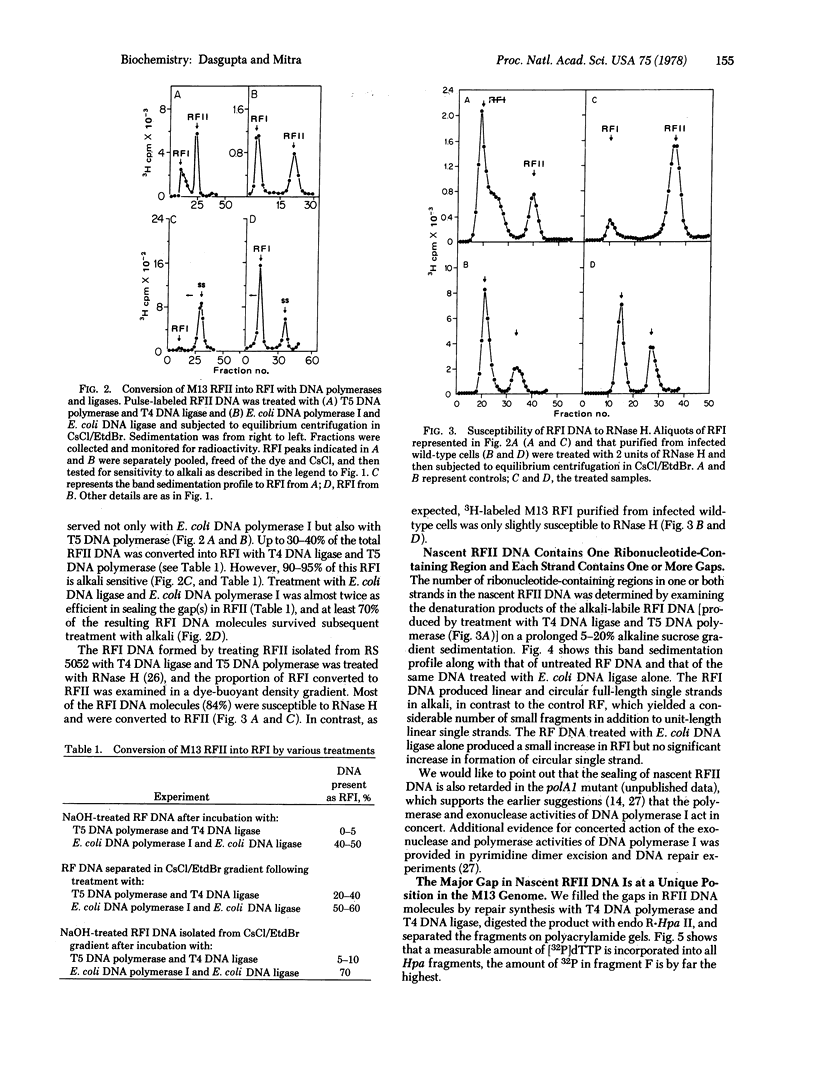
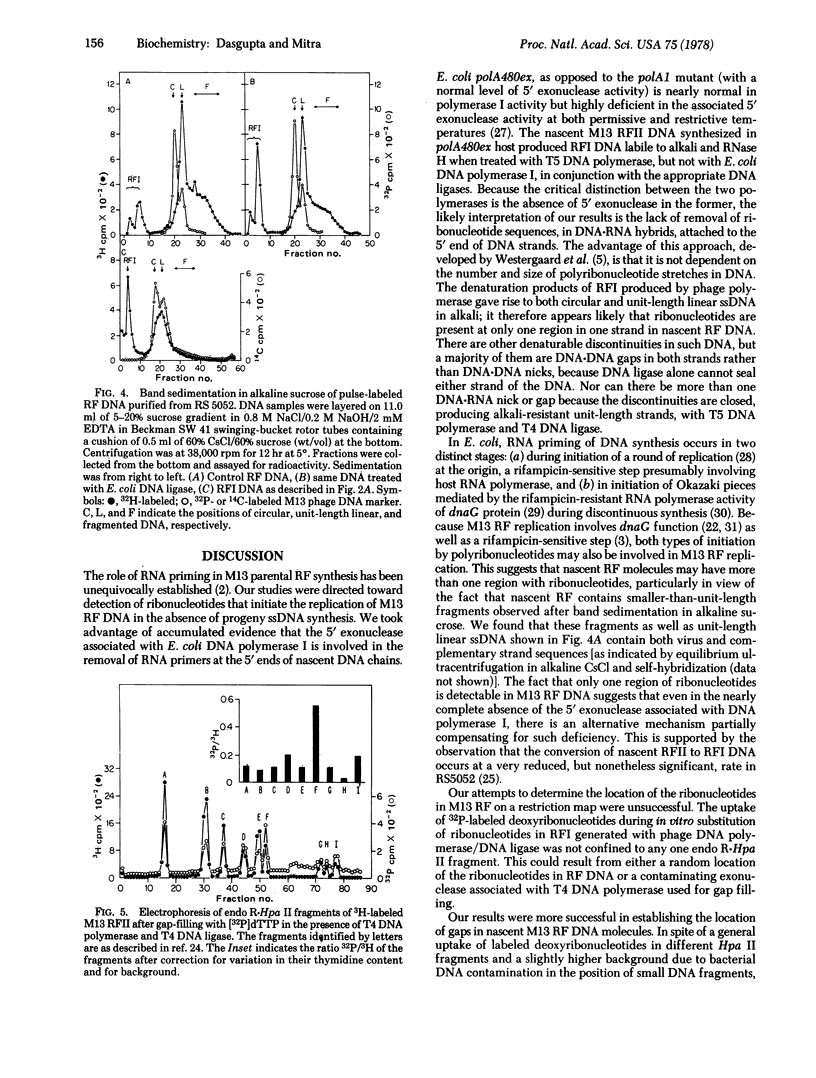
Nascent replicative form type II (RFII) DNA of coliphage M13 synthesized in an Escherichia coli mutant deficient in the 5' leads to 3' exonuclease associated uith DNA polymerase I contains ribonucleotides that are retained in the covalently closed RFI DNA sealed in vitro by the joint action of T5 phage DNA polymerase and T4 phage DNA ligase. These RFI molecules are labile to alkali and RNase H, unlike the RFI produced either in vivo or from RFII with E. coli DNA polymerase I and E. coli DNA ligase. The ribonucleotides are located at one site and predominantly in one strand of the nascent RF DNA. Furthermore, these molecules contain multiple small gaps, randomly located, and one large gap in the intracistronic region.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison D. P., Ganesan A. T., Olson A. C., Snyder C. M., Mitra S. Electron microscopic studies of bacteriophage M13 DNA replication. J Virol. 1977 Nov;24(2):673–684. doi: 10.1128/jvi.24.2.673-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Ray D. S. Replication of bacteriophage M13. X. M13 replication in a mutant of Escherichia coli defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. J Mol Biol. 1976 Sep 25;106(3):589–604. doi: 10.1016/0022-2836(76)90253-9. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Allison D. P., Snyder C. E., Mitra S. Base-unpaired regions in supercoiled replicative form DNA of coliphage M13. J Biol Chem. 1977 Aug 25;252(16):5916–5923. [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. The role of DNA polymerase I-associated 5'-exonuclease in replication of coliphage M13 replicative-form DNA. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1108–1113. doi: 10.1016/0006-291x(77)90535-6. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. The role of Escherichia coli dnaG function in coliphage M13 DNA synthesis. Eur J Biochem. 1976 Aug 1;67(1):47–51. doi: 10.1111/j.1432-1033.1976.tb10630.x. [DOI] [PubMed] [Google Scholar]
- Fujimura R. K., Roop B. C. Characterization of DNA polymerase induced by bacteriophage T5 with DNA containing single strand breaks. J Biol Chem. 1976 Apr 10;251(7):2168–2174. [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Horiuchi K., Zinder N. D. Origin and direction of synthesis of bacteriophage fl DNA. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2341–2345. doi: 10.1073/pnas.73.7.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Genetic control over the initiation of the synthesis of the short deoxynucleotide chains in E. coli. Nat New Biol. 1972 Dec 20;240(103):237–240. doi: 10.1038/newbio240237a0. [DOI] [PubMed] [Google Scholar]
- Lehman I. R., Uyemura D. G. DNA polymerase I: essential replication enzyme. Science. 1976 Sep 10;193(4257):963–969. doi: 10.1126/science.781842. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Dueber J., Suggs S. Replication of bacteriophage M13 IX. Requirement of the Escherichia coli dnaG function for M13 duplex DNA replication. J Virol. 1975 Aug;16(2):348–355. doi: 10.1128/jvi.16.2.348-355.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P., Eliasson R., Söderman G. Initiator RNA in discontinuous polyoma DNA synthesis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4901–4905. doi: 10.1073/pnas.71.12.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. Replication of colicin E1 plasmid DNA in cell extracts. II. Selective synthesis of early replicative intermediates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1403–1407. doi: 10.1073/pnas.71.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Replication of bacteriophage M13. XI. Localization of the origin for M13 single-strand synthesis. J Mol Biol. 1977 Feb 15;110(1):147–163. doi: 10.1016/s0022-2836(77)80103-4. [DOI] [PubMed] [Google Scholar]
- Sugino A., Okazaki R. RNA-linked DNA fragments in vitro. Proc Natl Acad Sci U S A. 1973 Jan;70(1):88–92. doi: 10.1073/pnas.70.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Griffith J., Geider K., Schaller H., Kornberg A. Initiation of deoxyribonucleic acid synthesis. VII. A unique location of the gap in the M13 replicative duplex synthesized in vitro. J Biol Chem. 1974 May 25;249(10):3049–3054. [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Studies on bacteriophage M13 DNA. 1. A cleavage map of the M13 genome. Eur J Biochem. 1975 May 6;53(2):547–558. doi: 10.1111/j.1432-1033.1975.tb04098.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Westergaard O., Brutlag D., Kornberg A. Initiation of deoxyribonucleic acid synthesis. IV. Incorporation of the ribonucleic acid primer into the phage replicative form. J Biol Chem. 1973 Feb 25;248(4):1361–1364. [PubMed] [Google Scholar]
- Williams H., Boyer H. W., Helsinki D. R. Size and base composition of RNA in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3744–3748. doi: 10.1073/pnas.70.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]


