Abstract
Glucosinolates (GLSs) are abundant in cruciferous vegetables and reported to have anti thyroidal effects. Four GLSs (sinigrin, progoitrin, glucoerucin, and glucotropaeolin) were administered orally to rats, and the breakdown products of GLSs (GLS-BPs: thiocyanate ions, cyanide ions, organic isothiocyanates, organic nitriles, and organic thiocyanates) were measured in serum. Thiocyanate ions were measured by colorimetric method, and cyanide ions were measured with CI-GC-MS. Organic isothiocyanates and their metabolites were measured with the cyclocondensation assay. Organic nitriles and organic thiocyanates were measured with EI-GC-MS. In all treatment groups except for progoitrin, thiocyanate ions were the highest among the five GLS-BPs. In the progoitrin treated group, a high concentration of organic isothiocyanates (goitrin) was detected. In the glucoerucin treated group, a relatively low amount of goitrogenic substances was observed. The metabolism to thiocyanate ions happened within five hours of the administration, and the distribution of GLSs varied with the side chain. GLSs with side chains that can form stable carbocation seemed to facilitate the degradation reaction and produce a large amount of goitrogenic thiocyanate ions. Because goitrogenic metabolites can be formed without myrosinase, the inactivation of myrosinase during cooking would have no effect on the anti-nutritional effect of GLSs in cruciferous vegetables.
Keywords: Glucosinolates, Goitrogenic metabolites, Thiocyanate ions, Goitrin, Thyroid
INTRODUCTION
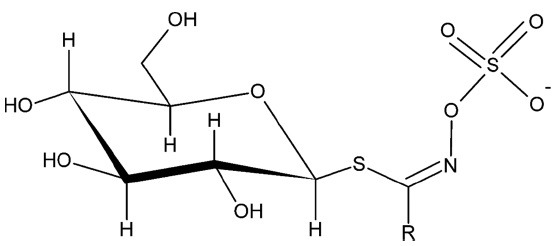
Glucosinolates (GLSs), β-thioglycosides N-hydroxysulfates, are secondary metabolites abundant in cruciferous vegetables such as cabbage, broccoli, and mustard (Fig. 1). Intact GLSs are stable and inactive, but when the plants get damaged, GLSs are released from vacuoles and enzymatically hydrolyzed by myrosinase (β-thioglucosidase) (1). This hydrolysis generates GLS breakdown products (GLS-BPs) including organic isothiocyanates, organic nitriles, organic thiocyanates, thiocyanate ions, etc. GLS-BPs vary depending on the hydrolysis conditions such as the pH, temperature and R-group of the GLSs (1,2).
Fig. 1. Glucosinolate structure.
During food processing, mastication, and digestion in vivo, GLSs generally come into contact with myrosinase and undergo degradation before they are absorbed in the body (1-3). During digestion, pepsin-HCl and colonic microflora especially play an important role in GLS-BPs formation (4). GLSBPs have a relatively higher logP value and lower molecular weight than their parent molecules, so they are rapidly absorbed in the body, but not all the GLS-BPs have identical absorption rates. According to preceding research, structurally different GLS-BPs show different absorption rates (5-8). However, most of the studies focused on the absorption of organic isothiocyanates only. Or the studies involving GLSs employed whole vegetables, which contain mixtures of GLSs as well as myrosinase. This made it hard to estimate the meta bolic conversion of individual GLS. In animal studies, administered rapeseed meal increased thyroid weight, decreased the circulating level of thyroid hormones, and revealed abnormalities in the liver and kidneys. These effects are expected to be caused by GLS-BPs. GLS-BPs such as goitrin and organic nitriles are known to be goitrogenic, which inhibit iodine uptake by the thyroid, enlarge the thyroid, and reduce the levels of circulating thyroid hormones (9). Moreover, thiocyanate ions are also reported to facilitate the export of iodine out of the thyroid and inhibit the synthesis of thyroid hormones. In addition, high concentrations of thiocyanate ions inhibit iodine uptake into the thyroid which is one of the steps in thyroid hormone synthesis (10).
Because different GLS-BPs have been found in damaged plant tissues, depending on the side chain of the GLSs, the absorption of GLS-BPs and their distribution and physiological effects in vivo would also be dependent on the side chain. However, studies on GLS degradation in vivo are limited. Therefore, in the present study, we selected four kinds of GLSs based on their structure and on the consumption of cruciferous vegetables in Korea. After the oral administration of isolated GLSs, the concentrations of the absorbed GLS-BPs (thiocyanate ions, cyanide ions, organic isothiocyanates, organic nitriles, and organic thiocyanates) in rat serum were measured to compare the breakdown and absorption of different GLSs depending on their side chains.
MATERIALS AND METHODS
Chemicals and apparatus. n-Hexane, methyl alcohol, 2-propanol and dichloromethane were purchased from Samchun Chemical Co., Ltd. (Seoul, Korea). DEAE-Sephadex A-25 chloride form, imidazole, formic acid, trifluoroacetic acid, potassium thiocyanate, potassium cyanide, tetrabutylammonium sulfate (TBAS; 50% w/w solution in water), 2,3,4,5,6-pentafluorobenzyl bromide (PFB-Br), sodium tetraborate decahydrate, potassium phosphate monobasic, potassium phosphate dibasic, benzyl isothiocyanate, allyl cyanide, and benzyl cyanide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium sulfate was purchased from Yakuri Pure Chemicals Co., Ltd. (Osaka, Japan). Acetonitrile was purchased from Avantor Performance Materials (Phillipsburg, NJ, USA). Iron (III) nitrate enneahydrate (Fe(NO3)3·9H2O) was purchased from Kanto Chemicals Co. Inc. (Tokyo, Japan). Nitric acid (HNO3) and magnesium sulfate anhydrous (MgSO4) were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Allyl isothiocyanate was purchased from Fluka (Buchs, Switzerland). Erucin and DL-goitrin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). 1,2-benzenedithiol and benzyl thiocyanate were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Allyl thiocyanate, 2-hydroxy-3-butenyl cyanide, 4-(methylthio)butyl cyanide, and 4-(methylthio) butyl thiocyanate were synthesized in the lab.
PVDF membrane filters (Durapore®, hydrophilic, 0,22 μm), amicon ultra centrifugal filter devices (3 K & 30 K), and PTFE membrane filters (hydrophilic, 0.5 μm) were obtained from Millipore Co. (Bedford, MA, USA). Autosampler vials were obtained from Thermo scientific (Waltham, MA, USA). The Mouse/Rat Triiodothyronine (T3) ELISA kit and Mouse/Rat Thyroxine (T4) ELISA kit were obtained from Calbiotech (Spring valley, CA, USA).
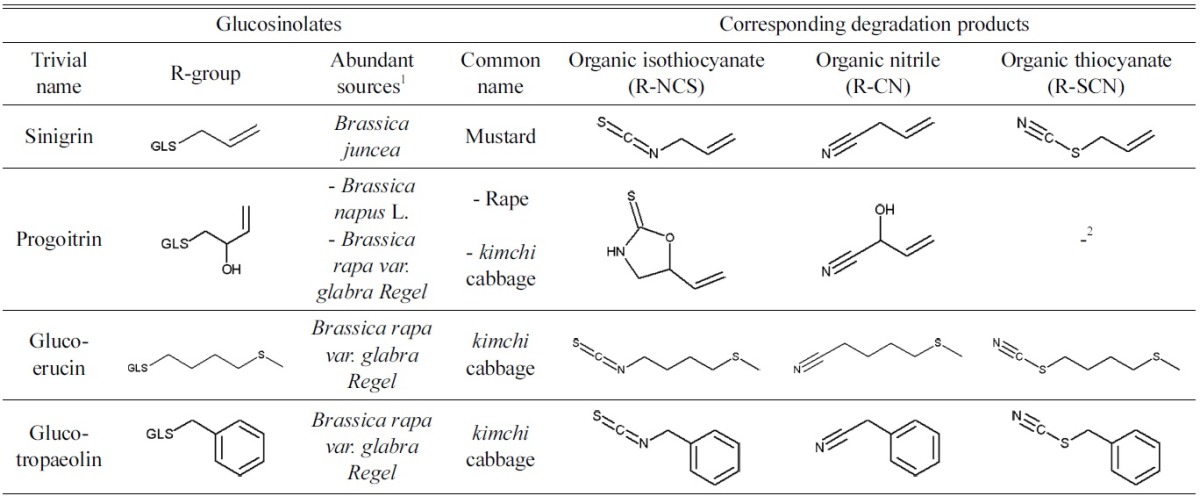
Seeds and standards. Four GLSs were selected according to the R-group and the consumption of cruciferous vegetables in Korea (Table 1). Sinigrin was obtained from Sigma-Aldrich (St. Louis, MO, USA). Progoitrin, Glucoerucin, and Glucotropaeolin were separated from seeds which contain the individual GLS predominantly. Progoitrin was separated from Brassica napus L. seeds (Asia Seed Co., Ltd., Seoul, Korea). Glucoerucin was separated from Eruca sativa Mill. seeds (Asia Seed Co., Ltd., Seoul, Korea). Glucotropaeolin was separated from Tropaeolum majus L. seeds (Garamone, Yongin, Korea). The seeds were stored in a refrigerator until used. GLS standards, progoitrin potassium salt, glucoerucin, and glucotropaeolin potassium salt, were obtained from ChromaDex (Irvine, CA, USA).
Table 1. Selected glucosinolates and their breakdown products.
Extraction and isolation of glucosinolates. GLSs were extracted according to Song et al. (11) and Rochfort et al. (12). Brassica napus L. and Eruca sativa Mill. seeds were heat-treated in a drying oven at 110℃ for 2 hr to inactivate myrosinase. Tropaeolum majus L. seeds were treated with boiling water because they are too thick to inactive the enzyme in a drying oven. After 100 g of Tropaeolum majus L. seeds were boiled in 1 L of boiling water for 10 min, the seeds were dried and ground to powder using a mortar and pestle. The ground seeds were defatted by extraction with 200 ml of hexane five times. The residual solvent was removed by rotary evaporator (Heidolph VV 2000; Heidolph, Kelheim, Germany). The defatted seeds were extracted with 70% methanol (1 : 10 w/w) in a 75℃ shaking water bath for 10 min (BS-21 Shaking & Heating bath; Jeio tech Co., Ltd., Daejeon, Korea), then centrifuged at 2,000 ×g for 15 min (Himac CP80MX; Hitachi Co., Tokyo, Japan). The supernatant was concentrated to approximately 30% of the original volume by rotary evaporator.
Ion-exchange chromatography was carried out to separate the GLSs from the concentrated extract according to Visentin et al. (13) and ISO norm (14). DEAE-Sephadex A-25 resin was mixed with 2 M acetic acid until the volume of the liquid reached twice as much as the volume of the sediment. A DEAE-Sephadex A-25 suspension was prepared 2 days prior to its use and stored in a refrigerator. A DEAESephadex A-25 column (2.5 × 30 cm) was prepared with the suspension. The column was conditioned with 150 ml of 6 M imidazole formate and washed with 150 ml of deionized water. One hundred-fifty milliliters of the concentrated extract were loaded, and washed with 200 ml of formic acid/2-propanol/water (3 : 2 : 5) and 300 ml of deionized water sequentially. Finally, the GLSs were eluted with 150 ml of 0.5 M K2SO4/5% 2-propanol and filtered through a PVDF membrane filter. Individual GLSs were isolated from the eluate using preparative HPLC (LC-9201; Japan Analytical Industry Co., Ltd., Tokyo, Japan) with a Supelcosil PLC-18 (25 cm × 21.2 mm, 12 μm) column (Supelco Inc., Bellefonte, PA, USA). All solvents for preparative HPLC were filtered through a PTFE membrane filter and degassed before use. Progoitrin was eluted at approximately 27~28 min with an isocratic mobile phase of 0.1% TFA in water. Glucoerucin was eluted at approximately 22~23 min with an isocratic mobile phase of 0.1% TFA in 75% water/25% methanol. Glucotropaeolin was eluted at approximately 22~23 min with an isocratic mobile phase of 0.1% TFA in 70% water/30% methanol. These three GLSs were eluted at a flow rate of 3 ml/min, and the peaks were detected at 229 nm. The final eluates were concentrated by vacuum concentrator (ScanSpeed MaxiVac Alpha; Labogene, Lynge, Denmark) and their concentrations were calculated using a corresponding standard calibration curve at 229 nm.
Animals. Seven-week-old male Wistar rats weighing 206.32 ± 1.86 g were obtained from the Institute of Laboratory Animal Resources at Seoul National University (Seoul, Korea). The animals were bred and housed at the Animal Center for Pharmaceutical Research at Seoul National University (Seoul, Korea). Twenty-five rats were randomly divided into 5 groups: the Control, Sinigrin, Progoitrin, Glucoerucin, and Glucotropaeolin groups. Two to three animals were housed in a cage, and they were adapted to their environment for 24 hr before the experiment. The animal room was kept at a temperature of 22 ± 2℃, relative humidity of 50 ± 5%, and on a 12-hr light/dark cycle (lights on 7:00~19:00). The animals were fed Purina irradiated laboratory chow 38057 (Purina Korea, Seoul, Korea) and autoclaved water ad libitum. The treatment groups were given a single dose of an individual GLS at 50 μmol/ml, and the control group was given a single dose of autoclaved water 1 ml by gavage after fasting for 16 hr. Five hours after gavage, the animals were anesthetized by carbon dioxide inhalation. Their blood was collected from the abdominal aorta, and the livers were removed. The liver samples were washed with 0.9% NaCl, and the weights were recorded. The blood samples were clotted at room temperature for 30 min and centrifuged at 2,000 ×g, 4o℃ for 10 min. Serum was collected and stored at −80℃ until used. This animal experiment was carried out with the approval of the Institutional Animal Care and Use Committee (IACUC) of Seoul National University (Approval No: SNU-130621-3-1).
Analysis of breakdown products in serum. Thiocyanate ions were measured according to Van Staden and Botha (15) and Shim et al. (16). Fe (III) reagent solution was prepared by dissolving 1400 mg of Fe(NO3)3·9H2O in 40 ml of deionized water and adding 3.7 ml of 70% HNO3 to it. Two-hundred microliters of serum sample were transferred to amicon ultra centrifugal filter devices (30 K) and centrifuged at 14,000 ×g for 15 min at 4℃ for deproteinization (Smart R17; Hanil Science industrial Co., Ltd., Seoul, Korea). One-hundred microliters of the filtrate were mixed with the Fe (III) reagent solution for 10 min, and then the absorbance was measured at 450 nm (Spectramax 190; Molecular Devices Co., Sunnyvale, CA, USA). Potassium thiocyanate was used as a standard.
Cyanide ions were measured according to Bhandari et al. (17). TBAS 10 mM in a saturated solution of sodium tetraborate decahydrate (pH 9.5) and a 20 mM solution of PFBBr in ethyl acetate were prepared. Eight-hundred microliters of 10 mM TBAS and 500 μl of a 20 mM PFB-Br solution were added to 100 μl of a serum sample and then vortexed for 2 min. The mixture was heated in a 70℃ water bath for 1 hr (BS-21 Shaking & Heating bath; Jeio tech Co., Ltd., Daejeon, Korea) and centrifuged at 9,300 ×g for 4 min (Smart R17; Hanil Science industrial Co., Ltd., Seoul, Korea). The supernatant organic layer was dehydrated with MgSO4 and analyzed by chemical ionization gas chromatography mass spectrometry (TSQ 8000 triple quadrupole GC-MS/MS equipped with a TRACE 1310 GC and TriPlus RSH Autosampler; Thermo scientific, Waltham, MA, USA). A DB-WAX (60 m × 0.25 mm I.D., 0.5 μm df) column (Agilent Technologies, Palo Alto, CA, USA) was used with helium as a carrier gas at a flow rate of 1 ml/min. Methane was used as a CI gas at a flow rate of 1.5 ml/min for positive ions with an electron energy of 150 eV. The injection volume was 1 μl, and the temperature of the injection port was maintained at 210℃. The temperatures of the ion source and MS transfer line were maintained at 200℃ and 220℃, respectively. The GC oven was programmed from 60℃ (2 min) to 230℃ (4 min) at a rate of 7℃/min. The final product, PFB-CN (m/z 208), was eluted approximately 23.18 min and monitored with selected ion monitoring (SIM). Potassium cyanide was used as a standard.
Organic isothiocyanates and their metabolites were measured with the cyclocondensation assay (18,19). Organic isothiocyanates are metabolized to dithiocarbamates in vivo, and the cyclocondensation assay can detect both of them. Potassium phosphate buffer (pH 8.5) 100 mM and 1,2-benzenedithiol 20 mM & 50 mM in acetonitrile were prepared. In the case of the sinigrin, glucoerucin, and glucotropaeolin groups, 200 μl of the serum sample, 200 μl of 100 mM potassium phosphate buffer, and 400 μl of 20 mM 1,2-benzenedithiol were combined in a 2.0 ml vial, and then the mixture was heated in a 65℃ water bath for 3 hr (BS-21 Shaking & Heating bath; Jeio tech Co., Ltd., Daejeon, Korea). In the case of the progoitrin group, 200 μl of the serum sample, 200 μl of 100 mM potassium phosphate buffer, and 400 μl of 50 mM 1,2-benzenedithiol were combined in a 2.0 ml vial, and then the mixture was heated in a 65℃ water bath for 12 hr. Goitrin, which is the breakdown product of progoitrin, reacts slowly because of its ring structure, thus the concentration of 1,2-benzenedithiol and the incubation time were increased. After cooling to room temperature, the mixture was centrifuged at 10,000 ×g for 2 min (Smart R17; Hanil Science industrial Co., Ltd., Seoul, Korea). Twenty microliters of the supernatant were analyzed with high-performance liquid chromatography (Dionex ultimate 3000 equipped with UV detector; Dionex Co., Sunnyvale, CA, USA). A reverse phase Luna C18 (250 × 4.6 mm, 5 μm, 100 Å) column (Phenomenex, Torrance, CA, USA) was used with an isocratic mobile phase of 80% methanol/20% water at a flow rate of 1 ml/min. The cyclocondensation product, 1,3-benzodithiole-2-thione, was eluted at approximately 9~10 min, and the peak was detected at 365 nm. The column was washed with 95% acetonitrile/5% water for 10 min between sample injections. Allyl isothiocyanate, erucin, benzyl isothiocyanate, and DL-goitrin were used as standards.
Organic nitriles and organic thiocyanates were measured with EI-GC-MS. Two-hundred microliters of serum sample were transferred to amicon ultra centrifugal filter devices (3 K) and centrifuged at 14,000 ×g for 30 min at 4℃ for deproteinization (Smart R17; Hanil Science industrial Co., Ltd., Seoul, Korea). One-hundred-thirty microliters of the filtrate were extracted with 130 μl of dichloromethane and dehydrated with MgSO4. The extract was analyzed with electron ionization gas chromatography mass spectrometry. The carrier gas was helium at a flow rate of 1.5 ml/min, and the electron energy was 70 eV. The injection volume was 1 μl, and the temperature of the ion source and MS transfer line were maintained at 250℃. In the case of the sinigrin group, two types of analysis methods were used because allyl thiocyanates are isomerized at high temperature. For the allyl thiocyanate analysis, the Agilent 6890 Series GC (Palo Alto, CA, USA) equipped with a JEOL JMS600W (Tokyo, Japan) and DB-5MS (30 m × 0.25 mm I.D., 0.5 μm df) column (Agilent Technologies, Palo Alto, CA, USA) were used. The temperature of the injection port was maintained at 50℃, and the GC oven temperature program was as follow: 35℃ (2 min), 5℃/min to 70℃, 25℃/min to 310℃ (5 min). For the allyl cyanide analysis, a Perkin-Elmer Clarus 680 GC equipped with a Clarus 600T CG-MS (Waltham, MA, USA) and HP-PLOT/Q column (30 m × 0.530 mm I.D., 40 μm df) (Agilent Technologies, Palo Alto, CA, USA) was used. The temperature of the injection port was maintained at 250℃, and the GC oven temperature program was as follow: 80℃ (2 min) and 10℃/min to 180℃ (5 min). In the case of the other groups, a Perkin-Elmer GC-MS equipped with a DB-5MS column was used. The temperature of the injection port was maintained at 250℃, and the GC oven temperature program was as follows: 40℃ (5 min), 10℃/min to 200℃, 25℃/min to 310℃ (5 min). Allyl thiocyanate (m/z 99) was eluted at approximately 7.90 min; allyl cyanide (m/z 67) was eluted at approximately 9.53 min; 2-hydroxy-3-butenyl cyanide (m/z 57) was eluted at approximately 10.61 min; 4-(methylthio)butyl cyanide (m/z 61) was eluted at approximately 14.72 min; 4-(methylthio)butyl thiocyanate (m/z 61) was eluted at approximately 18.04 min; benzyl cyanide (m/z 117) was eluted at approximately 13.76 min, and benzyl thiocyanate (m/z 91) was eluted at approximately 16.86 min, and all the peaks were compared with standards.
Thyroid hormones analysis. Total triiodothyronine (T3) and total thyroxine (T4) levels were examined in triplicate with the pooled serum samples of each group. T3 was analyzed with the Mouse/Rat Triiodothyronine (T3) ELISA kit and T4 was analyzed with the Mouse/Rat Thyroxine (T4) ELISA kit (Calbiotech, Spring valley, CA, USA).
Biochemical examination. γ-Glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin (TBIL), creatine phosphokinase (CPK), blood urea nitrogen (BUN), and creatinine (CRE) were examined with the Fuji drychem 3500s (Fuji film Co., Tokyo, Japan).
Statistical analysis. The control group was used as a blank. For the statistical analyses, SPSS Ver. 21 (SPSS, Inc., Chicago, IL) software was used. Data were evaluated with one-way variance analysis followed by Tuckey’s HSD test (p < 0.05). The results are expressed as the mean value ± the standard deviation.
RESULTS
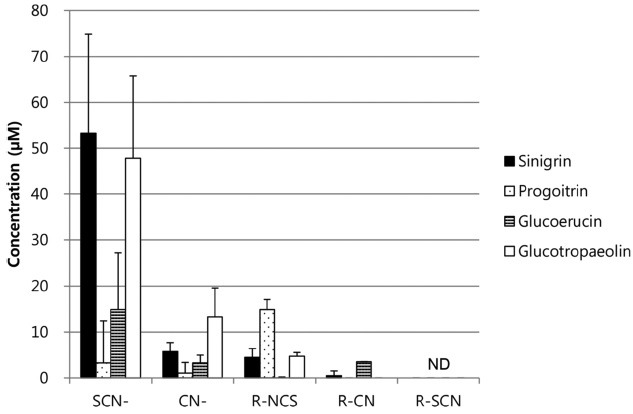
Glucosinolate breakdown products in rat serum. The serum was collected 5 hr after GLSs administration. In all treatment groups, thiocyanate ions, cyanide ions, and organic isothiocyanates were detected (Table 2). Moreover, in all the treatment groups except for the progoitrin group, the level of thiocyanate ions was the highest among the 5 breakdown product groups. In terms of the side chains, the highest concentration of thiocyanate ions was detected in the sinigrin (R = allyl) treated group (53.27 ± 21.62 μM), followed by the glucotropaeolin (benzyl), glucoerucin (methylthiobutyl), and progoitrin (hydroxyl-allyl) treated groups. The concentration of the serum thiocyanate ions is significantly different between the sinigrin/glucotropaeolin groups and glucoerucin/progoitrin groups. The highest concentration of cyanide ions was detected in the glucotropaeolin treated group (13.28 ± 6.29 μM), followed by the sinigrin, glucoerucin, and progoitrin treated groups. The glucotropaeolin treated group released significantly more cyanide ions than that of the other groups. The highest concentration of organic isothiocyanates was detected in the progoitrin treated group (14.81 ± 2.23 μM), followed by the glucotropaeolin and sinigrin groups, but there were negligible amounts of organic isothiocyanates in the glucoerucin treated group. Organic nitriles were detected from the sinigrin and glucoerucin treated groups, though the amount in the sinigrin group was very low. No organic thiocyanates were detected in any of the groups (Table 2 and Fig. 2).
Table 2. Glucosinolate metabolites in rat serum.
| SCN− (μM) | CN− (μM) | R-NCS (μM) | R-CN (μM) | R-SCN (μM) | |
|---|---|---|---|---|---|
|
| |||||
| Sinigrin | 53.27 ± 21.62b | 5.72 ± 2.00a | 4.47 ± 1.93b | 0.47 ± 1.06 | ND |
| Progoitrin | 3.24 ± 9.16a | 1.03 ± 2.33a | 14.81 ± 2.23c | ND | -1 |
| Glucoerucin | 14.84 ± 12.36a | 3.22 ± 1.72a | 0.03 ± 0.17a | 3.48 ± 0.05 | ND |
| Glucotropaeolin | 47.84 ± 17.90b | 13.28 ± 6.29b | 4.73 ± 0.80b | ND | ND |
Note. R-NCS: organic isothiocyanate, R-CN: organic nitrile, R-SCN: organic thiocyanate, ND: not detected.
Values in the same column with different superscripts (a, b, c) are significantly different (p<0.05).
1R-SCN of progoitrin is not reported to occur.
Fig. 2. Glucosinolate breakdown products in rat serum.
Thyroid hormones analysis. In the sinigrin, progoitrin, and glucotropaeolin treated groups, the concentration of T3 was lower than that of the control group. In addition, in the progoitrin and glucoerucin treated groups, the concentration of T4 was lower than that of the control group. However, there was no statistically significant difference between the control and treatment groups (Table 3).
Table 3. Serum T3 and T4 levels of rats treated with glucosinolates.
| Control | Sinigrin | Progoitrin | Glucoerucin | Glucotropaeolin | |
|---|---|---|---|---|---|
|
| |||||
| T3 (ng/ml) | 1.59 ± 0.95 | 1.27 ± 0.29 | 1.42 ± 0.46 | 1.95 ± 0.31 | 1.59 ± 0.69 |
| T4 (μg/dl) | 3.39 ± 1.30 | 4.22 ± 2.28 | 1.60 ± 0.62 | 1.11 ± 0.78 | 3.68 ± 1.91 |
Biochemical examination. There were no significant differences in the GGT, ALT, AST, ALP, TBIL, CPK, and CRE levels between the control and treatment groups. The BUN level was significantly increased in the progoitrin and glucoerucin treated groups compared to the control group (Table 4).
Table 4. Serum biochemistry of rats treated with glucosinolates.
| Control | Sinigrin | Progoitrin | Glucoerucin | Glucotropaeolin | |
|---|---|---|---|---|---|
|
| |||||
| GGT (U/L) | 3.8 ± 0.4 | 16.0 ± 24.7 | 6.0 ± 4.8 | 5.6 ± 4.8 | 3.8 ± 1.1 |
| ALT (U/L) | 19.4 ± 7.0 | 23.8 ± 8.5 | 19.6 ± 4.3 | 17.2 ± 4.5 | 17.4 ± 1.80 |
| AST (U/L) | 80.4 ± 46.0 | 95.2 ± 22.0 | 84.4 ± 27.5 | 75.6 ± 17.1 | 58.6 ± 12.5 |
| ALP (U/L) | 576 ± 190 | 720 ± 81 | 653 ± 180 | 555 ± 89 | 857 ± 290 |
| TBIL (mg/dl) | 0.5 ± 0.5 | 1.5 ± 0.7 | 1.1 ± 0.9 | 1.1 ± 0.7 | 0.7 ± 0.3 |
| CPK (U/L) | 260 ± 357 | 268 ± 68 | 208 ± 125 | 215 ± 45 | 137 ± 530 |
| BUN (mg/dl) | 13.2 ± 5.6 | 17.7 ± 2.3 | 20.5 ± 5.1* | 21.5 ± 3.3* | 11.1 ± 1.3 |
| CRE (mg/dl) | 0.3 ± 0.1 | 0.4 ± 0.4 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 |
* Significant differences in comparison with the control (p<0.05).
Liver weight. In the sinigrin and progoitrin treated groups, liver weight per 100 g of body weight was increased but not statistically significant compared to the control group. The other treatment groups did not show any significant differences compared to the control group as well (data not shown).
DISCUSSION
In the present study, a small amount of organic nitriles were detected only from the progoitrin and glucoerucin treated groups, and no organic thiocyanates were detected. However, organic isothiocyanates and thiocyanate ions were detected from all of the treated groups. Thiocyanate ions as well as organic isothiocyanates were reported when Brassica napus, B. campestris rapeseed and B. juncea mustard were incubated with botanical myrosinase, and the data from the present study indicate that the degradation of the GLSs in the animal body generates the same products without botanical myrosinase (20).
It is noteworthy that most of the treatment groups generated a high concentration of thiocyanate ions. According to previous studies on the metabolism of GLS-BPs in the body, organic isothiocyanates were reported to be conjugated with glutathione to form mercapturic acid and then excreted through the urine in rats and humans (21,22). Organic nitriles were observed to undergo cytochrome P450 metabolism and generate cyanide ions during the metabolism. The amount of cyanide ions generated depended on the structure of the organic nitriles (23). Besides, thiocyanate ions were detected in the serum of mice treated with organic nitriles, which supports that cyanide ions generated from organic nitriles can be converted into thiocyanate ions (24). Organic thiocyanates were shown to be metabolized by glutathione conjugation and produce cyanide ions during metabolism (25). In the current study, the concentrations of thiocyanate and cyanide ions were relatively higher than those of the other GLS-BPs. Meanwhile, organic nitriles and organic thiocyanates were rarely detected. It could be assumed that the metabolism of the GLS-BPs are relatively fast, so within five hours they may have been further degraded into thiocyanate and cyanide ions. More thiocyanate ions would be produced from organic nitriles with cyanide ions yet to be metabolized.
In the progoitrin treated group, serum organic isothiocyanates were at the highest level among the GLS-BPs. Progoitrin, which has a hydroxyl group on the β-carbon, spontaneously cyclizes to form 5-oxazolidine-2-thione (goitrin) upon hydorlysis (26). Goitrin is known to have strong antithyroidal effects. It was reported that goitrin increased liver and thyroid weights, and inhibited thyroid hormones synthesis (27,28). Similar to goitrin, thiocyanate ions have a goitrogenic effect, which can be alleviated by iodide supplementation. The anti-thyroidal effect of goitrin, however, cannot be alleviated by dietary iodide. Hence, goitrin is regarded as one of the most potent anti-thyroid substances (28).
The production of goitrogenic substances was relatively low from glucoerucin. In addition, the metabolism of the breakdown products seemed relatively slow because this is the only group in which a quantitable amount of organic nitriles was detected, while the final ion concentrations are low. Sinigrin and glucotropaeolin generated significantly more thiocyanate ions in rats than that of the other GLSs within 5 hr of the administration. The R-groups of sinigrin and glucotropaeolin are an allyl and benzyl group, respectively, which can form a relatively stable carbocation when it is further degraded to form thiocyanate ions. Progoitrin predominantly metabolized to organic isothiocyanates (goitrin). It seems that the ring structure of goitrin made the reaction with glutathione slow so the clearance of goitrin was delayed. Because goitrin is reported to have potent anti-nutritional effects on the thyroid, its retarded clearance may aggravate the situation.
The T3 and T4 concentrations in serum were examined in order to observe the effect of a single dose of GLSs on the thyroid hormone. In the sinigrin and glucotropaeolin treated groups, the T3 level was reduced, possibly due to the effect of the thiocyanate ions. In the progoitrin treated group, both the T3 and T4 levels were reduced, possibly due to the effect of goitrin. In the glucoerucin treated group, the T4 level was reduced though there were little goitrogenic substances. However, there were no significant differences between the control and treatment groups, thus more studies are needed to determine the effect of GLSs on thyroid hormones.
There were no significant differences in the liver weights of the animals 5 hr after the treatment with GLSs. The BUN levels in the progoitrin and glucoerucin treated groups were significantly increased compared to those of the control group. However, the CRE level, another marker for kidney function, did not change. Therefore, more careful interpretation and further investigation would be necessary regarding kidney function and GLS administration.
The present study has shown that the absorption and distribution of GLS-BPs in the blood may vary depending on the R-group of the intact GLS. It was observed that without botanical myrosinase, anti-thyroidal thiocyanate ions and goitrin can be generated in the animal body. Thus, the inactivation of myrosinase during cooking will have no effect on the anti-nutritional effect of GLSs in cruciferous vegetables. Additionally, the relative fast conversion to thiocyanate ions, which block the uptake of iodine into the thyroid, can explain the protection of simultaneous administration of iodine from thiocyanate ion induced T3 deficiency.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2011-0013686).
References
- 1.Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. (2001);56:5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 2.Fenwick G.R., Heaney R.K., Mullin W.J., Vanetten C.H. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. (1982);18:123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro T.A., Fahey J.W., Wade K.L., Stephenson K.K., Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts metabolism and excretion in humans. Cancer Epidemiol. Biomarkers Prev. (2001);10:501–508. [PubMed] [Google Scholar]
- 4.Maskell I., Smithard R. Degradation of glucosinolates during in vitro incubations of rapeseed meal with myrosinase (EC 3.2.3.1) and with pepsin (EC 3.4.23.1)-hydrochloric acid, and contents of porcine small intestine and caecum. Br. J. Nutr. (1994);72:455–466. doi: 10.1079/BJN19940047. [DOI] [PubMed] [Google Scholar]
- 5.Mennicke W., Kral T., Krumbiegel G., Rittmann N. Determination of N-acetyl-S-(N-alkylthiocarbamoyl)-L-cysteine, a principal metabolite of alkyl isothiocyanates, in rat urine. J. Chromatogr. B Biomed. Sci. Appl. (1987);414:19–24. doi: 10.1016/0378-4347(87)80020-8. [DOI] [PubMed] [Google Scholar]
- 6.Slominski B., Campbell L., Stanger N. Influence of cecectomy and dietary antibiotics on the fate of ingested intact glucosinolates in poultry. Can. J. Anim. Sci. (1987);67:1117–1124. doi: 10.4141/cjas87-117. [DOI] [Google Scholar]
- 7.Slominski B.A., Campbell L.D., Stanger N.E. Extent of hydrolysis in the intestinal tract and potential absorption of intact glucosinolates in laying hens. J. Sci. Food Agric. (1988);42:305–314. doi: 10.1002/jsfa.2740420404. [DOI] [Google Scholar]
- 8.Holst B., Williamson G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. (2004);21:425–447. doi: 10.1039/b204039p. [DOI] [PubMed] [Google Scholar]
- 9.Heaney R.K., Fenwick G.R. Natural toxins and protective factors in Brassica species, including rapeseed. Nat. Toxins. (1995);3:233–237. doi: 10.1002/nt.2620030412. [DOI] [PubMed] [Google Scholar]
- 10.Erdoğan M.F. Thiocyanate overload and thyroid disease. Biofactors. (2003);19:107–111. doi: 10.1002/biof.5520190302. [DOI] [PubMed] [Google Scholar]
- 11.Song L., Iori R., Thornalley P.J. Purification of major glucosinolates from Brassicaceae seeds and preparation of isothiocyanate and amine metabolites. J. Sci. Food Agric. (2006);86:1271–1280. doi: 10.1002/jsfa.2488. [DOI] [Google Scholar]
- 12.Rochfort S., Caridi D., Stinton M., Trenerry C., Jones R. The isolation and purification of glucoraphanin from broccoli seeds by solid phase extraction and preparative high performance liquid chromatography. J. Chromatogr. A. 2006;1120:205–210. doi: 10.1016/j.chroma.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Visentin M., Tava A., Iori R., Palmieri S. Isolation and identification of trans-4-(methylthio)-3-butenyl glucosinolate from radish roots (Raphanus sativus L.). J. Agric. Food Chem. 1992;40:1687–1691. doi: 10.1021/jf00021a041. [DOI] [Google Scholar]
- 14.ISO norm. Rapeseed- Determination of glucosinolate content-Part 1: Method using high-performance liquid chromatography. I.S.O. (1992);ISO 9167-1:1–9. [Google Scholar]
- 15.van Staden J., Botha A. Spectrophotometric determination of thiocyanate by sequential injection analysis. Anal. Chim. Acta. (2000);403:279–286. doi: 10.1016/S0003-2670(99)00651-0. [DOI] [Google Scholar]
- 16.Shim K.H., Kang K.S., Ahn C.W., Seo K.I. Quantitative analysis of glucosinolates and thermal degradation product of indole glucosinolates in radish. J. Korean Agric. Chem. Soc. (1993);36:23–28. [Google Scholar]
- 17.Bhandari R.K., Oda R.P., Youso S.L., Petrikovics I., Bebarta V.S., Rockwood G.A., Logue B.A. Simultaneous determination of cyanide and thiocyanate in plasma by chemical ionization gas chromatography massspectrometry (CI-GC-MS). Anal. Bioanal. Chem. (2012);404:2287–2294. doi: 10.1007/s00216-012-6360-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. The 1, 2-benzenedithiole-based cyclocondensation assay: a valuable tool for the measurement of chemopreventive isothiocyanates. Crit. Rev. Food Sci. Nutr. 2012;52:525–532. doi: 10.1080/10408398.2010.503288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye L., Dinkova-Kostova A.T., Wade K.L., Zhang Y., Shapiro T.A., Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta. (2002);316:43–53. doi: 10.1016/S0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 20.McGregor D. Thiocyanate ion, a hydrolysis product of glucosinolates from rape and mustard seed. Can. J. Plant Sci. (1978);58:795–800. doi: 10.4141/cjps78-118. [DOI] [Google Scholar]
- 21.Brusewitz G., Cameron B.D., Chasseaud L.F., Gorler K., Hawkins D.R., Koch H., Mennicke W.H. The metabolism of benzyl isothiocyanate and its cysteine conjugate. Biochem. J. (1977);162:99–107. doi: 10.1042/bj1620099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung F.L., Morse M.A., Eklind K.I. New potential chemopreventive agents for lung carcinogenesis of tobaccospecific nitrosamine. Cancer Res. (1992);52:S2719–2722. [PubMed] [Google Scholar]
- 23.Silver E., Kuttab S.H., Hasan T., Hassan M. Structural considerations in the metabolism of nitriles to cyanide in vivo. Drug Metab. Dispos. (1982);10:495–498. [PubMed] [Google Scholar]
- 24.Stea K. The Effect of Thyroxine Administration on the Formation of Thiocyanate from Acetonitrile in Mice. Acta Pharmacol. Toxicol. 1952;8:263–270. doi: 10.1111/j.1600-0773.1952.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 25.Hideo O., Casida J.E. Glutathione S-transferases liberate hydrogen cyanide from organic thiocyanates. Biochem. Pharmacol. 1971;20:1708–1711. doi: 10.1016/0006-2952(71)90303-0. [DOI] [PubMed] [Google Scholar]
- 26.Kjaer A., Christensen B., Hansen S. Isothiocyanates. 34. The absolute configuration of (-)-5-vinyl-2-oxazolidinethione (goitrin) and its glucosidic progenitor (progoitrin). Acta Chem. Scand. (1959);13:144–150. doi: 10.3891/acta.chem.scand.13-0144. [DOI] [Google Scholar]
- 27.Nishie K., Daxenbichler E. Hepatic effects of Rgoitrin in sprague-dawley rats. Food Chem. Toxicol. (1982);20:279–287. doi: 10.1016/S0278-6915(82)80294-9. [DOI] [PubMed] [Google Scholar]
- 28.Fenwick G., Heaney R. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983;11:249–271. doi: 10.1016/0308-8146(83)90074-2. [DOI] [Google Scholar]
- 29.Griffiths D.W., Deighton N., Birch A.E., Patrian B., Baur R., Stadler E. Identification of glucosinolates on the leaf surface of plants from the Cruciferae and other closely related species. Phytochemistry. 2001;57:693–700. doi: 10.1016/S0031-9422(01)00138-8. [DOI] [PubMed] [Google Scholar]
- 30.Park H.U. Degradation patterns of glucosinolates in ruciferous vegetables under food processing conditions. Department of Food and Nutrition, The graduate school, Seoul National University.; (2012). [Google Scholar]


