Abstract
Objective
Despite the significant prevalence of adolescent depression, little is known about the neuroanatomical basis of this disorder. Functional dysregulation in frontolimbic circuitry has been suggested as a key neural correlate of adult and adolescent depression impeding emotional regulation. However, less is known about whether this dysregulation is overlaid on impaired white matter microstructure. Guided by neuroimaging findings, we test the a priori hypotheses that adolescent depression is associated with alterations in white matter microstructure in the 1) uncinate fasciculus (UF) and 2) cingulum bundles.
Method
Diffusion tensor magnetic resonance imaging (DTI) data were obtained on 52 unmedicated adolescents with major depressive disorder (MDD) and 42 matched controls. We calculated fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) for bilateral UF and cingulum. We also completed a voxelwise comparison of participants with depression and control participants using tract-based spatial statistics (TBSS).
Results
Adolescents with depression had significantly lower FA and higher RD in bilateral UF; no significant differences were observed in cingulum. TBSS results additionally revealed lower FA values in the white matter associated with the limbic-cortical-striatal-thalamic circuit, corpus callosum, and anterior and superior corona radiata.
Conclusion
Unmedicated adolescent depression is associated with reduced fractional anisotropy in emotion regulatory networks, which may underlie the functional differences in frontolimbic circuitry characterizing depressive disorder. Given the relatively recent onset of depression in our sample, our findings in the context of the current literature provide preliminary evidence that reduced fractional anisotropy in the UF could be a predisposing risk factor for depression.
Keywords: Adolescent major depression, white matter, diffusion tensor imaging, uncinate fasciculus, emotion regulation
Introduction
Depression during adolescence affects 8-20% of all youth 1-3 and as much as quadruples the odds of developing adult depression. 4 Adolescence appears to be a particularly sensitive period in the development of depression as rates of this disorder increase dramatically. 5 Vulnerability to depression may therefore be related to atypical maturational changes in the brain. 6 Understanding the neuroanatomical basis of adolescent depression may yield important insights into the process by which normally developing neural circuitry may go awry in this disorder. Therefore, identifying the underlying etiology and neurobiology of adolescent depression has important clinical implications that may not only guide efforts to improve treatment, but may also provide insights for the prevention of adult depression and associated significant health and social problems.
Models of depression highlight the role of widely distributed yet interactive networks of cortical-striatal and cortical-limbic pathways involved in the regulation of mood. 7,8 Emotional and cognitive sequelae of depression may result from dysfunction in frontal regions (e.g. medial prefrontal cortex, mPFC) that poorly regulate reactivity of limbic structures (e.g. amygdala), which are involved in affective processing. 7 Indeed, functional magnetic resonance imaging (fMRI) studies of adult major depressive disorder (MDD) have demonstrated increased amygdala activity, 9,10 and reduced top-down modulation of amygdala reactivity by paralimbic regions and PFC in patients with depression. 11,12 While there is mounting evidence using fMRI that depression earlier in life may be similarly characterized, 13-18 much less is known about the underlying white matter microstructure associated with adolescent depression.
Diffusion tensor imaging (DTI) is a noninvasive imaging method that permits inference of brain anatomy based on the diffusion of water within the brain. 19 Fractional anisotropy (FA) measures the degree of diffusion anisotropy and is the most commonly used DTI metric. Reflecting fMRI evidence of dysregulated frontolimbic pathways, the few studies of adult depression that have targeted a priori, anatomically defined white matter tracts have focused primarily on the uncinate fasciculi (UF)20,21 and cingulum bundles.20 The UF connects the hippocampus and amygdala with the ventrolateral and orbitomedial regions of the PFC,7,8,22 while the cingulum bundles extend from the subgenual anterior cingulate cortex and connect distinct brain regions that are involved in a variety of emotional and motivational processes implicated in depression.7,22,23 There is one prior study of white matter microstructure in adolescents; however, while this study found some evidence for reduced FA in regions overlapping with UF, it was also confounded by the fact that the majority of participants with depression were receiving several different types and classes of pharmacological treatment.9,10,24
Based upon the reviewed literature, we hypothesized that adolescent depression is associated with reduced FA in the UF and cingulum bundles. We test our hypotheses using DTI in the largest sample to date of unmedicated adolescents with a primary diagnosis of MDD and healthy, well-matched controls. DTI data can be analyzed using both whole-brain and tract-specific methods; however, we emphasize a priori tract-based hypotheses for which there is a theoretical and empirical justification. We use probabilistic tractography to examine two anatomically defined white matter tracts: the bilateral UF and cingulum bundles. We also use a whole-brain, voxelwise approach to further explore depression-related differences in white matter.
Method
Participants
Ninety-four adolescents (52 with depression, 42 healthy controls) ranging in age from 13 – 17 years participated in this study, which was approved by the institutional review boards of University of California (UC), San Diego, UC San Francisco, Rady Children's Hospital, and the County of San Diego. All participants gave written informed assent, and their parents/legal guardians provided written informed consent. Participants were compensated for their time.
Healthy control adolescents were recruited from the San Diego area by means of posted flyers, e-mail, and the Internet. Participants with MDD were recruited from 35 adolescent psychiatric clinics spread widely throughout the San Diego County area.
Clinical Assessment
All adolescents with potential depression were administered the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS-PL),25 and diagnoses were determined by a board-certified child and adolescent psychiatrist. All participants with depression met full criteria for a primary diagnosis of MDD and were excluded if they had a comorbid diagnosis of psychosis, bipolar disorder, or substance abuse.
Healthy adolescents were excluded from the study if they had any Axis I psychiatric disorder, which was determined using the computerized Diagnostic Interview Schedule for Children version 4.0 (DISC-IV)26 and the Diagnostic Predictive Scale (DPS).27 Control participants were also excluded if they had any family history of mood or psychotic disorders in first- or second-degree relatives.
Severity of depression was assessed through both a clinician ratings scale, the Children's Depression Rating Scale-Revised (CDRS-R),28 and a self-report scale, the Reynolds Adolescent Depression Scale (RADS-2).29 CDRS-R scores were used to further characterize study groups; controls with scores higher than 54 and MDD participants with scores lower than 55 were excluded. Psychosocial functioning was assessed using the Children’s Global Assessment Scale (CGAS).30
Given the high rates of comorbid anxiety in adolescent depression, we also assessed anxiety symptoms31 with the Multidimensional Anxiety Scale for Children (MASC).32
Additional exclusion criteria for all participants included: a performance score of less than 70 on the Wechsler Abbreviated Scale of Intelligence (WASI),33 inability to fully understand and cooperate with study procedures, contraindications for MRI (e.g., metallic implants, claustrophobia, pregnancy or the possibility thereof), left-handedness, prepubertal status (Tanner stage 1 or 2), substance abuse, history of neurological disorders (e.g., head trauma, seizures), misuse of prescription drugs or more than two alcohol drinks per week, and the use of medications with a CNS effect within 2 weeks prior to scanning. Parental socioeconomic status was measured using the Hollingshead Two Factor Index of Social Position, 34 and participants self-reported their ethnicity (Hispanic, Non-Hispanic).
Demographic and Clinical Scales Analysis
All statistical analyses were conducted in R.35 Between-group differences were assessed by means of Welch t-tests for age, the performance subscale of the WASI, CDRS-R, MASC, and RADS-2. While all of our participants were fluent English speakers, for some, English was their second language. Therefore, we measured IQ solely on the basis of the performance measure of the WASI. All depression scales were standardized. Group differences in gender and number of rejected directions were assessed using a χ2 test of equal proportions. We used the Wilcoxon Rank Sum test to determine group differences in the Hollingshead Socioeconomic Index, CGAS, and Tanner Stage.
MR Data Acquisition
All scanning was conducted on a 3T MR750 GE (Milwaukee, WI) scanner at the UCSD Center for Functional Magnetic Resonance Imaging. High-resolution sagittal T1-weighted anatomical images were acquired using a fast spoiled gradient echo sequence: TR/TE=8.1ms/3.17ms, flip angle=12°, 256×256 matrix, 168 sagittal slices 1×1×1mm voxels. Diffusion data were measured along 30 directions using a dual spin echo, single-shot, echo planar imaging sequence: TR/TE=7200ms/86.5ms, flip angle=90°, 96x96 matrix, 50 axial slices, b-value: 1500s/mm2, 1.875×1.875×2.5mm voxels. We averaged 2 separate excitations (NEX=2) to maximize the signal to noise ratio. Gradient echo field maps were also acquired to permit compensation for magnetic field inhomogeneity: TR/TE1/TE2=1000ms/4.4ms/5.5ms, flip angle=12°, 128×128 matrix, 50 axial slices, 1.875×1.875×2.5mm voxels.
Data Analysis
MR Data Analysis
All analyses were conducted using FSL 4.1.9.36
Anatomical Analysis
T1-weighted images were bias-field corrected,37 skull stripped, and transformed to MNI152 space using an affine transform38 followed by non-linear refinement.39
Diffusion Data
DTI images were processed using FUGUE software to compensate for B0 inhomogeneity.40 Images were skull-stripped and outlier directions were censored from the analysis. Outliers were determined using 3dToutcount software from AFNI.41 A direction (volume) was rejected if the fraction of outliers in the volume was greater than 2 standard deviations from the mean outlier count across the entire 4D volume (For more details see Supplement 1, available online). Rejected directions were inspected visually, as were volumes near the 2 standard deviation threshold. A linear affine transformation was applied to diffusion-weighted images to correct for the distortions caused by motion and eddy currents.42 The diffusion vector was rotated to adjust for the registration step. Finally, a tensor model was fit to these images using FMRIB's Diffusion Toolbox (FDT),36 yielding fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) maps in native space. RD and AD are additional DTI metrics that may contribute further insight into the character of white matter differences. AD refers to diffusion in the same direction of the fiber tract, and RD refers to diffusion perpendicular to the fiber tract.
Probabilistic Tractography
For our a priori tracts (bilateral UF and cingulum bundles), masks in standard space were extracted from ICBM-DTI-81 atlas43 and reverse-transformed to native space by serially applying the inverse of the previously calculated nonlinear warpfield from T1 to MNI152 space and the inverse of the rigid-body transformation from DTI space to T1 space for the application of probabilistic tractography. Examples of these tracts in diffusion space for one representative subject can be seen in Figure S1, available online).
Probtrackx, run in single mask mode in DTI space, was used to delineate tracts of interest. Tractography was performed with the following constraints: minimum FA value of 0.15, curvature threshold of 78.5°, step length of 0.5mm, and within only one hemisphere. We chose a curvature threshold of 78.5° based on prior studies,24,44,45 including the only other study of white matter microstructure in adolescent MDD.24 A connectivity distribution was generated by seeding 5000 sample fibers from each voxel in the seed mask.
For all resulting tracts, images were thresholded such that those voxels with less than a 5% probability of being connected to the seed masks were eliminated. Eliminating voxels at this threshold is frequent in the literature44 and is also consistent with the only other published study of white matter microstructure in adolescent MDD.24 All connected components were identified (26-connectivity in 3D), and we retained only the largest connected component to exclude physiologically implausible tracts. Across all subjects, the largest disconnected component deleted was no greater than 5 voxels in size. An example of retained and eliminated tracts can be seen in Figure S2 (available online). All resulting tracts were visually inspected for anatomical plausibility. Mean FA, RD, and AD were extracted from voxels within this largest connected component.
Group and Correlational Analyses
Between-group differences in the mean FA extracted for each tract were assessed using linear mixed effects (LME) models implemented in R,35 where the participant was treated as a random effect. All models were adjusted for covariates that significantly differed between groups. If a significant FA difference was found, we examined whether there were also significant differences in RD and AD using the same modeling strategy.
Voxelwise Analysis
Voxelwise statistical analysis of the FA data was conducted using Tract-Based Spatial Statistics (TBSS).46 All participants’ FA images were aligned into a common space using the FNIRT nonlinear registration tool,39 which uses a b-spline representation of the registration warp field.47 Next, the mean FA image was created and thinned to make a mean FA skeleton that represents the centers of all tracts common to all participants. Each participant's aligned FA data was then projected onto this skeleton. The resulting data was subjected to voxelwise permutation-based nonparametric methods48 (5000 permutations) that corrected for multiple comparisons across space and incorporated threshold-free cluster enhancement (TFCE).49 Statistical maps were thresholded at p ≤ 0.05 (TFCE-corrected for family-wise errors) for the group main effects.
Sensitivity Analyses
We also conducted sensitivity analyses to examine whether head motion, outliers, or participants with MDD and comorbid attention-deficit/hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), enuresis, social phobia, oppositional defiant disorder (ODD), or conduct disorder (CD) influenced our FA results. To examine the effect of outliers, we re-ran LME models excluding those observations with FA values greater than two standard deviations from the mean. To examine the effect of comorbidity on our results, we re-ran our LME models and TBSS analyses excluding those participants in the depressed group with the above-listed comorbidities. Finally, to examine the effect of head motion on our results, we included average motion parameters for each subject in our LME models (see Supplement 1, Materials, available online, for details).
Results
Demographic and Clinical Scales
The mean age of our sample was 16.1 years old (SD=1.4, range 13.1-17.9) and 61% were female. Control participants did not differ significantly on age, gender, or socioeconomic status from those with depression. The MDD group reported marginally greater Tanner stage scores than the healthy controls (p=0.05). The controls reported significantly greater performance IQ than the participants with MDD (p=0.01). Therefore, in all group comparisons of white matter microstructure, we adjusted for performance IQ and Tanner stage. As expected, the participants with MDD endorsed significantly greater levels of depression and anxiety on all scales (CDRS-R, RADS-2, MASC) and lower levels of psychosocial functioning than the controls (all p<0.001; see Table 1). Mean duration of illness was 2.1 (SD=1.9) years. Many of our participants with MDD had additional comorbidities (see Table 1 for more details).
Table 1.
Descriptive Characteristics of the Analytic Sample
| Characteristic | NCLa | MDDa | DF | Statisticb, c | P-value |
|---|---|---|---|---|---|
| Number of participants (n) | 42 | 52 | |||
| Gender (M / F) | 16 / 26 | 21 / 31 | NA | X2=0.05 | 0.82 |
| # of directions rejected due to motion/outlier | 2 ± 0 (1-4) | 1.5 ± 0.7 (1-3) | NA | W=1274.00 | 0.12 |
| Age at time of scan (years) | 16 ± 0.2 (13.2-17.9) | 16.2 ± 0.2 (13.1-17.9) | 86.3 | t=−0.66 | 0.51 |
| Hollingshead socioeconomic score | 29 ± 20.8 (11-77)† | 40 ± 26.7 (11-70)† | NA | W=867.00 | 0.12 |
| Tanner score | 4 ± 0.7 (3-5)† | 4.5 ± 0.7 (3-5) [1]† | NA | W=803.50 | 0.05 |
| WASI performance | 105.4 ± 1.7 (84-125) [1] | 99.2 ± 1.7 (70-124) | 88.33 | t=2.61 | 0.01 |
| CGAS | 90 ± 6.7 (75-100) [1]† | 65 ± 14.8 (40-85)† | NA | W=2065.00 | <.0001 |
| CDRS-R | 33 ± 0.7 (30-48) [1] | 72.5 ± 1.1 (55-85) | 80.76 | t=−30.27 | <.0001 |
| RADS-2 total score | 41.7 ± 1.2 (30-56) [2] | 65.8 ± 1 (41-80) | 78.5 | t=−15.00 | <.0001 |
| MASC | 30.3 ± 2.1 (10-62) [3] | 57.3 ± 1.9 (22-87) [2] | 80.81 | t=−9.61 | <.0001 |
| Hispanic ethnicity (yes/no) | 23 / 42 | 28 / 52 | NA | X2=0.0002 | 0.99 |
| Comorbid Diagnoses in the MDD Group | |||||
| GAD | 16 | ||||
| Dysthymic Disorder | 9 | ||||
| ADHD | 5 | ||||
| Social Phobia | 2 | ||||
| Enuresis | 2 | ||||
| Anxiety Disorder NOS | 2 | ||||
| ODD | 1 | ||||
| Conduct Disorder | 1 | ||||
Note: ADHD = attention-deficit/hyperactivity disorder; CDRS-R = Children's Depression Rating Scale-Revised; CGAS = Children's Global Assessment Scale; DF = degrees of freedom; GAD = generalized anxiety disorder; MASC = Multidimensional Anxiety Scale for Children (Standardized); MDD = major depressive disorder; NA = not applicable; NCL = control; NOS = not otherwise specified; ODD = oppositional defiant disorder; RADS-2 = Reynolds Adolescent Depression Scale-2 (Standardized); WASI = Wechsler Abbreviated Scale of Intelligence
Entries are of the form: mean ± standard error of the mean (SEM; min - max) or median ± median absolute deviation (MAD; min - max) if indicated by †. The optional number in [] indicates the number of missing items of data.
Statistic: W, Wilcox rank sum test; χ2, χ2 test for equality of proportions; t, Student's T test.
Statistics for clinical scales refer only to participants surviving motion and outlier correction.
Tract-Specific Results
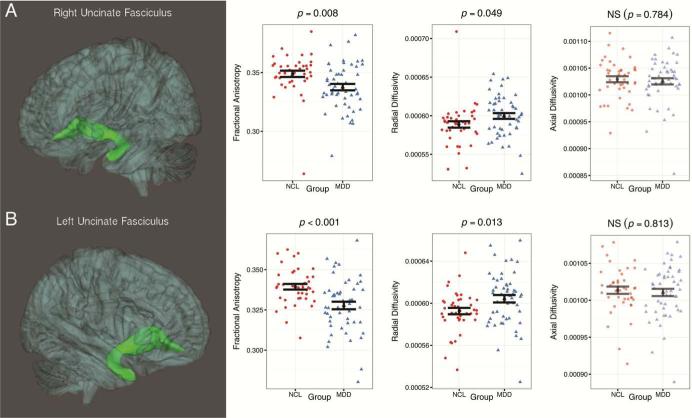
After adjusting for performance IQ and Tanner stage, adolescents with depression showed significantly reduced mean FA values in bilateral UF (right: F(1,88)=7.52, p=0.007; left: F(1, 88)=13.86, p<0.001-Figure 1). Participants with depression also showed reduced FA in bilateral cingulum (cingulate portion), though these results were not significant (right: F(1, 88)=3.34, p=0.07; left: F(1, 88)=3.64, p=0.057).
Figure 1.
Three-dimensional images of the probabilistic tractography results for (A) right uncinate fasciculus and (B) left uncinate fasciculus. Note: the images represent the group mean surface of the tracts. Scatterplots indicate distributions of fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) in the control group (NCL) and the group with major depressive disorder (MDD). The black shapes indicate the corresponding means of FA, RD, and AD for each group, while the black bars indicate standard error of the mean. Only a priori tracts with significant differences in FA values between groups are shown.
To further understand white matter microstructure within tracts demonstrating FA differences, we also investigated between-group differences in RD and AD (see Figure 1). Adolescents with depression showed significantly greater RD in left UF (F(1,88)=6.59, p=0.012), though only marginally so in the right UF (F(1, 88)=3.93, p=0.05). Similar to FA findings, RD in bilateral cingulum was greater, though not statistically significant (right: F(1,88)=3.09, p=0.07; left: F(1, 88)=3.48, p=0.06; results not shown in Figure 1). We observed no significant differences in AD in any of the tracts.
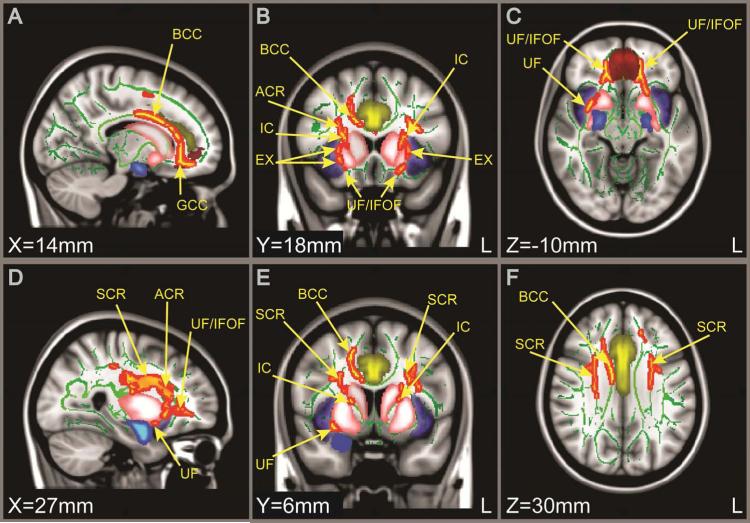
TBSS Results
We also conducted a TBSS voxelwise comparison between groups. We found many significant differences between control adolescents and those with depression (TFCE-corrected). In all regions where there was a significant group difference, participants with depression had reduced FA compared with controls. In addition to finding significant differences overlapping with our tract-based hypotheses (bilateral differences in UF shown in Figure 2C), we also found significant differences in the genu and body of the corpus callosum (Figure 2A), the anterior and superior corona radiata (Figure 2D,E,F), the inferior fronto-occipital fasciculi (Figure 2C), and the internal and external capsule (Figure 2B,E). Internal capsule and external capsule findings suggest involvement of the limbic-cortical-striatal-thalamic circuit. For interpretability, we have included visualization of selected cortical regions that have been found to be dysfunctional in depression when displaying our TBSS results. All images are in MNI152 space.
Figure 2.
Tract-based spatial statistics (TBSS) results comparing fractional anisotropy (FA) values in control adolescents and those with depression. Note: FA maps show sagittal, coronal, and axial views (from left to right). Green voxels represent the mean white matter skeleton from the entire sample, and red-yellow voxels represent white matter regions with lower FA in participants with major depressive disorder (MDD) compared with controls (p<.05, threshold-free cluster enhancement [TFCE]-corrected). In no regions were FA values significantly higher for participants with depression. Yellow (cingulate), blue (amygdala), purple (insula), red (frontal medial cortex), and pink (striatum) represent grey matter regions adjacent to the white matter tracts identified. ACR = anterior corona radiata; BCC = body of corpus callosum; EX = external capsule; GCC = genu of corpus callosum; IC = internal capsule; IFOF = inferior fronto-occipital fasciculus; SCR = superior corona radiate; UF = uncinate fasciculus.
Sensitivity Analyses
Our sensitivity analysis excluding outliers suggests that our findings are robust to the effects of extreme observations. Excluding participants with FA values greater than 2 standard deviations from the mean (3 MDD and 1 control) did not influence either the significance of our findings or the effect size (see Table S1, available online). Additionally, we re-ran our LME models and TBSS analyses excluding those MDD participants with MDD and comorbid ADHD, PTSD, enuresis, social phobia, ODD, or CD (MDD: n=33; control: n=42). With respect to the tract-based analyses, participants with MDD still had significantly reduced FA values and higher RD compared to controls in bilateral UF with comparable effect sizes. Cingulum FA findings remained nonsignificant (see Table S2). Our TBSS results on this more refined sample were not substantively different from the larger analysis; however, while the same white matter tracts were identified, some of the significant clusters were smaller (see Figure S3, available online). Finally, we also examined the effect of incorporating motion parameters into our adjusted models in the complete sample (MDD: n=52; control: n=42). Including these parameters had no effect on our results (see Table S3, available online).
Discussion
In the current study, we examined differences in white matter microstructure in a large sample of unmedicated adolescents with MDD and matched healthy controls. Our study yielded several novel findings. In our a priori probabilistic tracts, we found significantly reduced mean FA and higher mean RD values in bilateral UF in adolescents with clinical depression compared to healthy controls. Finally, our voxelwise analysis identified several other white matter tracts that may be involved in the pathophysiology of adolescent depression, including limbic-cortical-striatal-thalamic circuits.7,50
Our finding that adolescents with depression have reduced fractional anisotropy in bilateral UF provides initial evidence of structural deficits that may underlie the functional differences in frontolimbic circuits observed in adolescent depression. The UF is particularly relevant to the study of depression as it connects inferior frontal regions, such as the anterior cingulate cortex (ACC), mPFC and orbitofrontal cortex (OFC) with medial temporal regions, such as the amygdala and hippocampus.22 The ACC, OFC, and mPFC all play an important role in regulating amygdala reactivity,7,51 and many fMRI studies have highlighted these regions in both adolescent14-16,18,52 and adult depression.53 In the only other DTI study to date of adolescents with depression, Cullen et al. (2010) used TBSS and found evidence of reduced FA in a small number of voxels overlapping with the left UF. Two recent tract-based studies of white matter microstructure in adults with depression found evidence of reduced FA in the left UF20 and in an anterior, dorsal subsection of the UF.21 Interestingly, one small study of geriatric depression found lower FA in left UF only among those whose depression manifested at an early age (mid-to-late adolescence).54 Given the relatively recent onset of depression in our sample, our findings in the context of the current literature provide preliminary evidence that reduced fractional anisotropy in the UF could be a predisposing risk factor for depression.
Notably, in bilateral UF where FA values were significantly reduced in the group with depression compared to controls, RD values were significantly higher, but no differences in AD were observed. Previous studies in animal models measuring DTI in vivo suggest that the combination of higher RD with lower FA values is a plausible noninvasive biomarker of poor myelination (as opposed to axonal injury).55 One potential explanation for our findings is that tracts critical to emotion regulation are poorly myelinated in adolescent MDD, which may underlie a reduction in their capacity to moderate thoughts and feelings generated in limbic regions of the brain. A recent DTI study of adult MDD also found decreased FA and increased RD in right UF in participants with depression.20 The authors concluded that adult depression might result from a demyelination process. It is notable that we found the same pattern of differences in white matter microstructure in our sample of adolescents with depression and with relatively short disease histories, suggesting that there is a similar underlying neurobiological mechanism linking both adolescent- and adult-onset depression. Future longitudinal studies that 1) employ novel DTI metrics (such as the hindrance modulated orientational anisotropy [HMOA] index)56 that are more suited to examining myelination and 2) include participants with depression across the life course with varying age of onset and duration of illness may help clarify the exact nature of white matter differences that increase risk for adolescent depression.
Voxelwise analyses revealed several white matter tracts involved in mood regulation in which depressed subjects had significantly lower FA values than controls. Specifically, we found evidence of structural differences of the limbic-cortical-striatal-thalamic circuit in adolescent depression. This particular circuit has been highlighted due to the central role of the medial PFC in organizing emotional signals from the limbic system and in self-referential processing.7 Depression has been hypothesized to result from reduced transmission of information from the limbic system to the PFC via the striatum and its associated white matter tracts within the internal capsule.50 Our whole-brain analysis suggests that depressed adolescents have lower FA values in several tracts that facilitate communication within this network, including the internal capsule, external capsule, UF, and inferior fronto-occipital fasciculus.
However, not all differences identified in voxelwise analysis overlapped with white matter tracts specifically related to mood regulation. We also found large differences in both the body and genu of the corpus callosum, with significantly lower FA values in adolescents with depression relative to healthy controls. White matter differences in this area have been reported in several studies of adult MDD,57-59 and across multiple psychiatric conditions including schizophrenia,60 bipolar disorder,61 and ADHD.62 The corpus callosum is the largest white matter tract in the brain and provides interhemispheric communication and integration of emotional, cognitive, linguistic, and perceptual processing.63 The genu in particular integrates input from the left and right anterior cingulate, bridging attention and emotion.64 It also integrates signals from the bilateral insula incorporating internal and external homeostatically relevant information to inform behavior.65
Interestingly, corpus callosum differences have been observed in association with early life stress of varying severity (e.g. maltreatment, institutionalization, and stressful life events) in children (posterior corpus callosum),66 adolescents (posterior corpus callosum),67 and adults (genu).68 As early life stress is also a strong predictor of depression as well as other mood, substance, and behavior disorders,69 perhaps corpus callosum differences either mediate or precipitate effects of early life stress on mental health. As such, white matter differences in corpus callosum may serve as a sensitive, though not specific, indicator of psychiatric risk that could be used to identify candidates for primary prevention. These questions should be further explored in future research that includes measures of early life stress.
Although we did not find significant differences in the cingulum bundles from either our probabilistic tract-based or TBSS analyses, our tract-based findings trended in the anticipated direction, with adolescents with depression exhibiting lower FA than controls. However, in the subsample of participants with MDD without comorbidities, these results were no longer approaching significance. Given its role in connecting multiple regions of the brain that span many functions affected by depression (e.g. attention, memory, and emotion regulation), the cingulum has been the focus of several recent white matter studies of adult depression20 and depression risk.23,70 While one study found evidence that the cingulum may be involved in adolescent depression, these differences were identified in a small number of voxels that included participants with depression on a variety of medications, possibly confounding these results.24 Overall, few studies to date provide compelling evidence that cingulum differences are associated with clinical depression in adolescents.
Our results significantly contribute to the dearth of white matter microstructure studies addressing adolescent depression and have several potentially important clinical implications for the treatment and prevention of adolescent depression as well as other major pediatric psychiatric disorders. Our findings suggest that altered white matter microstructure of the UF may be a reliable biomarker of adolescent depression, and that changes to these structures may correlate with and predict treatment responsiveness. Additionally, our pattern of DTI findings in the UF indicates that white matter structures facilitating emotion regulation may be poorly myelinated in adolescent depression. This has implications for the study of factors that may contribute to poor myelination (e.g. genetics, adverse experience, nutritional exposures) as well as the development of novel preventive interventions and clinical therapies that could promote myelination during adolescent development. Finally, our whole-brain analysis indicates that many white matter tracts in the forebrain, including the corpus callosum, have reduced FA in depression. Such widespread differences suggest that generalized delayed white matter development may be characteristic of adolescent depression. Several studies of age-related change in white matter microstructure suggest that FA values increase during adolescence in the tracts identified in our voxelwise analysis.71-74 Future longitudinal studies will be essential to better understand the developmental time course of white matter microstructure in adolescents at risk for depression.
Our findings must be interpreted in light of the limitations of our study. We are unable to infer causal relations between underlying white matter microstructure and the development of adolescent depression due to our cross-sectional study design. However, the fact that all our participants with depression were unmedicated with relatively short durations of illness better suggests that our neuroanatomical findings are more likely to be related to depression than in similar studies without these characteristics. Although we recruited a large number of participants with depression that were identified with a rigorous screening protocol, it remains unclear how generalizable our findings are to other populations of adolescents, given the homogeneity of our sample (e.g. all participants reflect the demographics of San Diego County). Furthermore, while we conducted sensitivity analyses excluding participants with MDD and a wide range of comorbidities, we did not exclude participants with anxiety in this sub-analysis. Anxiety is highly comorbid with MDD in clinical populations,31 and excluding subjects with anxiety from our analyses would reduce our ability to generalize our findings to typical adolescents with MDD that are seen in clinical practice.
Additionally, we used a non-isotropic voxel size in acquiring our DTI scans. Recent reviews on DTI methodology suggest that an isotropic voxel size may be more ideal for fiber tracking.75 Nonisotropic voxel size is more likely to lead to an underestimate of FA values in tracts in which there are crossing fibers. Despite this limitation, we believe that this aspect of our DTI protocol would not have biased our results in a particular direction. Finally, we averaged the results of 2consecutive scans (NEX=2) prior to correcting each scan for motion, an approach that reduced our ability to identify artifacts. Future studies should acquire multiple repetitions of the diffusion directions and perform averaging in the data processing pipeline after correction for eddy currents and motion to avoid this problem.
In summary, we present the first evidence of widespread white matter differences in a large study of un-medicated adolescents with depression and matched healthy controls. To our knowledge, this is the largest DTI study to date of adolescent depression. White matter tracts associated with adolescent depression in our study included bilateral UF and white matter structures overlapping with the limbic-cortical-striatal-thalamic circuit. Notably, the pattern of DTI findings in the UF (i.e., reduced FA and increased RD in the depressed group) suggests that the neuroanatomical basis for adolescent depression may partially result from poor myelination of emotion regulatory circuitry. Our study population helps to inform potential risk factors for depression as adolescents with depression are less likely than adults to have experienced prolonged illness duration, which may have independent effects on brain structure.76 Our findings suggest that lower fractional anisotropy in tracts important to emotion regulation may be a predisposing risk factor for depression in early life. Whether this pattern of findings is due to a delay in white matter tract development in adolescents with depression that may rebound over time, or is a stable characteristic of the disorder, should be assessed in future longitudinal studies.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (NIMH: R01MH085734, R01MH085734-02S1, and R01MH085734-05S1, all TTY; 5K01MH097978-02, KZL), the Brain and Behavior Research Foundation (formerly NARSAD; TTY), from the Center of Excellence in Stress and Mental Health, and by a Veteran's Affairs Merit Grant (ANS). The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The authors would like to thank Robert Hendren, DO, of UCSF, for ongoing guidance and support. They also thank Napoleon Hoang, HS, Audrey Fortezzo, BS, Catherine Rios, BA, and Mary Luna, BA, of UCSD, Guang Yang, MS, of Leiber Institute of Brain Development, Dipavo Banerjee, BS, of the National Institutes of Health (NIH), and Kevin Hahn, BS, of Stanford University, for assistance with subject recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental material cited in this article is available online.
Mr. Drahos and Dr. Hoeft served as the statistical experts for this research.
Disclosure: Dr. Hoeft has received grant or research support from NIH. Drs. LeWinn, Connolly, Ho, Simmons, Yang, and Mssrs. Wu and Drahos report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Kaja Z. LeWinn, University of California, San Francisco (UCSF)..
Colm G. Connolly, University of California, San Francisco (UCSF)..
Jing Wu, University of California, San Francisco (UCSF)..
Miroslav Drahos, University of California, San Francisco (UCSF)..
Fumiko Hoeft, University of California, San Francisco (UCSF)..
Tiffany C. Ho, University of California, San Francisco (UCSF)..
Alan N. Simmons, University of California, San Diego (UCSD)..
Tony T. Yang, University of California, San Francisco (UCSF)..
References
- 1.Kessler RC, Walters EE. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress Anxiety. 1998;7(1):3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Cheung AH, Dewa CS. Canadian community health survey: major depressive disorder and suicidality in adolescents. Healthc Policy. 2006;2(2):76–89. [PMC free article] [PubMed] [Google Scholar]
- 3.Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–140. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent depression. J Adolesc Health. 2013;52(5):533–538. doi: 10.1016/j.jadohealth.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. J Am Acad Child Adolesc Psychiatry. 1994;33(6):809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1-2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 9.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 10.Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in Major Depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in Unipolar Depression: Related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Ho TC, Yang G, Wu J, et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho TC, Wu J, Shin DD, et al. Altered cerebral perfusion in executive, affective, and motor networks during adolescent depression. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1076–1091. doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connolly CG, Wu J, Ho TC, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman G, Simmons AN, Wu J, et al. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord. 2012;139(1):75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen KR, Gee DG, Klimes-Dougan B, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang TT, Simmons AN, Matthews SC, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49(1):42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhang A, Leow A, Ajilore O, et al. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2011;37(4):959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kwaasteniet B, Ruhe E, Caan M, et al. Relation between structural and functional connectivity in major depressive disorder. Biol Psychiatry. 2013;74(1):40–47. doi: 10.1016/j.biopsych.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 23.Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: The effect of family history and anhedonia. Biol Psychiatry. 2012;72(4):296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: A preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49(2):173–183. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Lucas CP, Zhang H, Fisher PW, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Poznanski EO. Children's Depression Rating Scale-Revised (CDRS-R). Western Psychological Services. 1996 [Google Scholar]
- 29.Reynolds WM. RADS-2, Reynolds Adolescent Depression Scale: Professional Manual. Psychological Assessment Resources. 2002 [Google Scholar]
- 30.Dyrborg J, Larsen FW, Nielsen S, Byman J, Nielsen BB, Gautre-Delay F. The Children's Global Assessment Scale (CGAS) and Global Assessment of Psychosocial Disability (GAPD) in clinical practice--substance and reliability as judged by intraclass correlations. Eur Child Adolesc Psychiatry. 2000;9(3):195–201. doi: 10.1007/s007870070043. [DOI] [PubMed] [Google Scholar]
- 31.Angold A, Costello EJ. Depressive comorbidity in children and adolescents: empirical, theoretical, and methodological issues. Am J Psychiatry. 1993;150(12):1779–1791. doi: 10.1176/ajp.150.12.1779. [DOI] [PubMed] [Google Scholar]
- 32.March JS, Parker JDA, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Abbreviated Scale of Intelligence Administration and Scoring Manual. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 34.Hollingshead AB. Two factor index of social position. Hollingshead; New Haven: 1957. [Google Scholar]
- 35.R Development Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2012 [Google Scholar]
- 36.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 38.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 39.Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation. FMRIB Center; Oxford, UK.: 2007. [Google Scholar]
- 40.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2002;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 41.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36(6):960–964. doi: 10.1002/mrm.1910360620. [DOI] [PubMed] [Google Scholar]
- 43.Mori S, van Zijl PCM, Oishi K, Faria AV. MRI Atlas of Human White Matter. Academic Press; Amsterdam: 2010. [Google Scholar]
- 44.Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73(1):85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 45.de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32(35):12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- 48.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith S, Nichols T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 51.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 52.Yang TT, Simmons AN, Matthews SC, et al. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. NeuroReport. 2009;20(4):440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegle GJ, Thompson WK, Collier A, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69(9):913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor WD, MacFall JR, Gerig G, Krishnan RR. Structural integrity of the uncinate fasciculus in geriatric depression: Relationship with age of onset. Neuropsychiatr Dis Treat. 2007;3(5):669. [PMC free article] [PubMed] [Google Scholar]
- 55.Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 56.Dell'Acqua F, Simmons A, Williams SCR, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp. 2013;34(10):2464–2483. doi: 10.1002/hbm.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo WB, Liu F, Xue Z-M, et al. Altered white matter integrity in young adults with first-episode, treatment-naïve, and treatment-responsive depression. Neurosci Lett. 2012;522(2):139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 58.Xu K, Jiang W, Ren L, et al. Impaired interhemispheric connectivity in medication-naive patients with major depressive disorder. J Psychiatry Neurosci. 2013;38(1):43–48. doi: 10.1503/jpn.110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38(1):49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitford TJ, Kubicki M, Schneiderman JS, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68(1):70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emsell L, Leemans A, Langan C, et al. Limbic and callosal white matter changes in euthymic Bipolar I Disorder: An advanced diffusion magnetic resonance imaging tractography Study. Biol Psychiatry. 2013;73(2):194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 62.Dramsdahl M, Westerhausen R, Haavik J, Hugdahl K, Plessen KJ. Adults with attention-deficit/hyperactivity disorder - a diffusion-tensor imaging study of the corpus callosum. Psychiatry Res. 2012;201(2):168–173. doi: 10.1016/j.pscychresns.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Hinkley LBN, Marco EJ, Findlay AM, et al. The role of corpus callosum development in functional connectivity and cognitive processing. PLoS ONE. 2012;7(8):e39804. doi: 10.1371/journal.pone.0039804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 65.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci USA. 2012;109(32):12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Bellis MD, Keshavan MS, Clark DB, Casey BJ. Developmental traumatology part II: Brain development. Biol Psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 68.Paul R, Henry L, Grieve SM, et al. The relationship between early life stress and microstructural integrity of the corpus callosum in a non-clinical population. Neuropsychiatr Dis Treat. 2008;4(1):193–201. doi: 10.2147/ndt.s1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kessler RC, McLaughlin KA, Green JG, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Brit J Psychiat. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2010;36(3):684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu M, Lu LH, Lowes A, et al. Development of superficial white matter and its structural interplay with cortical gray matter in children and adolescents. Hum Brain Mapp. 2014;35(6):2806–16. doi: 10.1002/hbm.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brouwer RM, Mandl RCW, Schnack HG, et al. White matter development in early puberty: a longitudinal volumetric and diffusion tensor imaging twin study. PLoS ONE. 2012;7(4):e32316. doi: 10.1371/journal.pone.0032316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 75.Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 76.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.