Abstract
Recent episodes of severe air pollution in eastern Asia have been reported in the scientific literature and news media. Therefore, there is growing concern about the systemic effects of air pollution on human health. Along with the other well-known harmful effects of air pollution, recently, several animal models have provided strong evidence that air pollutants can induce liver toxicity and act to accelerate liver inflammation and steatosis. This review briefly describes examples where exposure to air pollutants was involved in liver toxicity, focusing on how particulate matter (PM) or carbon black (CB) may be translocated from lung to liver and what liver diseases are closely associated with these air pollutants.
Keywords: Air pollution, Liver, Particulate matter, Carbon black, Lung
INTRODUCTION
A worldwide increase in particulate air pollution has been reported and severe episodes have been strongly associated with the increased incidence of several diseases. Particulate matter (PM) air pollutants such as carbon black (CB) created from incomplete combustion of fossil fuels and diesel exhaust particles (DEP) generated by diesel powered trucks and automobiles are the main constituents of atmospheric PM in urban areas. Many epidemiological studies have shown that exposure to ambient PM was positively correlated with increased human mortality by various causes including cardiovascular diseases (1,2) and respiratory diseases (3,4).
In experimental studies, tracheal instillation of PM has demonstrated that DEP induced tissue damage through oxidative stress including the generation of reactive oxygen species (5) by a non-enzymatic process (6)or by cytochrome P-450 catabolic enzymatic reactions (7). Similar to DEP, carbon black (CB) nanoparticle instillation caused pulmonary inflammation and genotoxicity in liver and lungs (8). Furthermore, airborne fine PM (PM2.5: particulate matter that is 2.5 μm in diameter and smaller) increased levels of lipid peroxidation in various organs including heart, liver, lungs, and testicles indicating that airborne PM2.5 acts as a systemic toxin (9).
In addition to direct tissue damage by PM and CB, increased mortality in patients with type 2 diabetes mellitus, which is associated with unhealthy lifestyles and complicated by numerous conditions including angiopathy (10), obesity (11), nephropathy and infection, is closely associated with ambient PM exposure suggesting that PM induces a wide range of toxic effects (12,13). These studies have revealed several mechanisms by which airborne PM or CB could be a probable cause of disease exacerbations, although more detailed mechanistic studies are needed to confirm the role of PM.
The present review is focused on the harmful effects of exposure to airborne PM or CB on liver, especially the following factors; 1) Direct toxic effects on the liver, 2) inflammation, lipid metabolism, and fatty liver disease, and 3) translocation of PM or CB from lung to liver. Our review will serve to alert physicians and the public, especially liver patients, of the potential hepatotoxic effects of PM air pollution. Most of the previous studies of the effects of PM or CB on human health have been in the fields of respiratory and cardiovascular disorders.
Translocation of PM from lung to liver. Although various organs including skin, eyes, and digestive tract can be exposed to ambient PM or CB, the main exposed area may be the pulmonary system. Whereas it is conceptually easy to visualize how air pollutants might translocate from the lung into systemic circulation, detailed mechanisms are less easily explained since the constituents of PM are quite diverse and the toxicodynamics of individual components could vary widely.
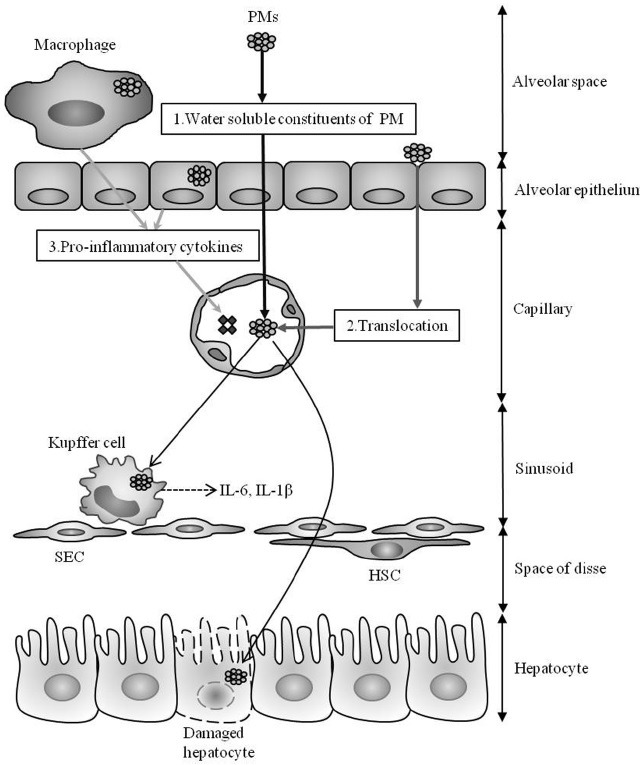
There are several scenarios whereby ambient PM could play a role in the progression of diseases in extra-pulmonary organs, especially liver. First, the possibility that water soluble fractions of PM could translocate into extra-pulmonary circulation has been examined (Fig. 1A). For example, intratracheal instillation of water-soluble metals such as vanadium and cadmium, and metal components of fly ash were detected in several extra-pulmonary organs, including liver, kidney, heart (14-16). These observations indicate that at least water-soluble constituents of PM could have a direct toxic role in extra-pulmonary organs. The second scenario (Fig. 1B) proposes that inhaled insoluble nanoparticles directly cross the alveolar-capillary barrier, circulate in the blood stream, and deposit on blood cells or on the surface of vascular endothelial cells in nonspecific organs (17). Such interactions might result in prothrombotic effects (18) on the hepatic microcirculation (19). The third scenario suggests that inhaled PM particles are first in contact with immune cells such as alveolar or bronchiolar macrophages and thereby stimulate innate immune responses, releasing pro-inflammatory cytokines into the blood stream (Fig. 1C). Such an inflammatory milieu could contribute to the disease progression in organs such as liver (17). However, the exact translocation pathways of the various constituents of PM or CB are not fully understood. Accordingly, understanding the role of PM translocation in triggering liver disease may provide insights into mechanisms and the development of protective and therapeutic strategies.
Fig. 1. Hypothetical mechanisms of liver toxicity for particulate air pollutants. Since little is known about the effects of air pollutants on the liver, some of these proposed mechanisms are extrapolated from research in lung and other organs. Hydrophilic constituents of inhaled air pollutants may translocate into the extra-pulmonary circulation (A) Water insoluble fractions of particulate matter (PM) or nanoparticles may directly cross the alveolar epithelial layer and translocate into the circulation (B) Once in the circulation, nanoparticles could interact with Kupffer cells, residential macrophages in the liver, leading to the production of pro-inflammatory cytokines, such as IL-6 and IL-1β, or have direct cytotoxic effects on hepatocytes by inducing multiple cellular stress responses. Inhaled PM particles could come in contact with immune cells or airway epithelial cells and provoke an innate immune response in the lungs, with subsequent release of pro-inflammatory cytokines into the blood stream (C) Such an inflammatory milieu and direct hepatotoxicity induced by translocated airborne PM could trigger the progression of various acute and chronic liver diseases. (SEC: sinusoidal endothelial cell, HSC: hepatic stellate cell).
Hepatotoxic effects of ambient PM. The harmful effects of ambient PM or CB on liver are well-documented (Table 1). Several animal models have clearly shown that exposure to PM or CB can cause direct hepatotoxicity. Classical studies from the Zhang and Meng laboratories using dust storm PM or airborne PM2.5 in rats provided convincing evidence for a causative role for PM in liver toxicity (9,20). They found that dust storm PM and normal airborne PM2.5 exposure in rats could lead to oxidative damage in the liver. Other models have been used to investigate the effects of wood smoke PM and CB in causing oxidative liver damage (21)and the effects of vanadium, present in crude oil as an organometallic complex, in producing increased lipid peroxidation in liver (22).
Table 1. Air pollutants in liver diseases.
| Diseases | Air pollutants | Mechanism of action | References |
|---|---|---|---|
|
| |||
| Hepatotoxicity | Coal fly ash | Lipid peroxidation, Hepatic megalocytosis, DNA damage | (22,37-39) |
| PM2.5 or CB | Generation of ROS, Lipid peroxidation, Genotoxicity, ER stress | (9,20,21,23,40) | |
| DEP | Genotoxicity, Generation of ROS | (5-7,23) | |
| NAFLD and Type II diabetes | PM2.5 or CB | Kupffer cell activation and Production of pro-inflammatory cytokine, Impaired hepatic glycogen storage, glucose intolerance and insulin resistance, Alteration of lipid homeostasis and Visceral adipose tissue inflammation, Imbalance in circulating leptin/adiponectin levels | (26-32,34,36,41) |
| DEP | Oxidative stress, DNA damage | (33,42-44) | |
| Liver fibrosis | The total extracts or the PAH fraction of airborneparticles | Mitochondrial and Hematogenic damage | (45) |
| Liver cancer | 2-NBA, 3-NBA | Genotoxicity, Mutagenic and Carcinogenic activity | (46-48) |
PM2.5: Particulate matter < 2.5 μm; CB: Carbon black; DEP: Diesel exhaust particles; ROS: Reactive oxygen species; NAFLD: Non-alcoholic fatty liver disease; ER: Endoplasmic reticulum; PAH: polycyclic aromatic hydrocarbon; 2-NBA: 2-nitrobenzanthrone; 3-NBA: 3-nitrobenzanthrone.
In addition, gastrointestinal exposure to CB and DEP generated oxidized DNA bases in liver, suggesting that such oxidative stress may be associated with an increased risk of liver cancer (23). While oxidative stress by PM is a widely accepted toxic mechanism, additional mechanisms have been reported. The Wallin group provided evidence demon-strating that pulmonary exposure to CB by inhalation in pregnant mice resulted in DNA strand breaks in the livers of both dams and offspring (24). A similar study has shown genotoxicity in the liver and lungs of mice after CB nanoparticle instillation (8). Whereas it is easy to describe how PM or CB could trigger oxidative stress and genotoxicity, the molecular mechanisms underlying PM-dependent induction of hepatotoxicity remain to be elucidated.
How does airborne PM exacerbate steatohepatitis? The increasing population of clinically obese people has been closely associated with increased incidence of several cardiovascular and metabolic diseases, including cardiac disease, hypertension, insulin resistance, diabetes, and nonalcoholic fatty liver disease (NAFLD) (11). NAFLD is defined as the excess accumulation of lipid in the liver of individuals who do not drink significant amounts of alcohol and do not have other known liver diseases (25). Among NAFLD patients, some develop a condition characterized by severe inflammation and ballooning degeneration of their hepatocytes, called non-alcoholic steatohepatitis (NASH) that can progress to cirrhosis or hepatocellular carcinoma. Unfortunately, connections between these risk factors and the mechanisms of onset of NAFLD and NASH are controversial and clear causation remains to be demonstrated.
As described earlier, airborne PM induced systemic effects including oxidative stress, inflammation, and genotoxicity. These factors are known to play important roles in the pathogenesis of NASH. Therefore, airborne PM may be acting as an inducer or promoter of NASH progression. Tan et al. (26)assessed whether ambient air PM activates Kupffer cells, resident hepatic macrophages, and subsequently exacerbates NAFLD via production of pro-inflammatory cytokines. PM exposure induced interleukin-6 (IL-6) secretion from isolated Kupffer cells in a concentrationdependent manner and this effect was reduced if Toll-like receptor 4 (TLR4) was absent, indicating that PM acts like a TLR4 ligand. The Monick group (27) obtained similar results although they used a macrophage cell line (not Kupffer cells) to demonstrate the pro-inflammatory effects of CB. They found that CB nanoparticle exposure activated caspase 1, increased interleukin-1β (IL-1β) production, and induced pyroptosis, an inflammasome-dependent form of cell death through TLR4 stimulation. In addition to these effects, chemotactic events may be triggered systemically. In rats, ambient PM2.5 exposure induced monocyte-macrophage congregation in liver sinusoids and formation of granulomas (28). These observations suggested that PM or CB are sufficient to trigger hepatitis and systemic inflammation.
While inflammation is an important factor for promoting the progression of NASH, the primary inducing factor for NASH is excessive lipid accumulation. In addition, metabolic dysregulations such as insulin resistance have been reported as NASH complications (29). Several groups have demonstrated that mice exposed to PM displayed impaired hepatic glycogen storage, glucose intolerance, and insulin resistance (30-32). For example, 10-week concentrated ambient PM2.5 exposure induced insulin resistance independent of obesity (32). However, there are also studies that provide evidence that PM also dysregulated lipid metabolism. The Uematsu group (33) found that in obese diabetic mice (db/db mice), pulmonary exposure to DEP increased the levels of liver enzymes including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and subsequently produced steatosis. It was also shown that intratracheal instillation of CB nanoparticles marginally increased total hepatic cholesterol (34)and produced steatosis independent of lipogenesis (35). Another recent study showed that PM2.5 exposure to C-C chemokine receptor 2 (CCR2)-/- or wild-type mice produced insulin resistance by producing visceral adipose tissue inflammation, decreased hepatic lipid metabolism, and decreased glucose utilization in skeletal muscle via CCR2 (36).
Finally, it is clear that PM and CB exposure created conditions favorable to NASH progression. From these data, it is clear protective or therapeutic strategies against air pollutant exposure need to be considered for further study.
CONCLUSIONS
Air pollutants have been associated with increased morbidity although direct evidence of causation has been lacking until recently. Several animal models provide strong evidence that PM or CB, indicators of air pollution, can induce various diseases and act to exacerbate existing lesions in organs that are accessible to the constituents of air pollution. Among those organs, the liver is one of the vulnerable target organs since its microvasculature allows ready access to hepatocytes and inhaled PM pollutants can be translocated from the alveolar space into the bloodstream. Direct effects of PM or CB on hepatocytes include the induction of oxidative stress and DNA strand breaks. In addition, airborne PMs contribute to the pathogenesis of steatohepatitis by alteration of lipid metabolism and induction of a pro-inflammatory milieu, resulting in exacerbation of NASH. Although direct evidence for these associations has been reported, more extensive and detailed studies are needed in liver models since the constituents of PM or CB are diverse and the roles of individual constituents on liver pathophysiology are currently unknown. Furthermore, therapeutic or prophylactic strategies for liver protection against exposure to air pollutants should be considered if we are not able to reduce air pollution in our environment.
Acknowledgments
This work was supported by a grant (No. 13182MFDS763) from Ministry of Food and Drug Safety in 2014.
References
- 1.Son J.Y., Lee J.T., Kim K.H., Jung K., Bell M.L. Characterization of fine particulate matter and associations between particulate chemical constituents and mortality in Seoul, Korea. Environ. Health Perspect. (2012);120:872–878. doi: 10.1289/ehp.1104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunekreef B., Beelen R., Hoek G., Schouten L., Bausch-Goldbohm S., Fischer P., Armstrong B., Hughes E., Jerrett M., van den Brandt P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res. Rep. Health Eff. Inst. (2009);139:5–71. [PubMed] [Google Scholar]
- 3.Nachman K.E., Parker J.D. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: a cross-sectional study. Environ. Health. (2012);11:25. doi: 10.1186/1476-069X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko F.W., Hui D.S. Air pollution and chronic obstructive pulmonary disease. Respirology. (2012);17:395–401. doi: 10.1111/j.1440-1843.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- 5.Siegel P.D., Saxena R.K., Saxena Q.B., Ma J.K., Ma J.Y., Yin X.J., Castranova V., Al-Humadi N., Lewis D.M. Effect of diesel exhaust particulate (DEP) on immune responses: contributions of particulate versus organic soluble components. Toxicol. Environ. Health A. (2004);67:221–231. doi: 10.1080/15287390490266891. [DOI] [PubMed] [Google Scholar]
- 6.Bai Y., Suzuki A.K., Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radicals Biol. Med. (2001);30:555–562. doi: 10.1016/S0891-5849(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 7.Ma J.Y., Ma J.K. The dual effect of the particulate and organic components of diesel exhaust partic leson the alteration of pulmonary immune/inflammatory responses and metabolic enzymes. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. (2002);20:117–147. doi: 10.1081/GNC-120016202. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Meng Z. Effects of airborne fine particulate matter on antioxidant capacity and lipid peroxidation in multiple organs of rats. Inhalation Toxicol. (2005);17:467–473. doi: 10.1080/08958370590964467. [DOI] [PubMed] [Google Scholar]
- 9.Bourdon J.A., Saber A.T., Jacobsen N.R., Jensen K.A., Madsen A.M., Lamson J.S., Wallin H., Møller P., Loft S., Yauk C.L., Vogel U.B. Carbon black nanoparticle instillation induces sustained inflammation and genotoxicity in mouse lung and liver. Part. Fibre Toxicol. (2012);9:5. doi: 10.1186/1743-8977-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shomali M. Diabetes treatment in 2025: can scientific advances keep pace with prevalence? Ther. Adv. Endocrinol. Metab. (2012);3:163–173. doi: 10.1177/2042018812465639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarantino G., Capone D., Finelli C. Exposure to ambient air particulate matter and non-alcoholic fatty liver disease. World J. Gastroenterol. (2013);19:3951–3956. doi: 10.3748/wjg.v19.i25.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puett R.C., Hart J.E., Schwartz J., Hu F.B., Liese A.D., Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ. Health Perspect. (2011);119:384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider A., Alexis N.E., Diaz-Sanchez D., Neas L.M., Harder S., Herbst M.C., Cascio W.E., Buse J.B., Peters A., Devlin R.B. Ambient PM2.5 exposure up-regulates the expression of costimulatory receptors on circulating monocytes in diabetic individuals. Environ. Health Perspect. (2011);119:778–783. doi: 10.1289/ehp.1002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallenborn J.G., Kovalcik K.D., McGee J.K., Landis M.S., Kodavanti U.P. Systemic translocation of 70zinc: kinetics following intratracheal instillation in rats. Toxicol. Appl. Pharmacol. (2009);234:25–32. doi: 10.1016/j.taap.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Sharma R.P., Flora S.J., Drown D.B., Oberg S.G. Persistence of vanadium compounds in lungs after intratracheal instillation in rats. Toxicol. Ind. Health. (1987);3:321–329. doi: 10.1177/074823378700300304. [DOI] [PubMed] [Google Scholar]
- 16.Mani U., Prasad A.K., Suresh Kumar V., Lal K., Kanojia R.K., Chaudhari B.P., Murthy R.C. Effect of fly ash inhalation on biochemical and histomorphological changes in rat liver. Ecotoxicol. Environ. Saf. (2007);68:126–133. doi: 10.1016/j.ecoenv.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Mills N.L., Donaldson K., Hadoke P.W., Boon N.A., Mac-Nee W., Cassee F.R., Sandström T., Blomberg A., Newby D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. (2009);6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 18.Khandoga A., Stoeger T., Khandoga A.G., Bihari P., Karg E., Ettehadieh D., Lakatos S., Fent J., Schulz H., Krombach F. Platelet adhesion and fibrinogen deposition in murine microvessels upon inhalation of nanosized carbon particles. J. Thromb. Haemostasis. (2010);8:1632–1640. doi: 10.1111/j.1538-7836.2010.03904.x. [DOI] [PubMed] [Google Scholar]
- 19.Khandoga A., Stampfl A., Takenaka S., Schulz H., Radykewicz R., Kreyling W., Krombach F. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. (2004);109:1320–1325. doi: 10.1161/01.CIR.0000118524.62298.E8. [DOI] [PubMed] [Google Scholar]
- 20.Meng Z.Q., Zhang Q.X. Effects of dust storm fine particles instillation on oxidative damage in hearts, livers, lungs of rats. Weisheng Yanjiu. (2006);35:690–693. [PubMed] [Google Scholar]
- 21.Danielsen P.H., Loft S., Jacobsen N.R., Jensen K.A., Autrup H., Ravanat J.L., Wallin H., Møller P. Oxidative stress, inflammation, and DNA damage in rats after intratracheal instillation or oral exposure to ambient air and wood smoke particulate matter. Toxicol. Sci. (2010);118:574–585. doi: 10.1093/toxsci/kfq290. [DOI] [PubMed] [Google Scholar]
- 22.Fortoul T.I., Rodriguez-Lara V., Gonzalez-Villalva A., Rojas-Lemus M., Cano-Gutierrez G., Ustarroz-Cano M., Colin-Barenque L., Montaño L.F., García-Pelez I., Bizarro-Nevares P., Lopez-Valdez N., Falcon-Rodriguez C.I., Jimenez-Martínez R.S., Ruiz-Guerrero M.L., López-Zepeda L.S., Morales-Rivero A., Muñiz-Rivera-Cambas A. Vanadium inhalation in a mouse model for the understanding of air-suspended particle systemic repercussion. J. Biomed. Biotechnol. (2011);2011:11. doi: 10.1155/2011/951043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller P., Folkmann J.K., Danielsen P.H., Jantzen K., Loft S. Oxidative stress generated damage to DNA by gastrointestinal exposure to insoluble particles. Curr. Mol. Med. (2012);12:732–745. doi: 10.2174/156652412800792624. [DOI] [PubMed] [Google Scholar]
- 24.Jackson P., Hougaard K.S., Boisen A.M., Jacobsen N.R., Jensen K.A., Møller P., Brunborg G., Gutzkow K.B., Andersen O., Loft S., Vogel U., Wallin H. Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: effects on liver DNA strand breaks in dams and offspring. Nanotoxicology. (2012);6:486–500. doi: 10.3109/17435390.2011.587902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vuppalanchi R., Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. (2009);49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan H.H., Fiel M.I., Sun Q., Guo J., Gordon R.E., Chen L.C., Friedman S.L., Odin J.A., Allina J. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J. Immunotoxicol. (2009);6:266–275. doi: 10.3109/15476910903241704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reisetter A.C., Stebounova L.V., Baltrusaitis J., Powers L., Gupta A., Grassian V.H., Monick M.M. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J. Biol. Chem. (2011);286:21844–21852. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D., Huang N., Wang Q., Liu H. [Study of ambient PM2.5 on the influence of the inflammation injury and the immune function of subchronic exposure rats]. Weisheng Yanjiu. (2008);37:423–428. [PubMed] [Google Scholar]
- 29.Chitturi S., Abeygunasekera S., Farrell G.C., Holmes-Walker J., Hui J.M., Fung C., Karim R., Lin R., Samarasinghe D., Liddle C., Weltman M., George J. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. (2002);35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Z., Xu X., Zhang X., Wang A., Zhang C., Hüttemann M., Grossman L.I., Chen L.C., Rajagopalan S., Sun Q., Zhang K. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J. Hepatol. (2013);58:148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X., Yavar Z., Verdin M., Ying Z., Mihai G., Kampfrath T., Wang A., Zhong M., Lippmann M., Chen L.C., Rajagopalan S., Sun Q. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler. Thromb. Vasc. Biol. (2010);30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conklin D.J. From lung to liver: how does airborne particulate matter trigger NASH and systemic insulin resistance? J. Hepatol. (2013);58:8–10. doi: 10.1016/j.jhep.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomaru M., Takano H., Inoue K., Yanagisawa R., Osakabe N., Yasuda A., Shimada A., Kato Y., Uematsu H. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int. J. Mol. Med. (2007);19:17–22. [PubMed] [Google Scholar]
- 34.Bourdon J.A., Halappanavar S., Saber A.T., Jacobsen N.R., Williams A., Wallin H., Vogel U., Yauk C.L. Hepatic and pulmonary toxicogenomic profiles in mice intratracheally instilled with carbon black nanoparticles reveal pulmonary inflammation, acute phase response, and alterations in lipid homeostasis. Toxicol Sci. (2012);127:474–484. doi: 10.1093/toxsci/kfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesterdal L.K., Danielsen P.H., Folkmann J.K., Jespersen L.F., Aguilar-Pelaez K., Roursgaard M., Loft S., Møller P. Accumulation of lipids and oxidatively damaged DNA in hepatocytes exposed to particles. Toxicol. Appl. Pharmacol. (2014);274:350–360. doi: 10.1016/j.taap.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu C., Xu X., Bai Y., Wang T.Y., Rao X., Wang A., Sun L., Ying Z., Gushchina L., Maiseyeu A., Morishita M., Sun Q., Harkema J.R., Rajagopalan S. Air pollutionmediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ. Health Perspect. (2014);122:17–26. doi: 10.1289/ehp.1306841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano-Gutiérrez G., Acevedo-Nava S., Santamaría A., Altamirano-Lozano M., Cano-Rodríguez M.C., Fortoul T.I. Hepatic megalocytosis due to vanadium inhalation: participation of oxidative stress. Toxicol. Ind. Health. (2012);28:353–360. doi: 10.1177/0748233711412424. [DOI] [PubMed] [Google Scholar]
- 38.Shim I., Oh E., Yang S., Ryu T., Soh J., Sul D., Kim P. Subacute inhalation toxicity assessment of fly ash from industrial waste incinerators. Inhalation Toxicol. (2012);24:741–750. doi: 10.3109/08958378.2012.716869. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan S.S., Chaudhary V.K., Narayan S., Misra U.K. Cytotoxicity of inhaled coal fly ash in rats. Environ. Res. (1987);43:1–12. doi: 10.1016/S0013-9351(87)80050-6. [DOI] [PubMed] [Google Scholar]
- 40.Laing S., Wang G., Briazova T., Zhang C., Wang A., Zheng Z., Gow A., Chen A.F., Rajagopalan S., Chen L.C., Sun Q., Zhang K. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am. J. Physiol. Cell Physiol. 2010;299:C736–749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C., Bai Y., Xu X., Sun L., Wang A., Wang T.Y., Maurya S.K., Periasamy M., Morishita M., Harkema J., Ying Z., Sun Q., Rajagopalan S. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part. Fibre Toxicol. (2014);11:27. doi: 10.1186/1743-8977-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manners S., Alam R., Schwartz D.A., Gorska M.M. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J. Allergy Clin. Immunol. (2014);134:63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risom L., Møller P., Dybdahl M., Vogel U., Wallin H., Loft S. Dietary exposure to diesel exhaust particles and oxidatively damaged DNA in young oxoguanine DNA glycosylase 1 deficient mice. Toxicol. Lett. (2007);175:16–23. doi: 10.1016/j.toxlet.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Danielsen P.H., Risom L., Wallin H., Autrup H., Vogel U., Loft S., Møller P. DNA damage in rats after a single oral exposure to diesel exhaust particles. Mutat. Res. (2008);637:49–55. doi: 10.1016/j.mrfmmm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Meiss R., Heinrich U., Offermann M., Themann H. g-term study of liver damage following subcutaneous injection of airborne particle extracts and polycyclic aromatic hydrocarbon fractions. Int. Arch. Occup. Environ. Health. (1982);49:305–314. doi: 10.1007/BF00377939. [DOI] [PubMed] [Google Scholar]
- 46.Mizerovská J., Draínská H., Frei E., Schmeiser H.H., Arlt V.M., Stiborová M. Induction of biotransformation enzymes by the carcinogenic air-pollutant 3-nitrobenzanthrone in liver, kidney and lung, after intra-tracheal instillation in rats. Mutat. Res. (2011);720:34–41. doi: 10.1016/j.mrgentox.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Landvik N.E., Arlt V.M., Nagy E., Solhaug A., Tekpli X., Schmeiser H.H., Refsnes M., Phillips D.H., Lagadic-Gossmann D., Holme J.A. Nitrobenzanthrone and 3-aminobenzanthrone induce DNA damage and cell signalling in Hepa1c1c7 cells. Mutat. Res. (2010);684:11–23. doi: 10.1016/j.mrfmmm.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Arlt V.M., Glatt H., Gamboa da Costa G., Reynisson J., Takamura-Enya T., Phillips D.H. Mutagenicity and DNA adduct formation by the urban air pollutant 2-nitrobenzanthrone. Toxicol. Sci. (2007);98:445–457. doi: 10.1093/toxsci/kfm103. [DOI] [PubMed] [Google Scholar]


