Abstract
Objectives
The pathophysiology of shrinking lung syndrome (SLS) is poorly understood. We sought to define the structural basis for this condition through the study of pulmonary mechanics in affected patients.
Methods
Since 2007, most patients evaluated for SLS at our institutions have undergone standardized respiratory testing including esophageal manometry. We analyzed these studies to define the physiological abnormalities driving respiratory restriction. Chest computed tomography data were post-processed to quantitate lung volume and parenchymal density.
Results
Six cases met criteria for SLS. All presented with dyspnea as well as pleurisy and/or transient pleural effusions. Chest imaging was free of parenchymal disease and corrected diffusing capacities were normal. Total lung capacities were 39-50% of predicted. Maximal inspiratory pressures were impaired at high lung volumes, but not low lung volumes, in 5 patients. Lung compliance was strikingly reduced in all patients, accompanied by increased parenchymal density.
Conclusion
Patients with SLS exhibited symptomatic and/or radiographic pleuritis associated with two characteristic physiological abnormalities: 1) impaired respiratory force at high but not low lung volumes, and 2) markedly decreased pulmonary compliance in the absence of identifiable interstitial lung disease. These findings suggest a model in which pleural inflammation chronically impairs deep inspiration, for example via neural reflexes, leading to parenchymal reorganization that impairs lung compliance, a known complication of persistently low lung volumes. Together these processes could account for the association of SLS with pleuritis as well as the gradual symptomatic and functional progression that is a hallmark of this syndrome.
Keywords: Shrinking Lung Syndrome, Pleuritis, Pleurisy, Systemic Lupus Erythematosus, Lung
Introduction
Shrinking lung syndrome (SLS) was first described as a complication of systemic lupus erythematosus (SLE) by Hoffbrand and Beck (1). The term identifies a constellation of respiratory manifestations including dyspnea, reduced lung volumes and/or elevated hemidiaphragms on chest radiograph (CXR), and a restrictive ventilatory defect assessed by pulmonary function tests (PFTs). Serial CXRs demonstrate steadily declining lung volumes as SLS patients become more dyspneic, rendering the impression that the lungs are vanishing. SLS is rare, with an estimated prevalence of <1% among patients with SLE, though several recent reports have suggested a higher prevalence (2-4). The syndrome has been reported in other rheumatologic disorders including scleroderma, Sjögren's syndrome, rheumatoid arthritis, and undifferentiated connective tissue disorder (5-8).
The pathogenesis of SLS remains unknown. Chest imaging shows no evidence of interstitial lung disease or vascular pathology, although chest pain and small pleural effusions are common (9). While patients may become markedly dyspneic, mortality is rare. The literature contains a single autopsy report of an SLS patient who expired following prolonged mechanical ventilation for pneumonia, making findings difficult to interpret (10,11). Since pathologic data are unavailable, theories of the etiology of SLS are speculative and include surfactant deficiency (1), diaphragmatic myopathy (12), phrenic neuropathy (13), and chest wall dysfunction (9,14,15).
The hallmark abnormality in SLS is restrictive lung disease with a marked reduction in total lung capacity (TLC). The physiological basis for this restriction is unclear. Pulmonary restriction may arise through parenchymal changes that impair lung compliance (intrinsic restriction). Alternatively, restriction may originate outside the lung (extrinsic restriction), reflecting either structural limits on the chest wall (e.g., obesity, scoliosis, pleural adhesions) or functional compromise due to muscle disease, neuropathy, or reflex inhibition of muscle engagement.
Conventional PFTs fail to discriminate among mechanisms of ventilatory restriction because pressure measured at the mouth reflects both the force generated by the chest wall and the ability of the lung to expand. This limitation is addressed by concomitant esophageal manometry. Because the esophagus is thin-walled, pressure within the distal esophagus mirrors the pressure in the adjacent pleural space, permitting direct measurement of the maximal inspiratory force developed by the chest wall (MIPes) (16). Lung compliance can be determined by coupling MIPes data with measurement of airflow and pressure at the mouth. Importantly, MIPes can be assessed over a range of lung volumes, and changes across the volume spectrum have diagnostic significance. Low MIPes values throughout the lung volume range suggest a respiratory myopathy. In contrast, MIPes values that are normal at low lung volumes but decreased at high volumes indicate either constrained chest wall expansion or inhibition of respiratory muscle activation by volitional or reflex mechanisms, as occurs in pleurisy. Thus, esophageal manometry permits definition of the contributions of intrinsic and extrinsic factors to pulmonary restriction.
We report here the result of esophageal manometry studies in SLS. One patient underwent manometry before and after treatment, affording the opportunity to determine the physiological correlates of clinical improvement. The novel insights that emerge from these studies suggest a new pathophysiologic model of SLS.
Materials and Methods
Case Definition
SLS was defined as encompassing patients with a systemic rheumatic disease who presented with: (1) dyspnea; (2) decreased lung volumes on CXR; (3) PFTs demonstrating a restrictive ventilatory defect with normal diffusing capacity of the lung for carbon monoxide (DLCO) corrected for alveolar volume and hemoglobin concentration; and (4) absence of parenchymal pathology on chest computed tomography (CT). Rheumatic disease categorization was by American College of Rheumatology definitions (17).Patients who met criteria for SLS and underwent pulmonary evaluation with esophageal manometry between 2007 and 2011 were identified at the Brigham and Women's Hospital (BWH) and Boston Children's Hospital (BCH). All diagnoses of SLS were confirmed through chart review by an outside reviewer with expertise in SLE(CS).Clinical data were obtained from medical records. Patients with active SLS evaluated during this time interval but who had not undergone pulmonary mechanics evaluation were identified at BCH by a survey of attending rheumatologists and at BWH through an electronic search of rheumatology clinic notes for the term “shrinking lung.”
This study was performed in accordance with the Partners Institutional Review Board (2010P002092).
Esophageal Balloon Manometry
Pulmonary studies were conducted by a single investigator (SHL). Measurements were made with the patient awake and seated. An esophageal balloon catheter (Jaeger-Toenneis, Viasis, Yorba Linda, CA) was passed by nose to position the tip of the balloon at 35-40cm from the nares. The balloon was 10cm long, 2cm in perimeter, and inflated with 0.5mL of air. Correct placement of the catheter in the distal esophagus was confirmed by negative pressure deflections during inspiration and a negligible change in transpulmonary pressure during inspiratory or expiratory efforts against an occluded airway. Flow measured with a pneumotachograph (Fleisch #2, Phipps & Bird, Richmond, VA) was integrated to indicate relative volume. Pressures, flow, and volume were recorded utilizing custom-made software (Npulmo, by Emil Millet) and commercial hardware (Dataq Instruments, Akron, OH). Static deflation curves of the lung measured during interrupted expiration from TLC were used to estimate static lung compliance (Cst) near functional residual capacity (FRC). Dynamic lung compliance (Cdyn) was measured while the subject breathed at 20 breaths per minute to a metronome. Chest wall function and ventilatory muscle strength were assessed by measurement ofMIPes at lung volumes from FRC to TLC.
Chest CT Reformatting
All available chest imaging was reviewed for parenchymal pathology by a single radiologist (RRG). Chest CT digital imaging and communication in medicine (DICOM) data were post-processed on the Vitrea Enterprise suite 6.0 (Vital Images, MN) to generate volume-rendered images depicting lung volume and density in Hounsfield units (HU). A 2-dimensional image was generated from 3-dimensional volume data by classification and composition of individual sample points of the view-aligned proxy geometry slices, allowing quantification of lung volume and density (18-20).
Results
Clinical Presentation of SLS
Nine patients met the criteria for active SLS from 2007 to 2011. The age at onset of SLS ranged from 12 to 56 years with the wide span reflecting the demographic population of the participating study centers, one adult and one pediatric hospital. Of the 9 patients with SLS, 6 of these individuals (4 females and 2 males), underwent pulmonary mechanics evaluation as part of routine clinical care (Table 1). Three patients were not studied. Of these, 2 presented before pulmonary mechanics evaluation became routine, and the third presented at the end of the study period and was not evaluated because of concomitant acute medical issues; however, the 3 non-studied patients were clinically similar to the 6 evaluated cases (Table S1). All studied patients presented with dyspnea lasting from 6 to 15 months; 2 were also dyspneic at rest. There was evidence of pleural inflammation in all patients: 5 reported pleuritic chest pain, and 5 had transient, typically small pleural effusions, including the patient without pleuritic chest pain (SVideo 1). Anti-Ro antibodies have been documented in high percentages of SLS patients, and anti-RNP antibodies are associated with earlier pulmonary damage in SLE (2,15). Four patients included in this study had extensive autoantibody testing, and all were positive for either anti-Ro (3/4) or anti-RNP (3/4) antibodies. Chest imaging revealed decreased lung volumes without evidence of interstitial lung disease or pulmonary adhesions. Chest fluoroscopy or ultrasound performed in 4 patients demonstrated decreased diaphragmatic excursion bilaterally. In Case 6, a dedicated chest ultrasound was performed to look for adhesions between the lung and diaphragm or chest wall; no evidence for adhesive restriction of diaphragm motion was found. The mean TLC was 45% of predicted, and the corrected DLCO was normal in all patients (Table 2). All cases had normal muscle enzymes at presentation with SLS.
Table 1. Presentation of Shrinking Lung Syndrome.
| Rheum Dx | Sex | Age at Rheum Dx | Age at SLS Dx | Symptoms of SLS | Chest Imaging | SLS Treatment | SLS Outcome | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | MCTD | F | 14 | 15 | Dyspnea, Pleuritic CP, Orthopnea | Low lung volumes, atelectasis, small R effusion, decreased diaphragm excursion | Methylpred 1gm × 3, Pred 80mg/day, Rituximab 1 gm × 2 | Remission |
| Case 2 | MCTD | M | 15 | 15 | Dyspnea, Pleuritic CP, Orthopnea | Low lung volumes, bilat atelectasis, small bilat effusions | Methylpred 1gm × 3, Pred 10mg/ day, Cy 750mg IV Q2 wks, Theophylline 300mg/day | Remission |
| Case 3 | SLE | F | 18 | 22 | Dyspnea | Low lung volumes, paraspinal atelectasis, small bilat effusions | Pred 10mg/day, Theophylline 600mg/day, Meloxicam 7.5mg/day | N/A |
| Case 4 | SLE | F | 15 | 18 | Dyspnea, Pleuritic CP | Low lung volumes, bilat atelectasis, small bilat effusions, bilat decreased diaphragm excursion | Pred 40mg/day, MMF 1000mg/day | N/A |
| Case 5 | SLE | M | 43 | 45 | Dyspnea, Pleuritic CP, Orthopnea | Low lung volumes, bilat, atelectasis, moderate bilat effusions, bilat decreased diaphragm excursion | Pred 60mg/day, MMF 3000mg/day | Remission |
| Case 6 | SLE | F | 12 | 14 | Dyspnea, Pleuritic CP, Orthopnea | Elevated R hemi-diaphragm | Pred 60mg/day, Rituximab with Cytoxan × 2, Naproxen 1000mg/day, | Active |
Six subjects, who had active SLS symptoms during the study interval and underwent pulmonary mechanics evaluation, are presented. Rheum Dx = rheumatologic diagnosis; SLS = shrinking lung syndrome; Dx= diagnosis; MCTD = mixed connective tissue disease; SLE = systemic lupus erythematosus; F = female; M = male; CP = chest pain; R = right; bilat = bilateral; methylpred = intravenous methylprednisolone; pred = prednisone; Cy = cyclophosphamide; wks = weeks; MMF = mycophenolatemofetil; N/A = not available
Table 2. Pulmonary Mechanics.
| FVC % pred | TLC % pred | Corr DLCO % pred | Cst mL/cmH2O | Cdyn mL/cmH2O | MIPes FRC cmH2O | MIPes TLC cmH2O | |
|---|---|---|---|---|---|---|---|
| Normal | >80 | >80 | >80 | 226-428 | 150-390 | >50 | 25-35 |
| Case 1 | 37 | 50 | 109 | 73.1 | 63.9 | 42.6 | 14.5 |
| Case 2 | 42 | 42 | 97 | 76.4 | 47.7 | 74.0 | 23.7 |
| Case 3 | 33 | 50 | 114 | 72.8 | 41.4 | 38.5 | 12.7 |
| Case 4 | 38 | 44 | 124 | 55.2 | 28.4 | 66.0 | 33.9 |
| Case 5 | 37 | 42 | 106 | 165.8 | 115.3 | 41.5 | 12.2 |
| Case 6 | 36 | 39 | 102 | 67.6 | 44.0 | 40.2 | 15.0 |
MIPes are recorded by the esophageal balloon catheter as negative pressures since they are measured in the thorax during inspiration; however, per convention, the absolute value is reported. FVC = forced vital capacity; % pred = % predicted; TLC = total lung capacity; Corr = corrected; DLCO = diffusing capacity of the lung for carbon monoxide; Cst = static lung compliance; Cdyn = dynamic lung compliance; MIPes = maximal inspiratory esophageal pressure; FRC = functional residual capacity; NA = not available.
Esophageal Manometry
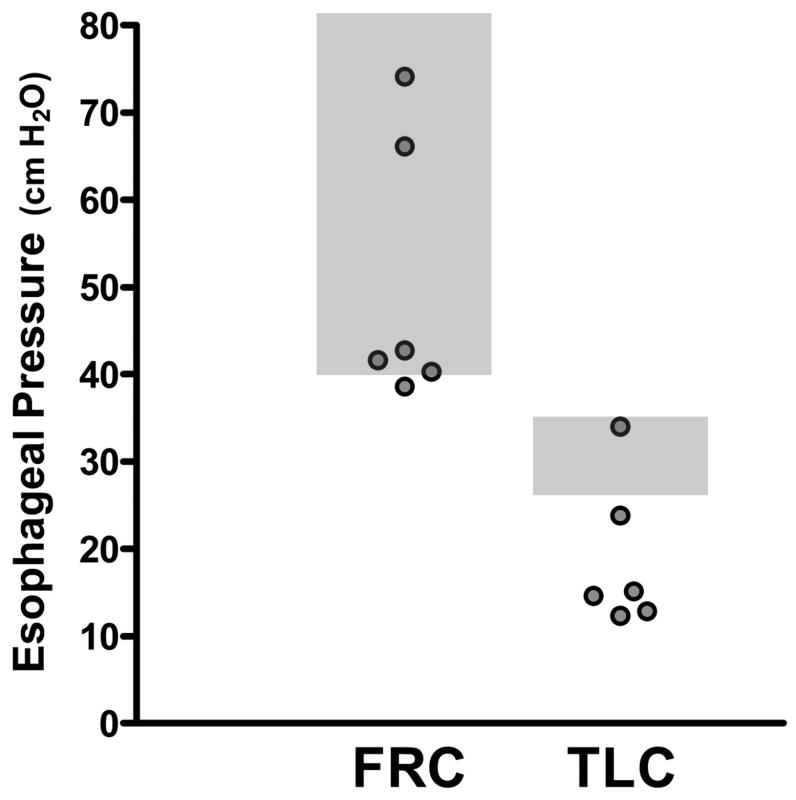
MIPes obtained at FRC offer an assessment of inspiratory muscle strength. Conventional normal values at FRC are >50cm H2O, with 40-50cm H2O representing marginal strength. Measurements <30cm H2O are considered clearly abnormal (21-23). In all subjects, MIPes at FRC were marginal or normal, ranging from 38.5 to 74cm H2O (Figure 1, Table 2). In contrast, inspiratory force at higher lung volumes was markedly reduced. MIPes at TLC were low in all but one subject, ranging from 12.2 to 23.7cm H2O (Figure 1, Table 2) (normal: 25-35cm H2O) (24,25). This pattern of relatively well preserved MIPes at FRC but reduced MIPes at TLC indicates limited lung expansion not due to intrinsic lung disease or respiratory myopathy but rather to either mechanical limitation of chest wall expansion or decreased respiratory muscle engagement.
Figure 1. Maximal Inspiratory Pressures Measured by Esophageal Manometry at Low and High Lung Volumes.
Maximal inspiratory pressures at functional residual capacity (FRC) and at total lung capacity (TLC). The gray boxes represent normal values for maximal inspiratory pressures as measured by the esophageal balloon (MIPes). MIPes are recorded by the esophageal balloon catheter as negative pressures since they are measured in the thorax during inspiration; however, the absolute value is reported.
Static (Cst) and dynamic (Cdyn) lung compliances were assessed to determine parenchymal distensibility. Cst, measured during periods of zero airflow from airway occlusions during passive exhalation beginning at TLC, is insensitive to airway resistance, while Cdyn, measured during spontaneous breathing, reflects a component of airway resistance from air flow (26). Cst ranged from 55.2 to 165.8mL/cm H2O. Normal values for Cst near FRC range from 226 to 428mL/cm H2O (Table 2) (27). Cdyn was reduced in all patients, ranging from 28.5 to 115.3mL/cm H2O (normal: 150-390mL/cm H2O) (27). The results confirm “stiff lungs” in these patients despite the absence of parenchymal disease on imaging.
Chest CT Reformatting
In all patients with available digital CT imaging, lung volumes near TLC were markedly low compared with predicted values (Table S2) (18). Lung density was increased uniformly throughout the lung fields, a finding also evident in both non-studied SLS patients with available CT data. Increased lung density is indicative of pulmonary edema, atelectasis, or parenchymal remodeling (28,29).
Clinical Course
Clinical outcomes were available for 4 patients (Table1). After treatment, SLS symptoms resolved in Cases 1, 2, and 5. Case 1 is discussed further below. Case 2 improved after cyclophosphamide and theophylline, having failed rituximab. Case 5 improved on corticosteroids and mycophenolatemofetil. Case 6 remains symptomatic, having failed therapy with rituximab and cyclophosphamide. Cases 3 and 4 were lost to follow-up.
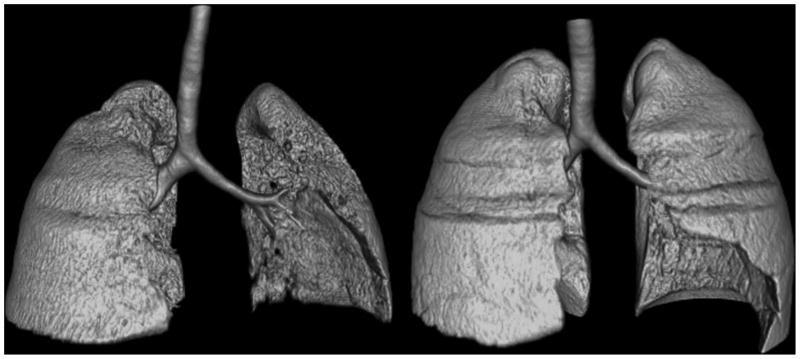
Manometry data both before and after successful therapy were available for Case 1, an adolescent with MCTD. Following failure to respond to intravenous methylprednisolone, oral corticosteroids (80mg/day) and theophylline, she received rituximab 1g intravenously, methylprednisolone 100mg, and oral corticosteroids. Her dyspnea improved within 7 weeks of therapy. Repeat study when asymptomatic (22 months after initial evaluation), found that her TLC had increased from 50% to 66% of predicted. MIPes at FRC increased from 42.6 to 84.4cm H2O, and MIPes at TLC from 14.5 to 21.6cm H2O, reflecting an increase in respiratory muscle strength and improved chest wall expansion at high lung volumes. However, Cst and Cdyn remained low. Repeat chest imaging showed resolution of the right-sided pleural effusion; lung volumes increased and parenchymal density decreased but failed to normalize (Figure 2, Table S2).
Figure 2. Chest CT Reformatted Images from a Case 1.
Volume-rendered images depicting lung volumes during symptomatic SLS and after recovery in Case 1. Irregularity of the surface of both lungs during symptomatic disease (left panel) reflects reduced lung volumes due to pleural effusions and potentially other factors. Resolution of pleural effusions in the recovery phase (right panel) was accompanied by improved lung volumes and densities.
Discussion
SLS is a recognized complication of SLE and related autoimmune disorders, but its pathophysiology remains essentially unknown. We found several concurrent respiratory abnormalities in almost all patients studied. These included significantly impaired inspiratory pressures at TLC in 5 patients and reduced lung compliance in all. Thus, patients exhibited both intrinsic (parenchymal) and extrinsic (extrapulmonary) causes of restrictive lung disease. Importantly, in each case, pleural inflammation was evident early in the disease course.
As noted, extrinsic restriction can be either structural or functional. Structural causes of extrinsic restriction include chest wall deformities (not present in these patients) or pleural adhesions as seen after pleurodesis or in asbestos-related fibrosis (30,31). Such patients typically have radiographically obvious pleural disease (31). Although most patients in this series had small pleural effusions at some point in their course, as is typical for SLS, no patient exhibited chronic pleural findings. Additionally, 3 cases followed through recovery had resolution of dyspnea and improvement in TLC, weighing against fixed adhesions. Finally, in one case, adhesions between the lung and diaphragm or chest wall were specifically excluded by ultrasound imaging. Based on these observations, we conclude that the extrinsic restriction observed in our patients was not structural.
Functional extrinsic restriction arises from impaired activity of the respiratory muscles. Diffuse respiratory myopathy is unlikely since our patients had normal muscle enzymes, no clinical evidence for concurrent myositis elsewhere, and adequate inspiratory strength at FRC. Rather, inspiratory pressures were impaired selectively at high lung volumes, suggesting an alternate pathophysiology. Respiratory muscle function is regulated not only by volition but also through neuronal reflex arcs. These include the intercostal-phrenic and pleural-phrenic reflexes, which are activated by stimulation of intercostal and pleural afferents, respectively, leading to phrenic nerve inhibition (32-38). In animals, the intercostal-phrenic reflex can be triggered by chest wall compression and rib vibration. After such stimuli, phrenic nerve electrical activity and diaphragm electromyographic recordings are decreased (37). The pleural-phrenic reflex can also be activated by exposure of the pleura to inflammatory cytokines (38). These reflexes have been documented in humans and may be responsible for decreased inspiratory capacity in post-operative patients (39). They are particularly potent at higher lung volumes (37). Since all cases had evidence of pleuritis, an observation noted in other series (9,14,15), pleural inflammation appears the most likely basis for engagement of these neural arcs, resulting in reflex (and potentially volitional) limitation of chest expansion. This possibility is supported by the observation that recovery in 3 patients coincided with resolution of pleuritic chest pain and effusions on chest imaging, and with normalization of MIPes at TLC in the single patient re-tested in remission.
Importantly, lung compliance was reduced in all individuals, and lung density was increased in all patients evaluated by chest CT reformatting. The basis for these findings is uncertain. Since all patients had intact corrected DLCO values and radiographically normal lung parenchyma, substantial interstitial lung disease is unlikely. Elevated lung density may simply reflect lower lung volumes. However, impairment of lung compliance, not explained by interstitial disease or atelectasis, has been observed in patients who are chronically restricted to lower lung volumes due to spinal cord injury or muscular dystrophy; tissue remodeling with changes in elasticity is proposed as the underlying pathophysiology (40-42). Such hypoinflation-induced decrease in compliance could contribute to the progressive nature of SLS, since stiff lungs are more difficult to inflate. Notably, the single patient with intact MIPes at TLC had the lowest lung compliance, suggesting that the contribution of different aspects of respiratory dysfunction in SLS may vary from patient to patient.
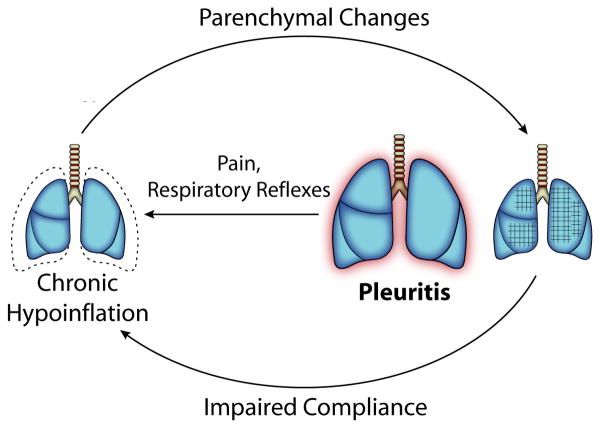
Together, these physiologic findings suggest a new model of the pathogenesis of SLS (Figure 3). Pleural inflammation triggers inhibition of deep inspiration by neural reflexes and pain (demonstrated by decreased MIPes at TLC) resulting in chronic lung hypoinflation, which in predisposed patients leads to parenchymal remodeling that decreases lung compliance. Impaired compliance worsens hypoinflation, initiating a positive feedback loop that helps to explain the gradual progression of SLS. Since this defect is primarily functional, the patient's ventilatory drive would be expected to limit further respiratory deterioration, accounting for the low mortality of SLS despite its alarming clinical presentation.
Figure 3. Model of the Pathophysiology of Shrinking Lung Syndrome.
We propose that SLS begins with pleural inflammation due to the underlying rheumatic disease. Activation of local neural reflexes, and/or volitional splinting because of pain, leads to chronic hypoinflation of the lung, which gradually impairs lung compliance through undefined parenchymal changes. The less compliant lung is more difficult to inflate, leading to a slowly progressive spiral of declining inflation until the positive feedback cycle is halted, likely by the patient's central respiratory drive.
Our results are inconsistent with conventional hypotheses implicating isolated diaphragmatic weakness or phrenic dysfunction in the pathogenesis of SLS. While it has been previously suggested that SLS may arise from extrinsic restriction mediated by neuronal reflexes (9,14,15),this study is the first to provide physiologic evidence supporting this hypothesis, through documentation of reduced MIPes at TLC. Additionally, we have demonstrated that patients with SLS have impaired pulmonary compliance, representing a key new finding that implicates parenchymal remodeling in SLS, and without which the gradual deterioration characteristic of SLS would remain unexplained. Importantly, lung compliance in SLS patients did not necessarily normalize even in patients who had symptomatically recovered. This result suggests that structural changes may persist even after symptomatic recovery, a new concept in SLS that will need to be considered in future research.
This study has several limitations. Most importantly, the series is small. While a small sample size typically raises concern about sampling error and generalizability, we are reassured by several considerations. First, SLS is an extremely rare condition in most surveys of SLE and related autoimmune diseases, and the cases studied in detail represented two-thirds of all the cases seen by >50 practicing rheumatologists in 2 large tertiary care referral centers over the course of 4 years. The 3 cases not studied were phenotypically similar to the studied cases; both non-studied patients with CT data exhibited lung density findings concordant with those of patients receiving esophageal manometry; and the grounds for failure to study the 3 missed patients are well understood and do not raise concern for selection bias. Second, among SLS patients studied fully, our findings are remarkably consistent: 5 of 6 had selectively impaired inspiratory force at high lung volumes, while all 6 had markedly impaired parenchymal compliance in the absence of abnormalities on conventional high-resolution CT imaging. Among the 5 patients for whom reformatted CTs were available, all 5 exhibited abnormally high lung density confirming the parenchymal abnormality identified via manometry. Therefore, despite a small sample size, our results are highly likely to reflect the physiology of most patients with SLS, at least as seen at similar referral centers.
Other limitations of our study arise out of the methodology used to evaluate our patients. As pleural adhesions can be masked on imaging by the presence of pleural effusions, direct thoracoscopic visualization of the pleura would have been preferable, but was not considered clinically justified. Further, pulmonary physiologic tests are dependent on patient effort, and it would have been of interest to assess maximal respiratory muscle strength by magnetic phrenic nerve stimulation and diaphragmatic electromyography. However, such studies performed in SLS have demonstrated no limitations, justifying their omission here (9,43).
Several key questions remain unanswered. Pleuritis is common in rheumatologic and non-rheumatologic conditions, yet SLS is rare. Lupus pleuritis usually responds to corticosteroids, while corticosteroids are often ineffective in SLS (44). It may be that the duration of pleural inflammation, the location of the inflammation within the pleura (apical vs. the zone of apposition), inter-subject variability in the potency of respiratory reflexes, or differential susceptibility to hypoinflation-induced parenchymal changes could explain these observations. The nature of the changes in pulmonary compliance and its potential reversibility remain unknown. Pathologic samples from patients with active SLS and in remission will be essential to understand these alterations in the lung parenchyma.
In summary, our data support the hypothesis that SLS represents an unusual complication of pleuritis, whereby inhibited respiratory muscle engagement limits inflation and thereby leads to progressive loss of lung compliance. The proposed model of SLS has implications for future research and therapy. If the primary driver of the disease is pleuritis, then anti-inflammatory therapy should be implemented early. If impaired lung expansion contributes to impaired lung compliance, pleuritic chest pain should be addressed with analgesia and pulmonary rehabilitation. These possibilities will require clinical validation.
Supplementary Material
Supplemental Video 1. Images are rendered as a video to permit 360° visualization of the lungs and pleural effusions during active SLS in Case 1.
Supplementary Table 1. Presentation of Shrinking Lung Syndrome in Subjects who did not Undergo Pulmonary Mechanics Evaluation
Supplementary Table 2. Total Lung Volume and Lung Densities
Acknowledgments
We are grateful to Dr. Bartolome Celli for careful review of the manuscript and to Dr. Luis Leon for graphical assistance with Figure 3.
P.A. Nigrovic is supported by the Cogan Family Fund and the SamaraJanTurkelCenter for Autoimmune Diseases. Dr. Dellaripa participates in clinical trials conducted by Genentech; however, no financial support was provided for this work. Dr. Loring was supported by HL52586 from the National Institutes of Health. Dr. Liao is supported by K08 AR060257 from the National Institutes of Health and the Katherine Swan Ginsburg Fund.
Footnotes
L.A. Henderson, M.D. , S. Kim, M.D., M.P.H., M.F.Son, M.D., P.A. Nigrovic, M.D., Division of Immunology, Boston Children's Hospital; P.A. Nigrovic, M.D., K.P. Liao, M.D, M.P.H., R. Ishizawar, M.D., Ph.D., P.F. Dellaripa, M.D., Division of Rheumatology, Immunology, and Allergy, Brigham and Women's Hospital; S.H. Loring, M.D., Department of Anesthesia and Critical Care, Beth Israel Deaconess Medical Center; R.R. Gill, M.D., R. Perlmutter-Goldenson, M.D., Division of Radiology, Brigham and Women's Hospital; D. Rothman, M.D., Pediatric Rheumatology, Shriners Hospitals for Children, Springfield; M. L. Stoll, MD, MSCS, Division of Pediatric Rheumatology, University of Alabama at Birmingham; L.S. Zemel, M.D., Pediatric Rheumatology, Connecticut Children's Medical Center; Christy Sandborg, M.D., Pediatric Rheumatology, Lucile Packard Children's Hospital at Stanford.
References
- 1.Hoffbrand BI, Beck ER. “Unexplained” dyspnoea and shrinking lungs in systemic lupus erythematosus. Br Med J. 1965;1:1273–7. doi: 10.1136/bmj.1.5445.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoli AM, Vilá LM, Apte M, Fessler BJ, Bastian HM, Reveille JD, et al. Systemic lupus erythematosus in multiethnic US cohort LUMINA XLVIII: factors predictive of pulmonary damage. Lupus. 2007;16:410–7. doi: 10.1177/0961203307079042. [DOI] [PubMed] [Google Scholar]
- 3.Allen D, Fischer A, Bshouty Z, Robinson D, Peschken C, Hitchon C, et al. Evaluating systemic lupus erythematosus patients for lung involvement. Lupus. doi: 10.1177/0961203312454343. in press. [DOI] [PubMed] [Google Scholar]
- 4.Gheita TA, Azkalany GS, El-Fishawy HS, NourEldin AM. Shrinking lung syndrome in systemic lupus erythematosus patients; clinical characteristics, disease activity and damage. Int J Rheum Dis. 2011 Oct;14(4):361–8. doi: 10.1111/j.1756-185X.2011.01651. cited 2011 Aug 31. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed S, Herrick A, O'Driscoll BR. Shrinking lung syndrome in patients without systemic lupus erythematosus. Arthritis Rheum. 2001;44:243–5. doi: 10.1002/1529-0131(200101)44:1<243::aid-anr36>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Scirè CA, Caporali R, Zanierato M, Mojoli F, Braschi A, Montecucco C. Shrinking lung syndrome in systemic sclerosis. Arthritis Rheum. 2003;48:2999–3000. doi: 10.1002/art.11393. [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Huang W, Menon Y, Espinoza LR. Shrinking lung syndrome in systemic lupus erythematosus and Sjögren's syndrome. J Clin Rheumatol. 2002;8:340–5. doi: 10.1097/00124743-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Tavoni A, Vitali C, Cirigliano G, Frigelli S, Stampacchia G, Bombardieri S. Shrinking lung in primary Sjögren's syndrome. Arthritis Rheum. 1999;42:2249–50. doi: 10.1002/1529-0131(199910)42:10<2249::AID-ANR31>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Laroche CM, Mulvey DA, Hawkins PN, Walport MJ, Strickland B, Moxham J, et al. Diaphragm strength in the shrinking lung syndrome of systemic lupus erythematosus. Q J Med. 1989;71:429–39. [PubMed] [Google Scholar]
- 10.Rubin LA, Urowitz MB. Shrinking lung syndrome in SLE-a clinical pathologic study. J Rheumatol. 1983;10:973–6. [PubMed] [Google Scholar]
- 11.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CJ, Edmonds JP, Hughes GR. Diaphragm function and lung involvement in systemic lupus erythematosus. Am J Med. 1977;63:926–32. doi: 10.1016/0002-9343(77)90547-2. [DOI] [PubMed] [Google Scholar]
- 13.Hardy K, Herry I, Attali V, Cadranel J, Similowski T. Bilateral phrenic paralysis in a patient with systemic lupus erythematosus. Chest. 2001;119:1274–7. doi: 10.1378/chest.119.4.1274. [DOI] [PubMed] [Google Scholar]
- 14.Toya SP, Tzelepis GE. Association of the shrinking lung syndrome in systemic lupus erythematosus with pleurisy: a systematic review. Semin ArthritisRheum. 2009;39:30–7. doi: 10.1016/j.semarthrit.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Neves SF, da Silva TH, Paviani G, Zimmerman FA, deCastro GR, Pereira AI. Reinforcing a medical hypothesis with a new question: is there a subgroup of shrinking lungs syndrome that is induced by pleurisy in systemic lupus erythematosus and is this subgroup marked by anti-Ro/SSA? ClinRheumatol. 2010;29:777–9. doi: 10.1007/s10067-010-1427-1. [DOI] [PubMed] [Google Scholar]
- 16.Mead J, Mcllroy MB, Selverstone NJ, Kriete BC. Measurement of intraesophageal pressure. J ApplPhysiol. 1955;7:491–5. doi: 10.1152/jappl.1955.7.5.491. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell CR, Bankier AA, Stiebellehner L, Reilly JJ, Brown R, Loring SH. Comparison of plethysmographic and helium dilution lung volumes: which is best for COPD? Chest. 2010;137:1108–15. doi: 10.1378/chest.09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irion KL, Marchiori E, Hochhegger B, Porto NS, Moreira JS, Anselmi CE, et al. CT quantification of emphysema in young subjects with no recognizable chest disease. Am J Roentgenol. 2009;192:W90–6. doi: 10.2214/AJR.07.3502. [DOI] [PubMed] [Google Scholar]
- 20.Camargo JJ, Irion KL, Marchiori E, Hochhegger B, Porto NS, Moraes BG, et al. Computed tomography measurement of lung volume in preoperative assessment for living donor lung transplantation: volume calculation using 3D surface rendering in determination of size compatibility. Pediatr Transplant. 2009;13:429–39. doi: 10.1111/j.1399-3046.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 21.Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 22.Harkin-Khan RI, Wise RA, Fozard JL. Determinants of maximal inspiratory pressure. The Baltimore Longitudinal Study of Aging. Am J RespirCrit Care Med. 1998;158:1459–64. doi: 10.1164/ajrccm.158.5.9712006. [DOI] [PubMed] [Google Scholar]
- 23.Steier J, Kaul S, Seymour J, Joley C, Rafferty G, Man W, et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–80. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colebatch HJ, Finucane KE, Smith MN. Pulmonary conductance and elastic recoil relationships in asthma and emphysema. J ApplPhysiol. 1973;34:143–53. doi: 10.1152/jappl.1973.34.2.143. [DOI] [PubMed] [Google Scholar]
- 25.Colebatch HJ, Greaves IA, Ng CK. Exponential analysis of elastic recoil and aging in healthy males and females. J ApplPhysiol. 1979;47:683–91. doi: 10.1152/jappl.1979.47.4.683. [DOI] [PubMed] [Google Scholar]
- 26.Gibson GJ. Lung volumes and elasticity. Clin Chest Med. 2001;22:623–35. doi: 10.1016/s0272-5231(05)70056-3. [DOI] [PubMed] [Google Scholar]
- 27.Galetke W, Feier C, Muth T, Ruehle KH, Borsch-Galetke E, Randerath W. Reference values for dynamic and static pulmonary compliance in men. Respir Med. 2007;101:1783–9. doi: 10.1016/j.rmed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Camiciottoli G, Orlandi I, Bartolucci M, Meoni E, Nacci F, Diciotti S, et al. Lung CT densitometery in systemic sclerosis: correlation with lung function, exercise testing, and quality of life. Chest. 2007;131:672–81. doi: 10.1378/chest.06-1401. [DOI] [PubMed] [Google Scholar]
- 29.Metry G, Wegenius G, Wikstrom B, Kallskog V, Hansell P, Lindgren PG, et al. Lung density for assessment of hydration status in hemodialysis patients using the computed tomographic densitometry technique. Kidney Int. 1997;52:1635–44. doi: 10.1038/ki.1997.496. [DOI] [PubMed] [Google Scholar]
- 30.Loring SH, Kurachek SC, Wohl ME. Diaphragmatic excursion after pleural sclerosis. Chest. 1989;95:374–8. doi: 10.1378/chest.95.2.374. [DOI] [PubMed] [Google Scholar]
- 31.Singh B, Eastwood PR, Finucane KE, Panizza JA, Musk AW. Effect of asbestos-related pleural fibrosis on excursion of the lower chest wall and diaphragm. Am J RespirCrit Care Med. 1999;160:1507–15. doi: 10.1164/ajrccm.160.5.9806135. [DOI] [PubMed] [Google Scholar]
- 32.Sant'Ambrogio G, Sant'Ambrogio FB. Reflexes from the upper airway, lungs, chest wall, and limbs. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. Philadelphia: Lippincott-Raven; 1997. pp. 1805–19. [Google Scholar]
- 33.Butler JE, McKenzie DK, Gandevia SC. Reflex inhibition of human inspiratory muscles in response to contralateral phrenic nerve stimulation. RespirPhysiolNeurobiol. 2003;138:87–96. doi: 10.1016/s1569-9048(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 34.Bellingham MC. Synaptic inhibition of cat phrenic motor neurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82:1224–32. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- 35.Homma I. Inspiratory inhibitory reflex caused by the chest wall vibration in man. RespirPhysiol. 1980;39:345–53. doi: 10.1016/0034-5687(80)90065-1. [DOI] [PubMed] [Google Scholar]
- 36.Knill R, Bryan AC. An intercostal-phrenic inhibitory reflex in human newborn infants. J Apply Physiol. 1976;40:352–6. doi: 10.1152/jappl.1976.40.3.352. [DOI] [PubMed] [Google Scholar]
- 37.Remmers JE. Inhibition of inspiratory activity by intercostal muscle afferents. RespirPhysiol. 1970;10:358–83. doi: 10.1016/0034-5687(70)90055-1. [DOI] [PubMed] [Google Scholar]
- 38.Jammes Y, Delpierre S. Respiratory and circulatory effects of parietal pleural afferent stimulation in rabbits. J ApplPhysiol. 2006;100:1539–46. doi: 10.1152/japplphysiol.01422.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ayoub J, Cohendy R, Prioux J, Ahmaidi S, Bourgeois JM, Dauzat M, et al. Diaphragm movement before and after cholecystectomy: a sonographic study. AnesthAnalg. 2001;92:755–61. doi: 10.1097/00000539-200103000-00038. [DOI] [PubMed] [Google Scholar]
- 40.Gibson GJ, Pride NB, Davis JN, Loh LC. Pulmonary mechanics in patients with respiratory muscle weakness. Am Rev Respir Dis. 1997;115:389–95. doi: 10.1164/arrd.1977.115.3.389. [DOI] [PubMed] [Google Scholar]
- 41.De Troyer A, Borenstein S, Cordier R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax. 1980;35:603–10. doi: 10.1136/thx.35.8.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estenne M, Gevenois PA, Kinnear W, Soudon P, Heilpor A, Troyer A. Lung volume restriction in patients with chronic respiratory muscle weakness: the role of microatelectasis. Thorax. 1993;48:698–701. doi: 10.1136/thx.48.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins P, Davison AG, Dasgupta B, Moxham J. Diaphragm strength in acute systemic lupus erythematosus in a patient with paradoxical abdominal motion and reduced lung volumes. Thorax. 2001;56:329–30. doi: 10.1136/thorax.56.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Man BL, Mok CC. Serositis related to systemic lupus erythematosus: prevalence and outcome. Lupus. 2005;14:822–6. doi: 10.1191/0961203305lu2187oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Images are rendered as a video to permit 360° visualization of the lungs and pleural effusions during active SLS in Case 1.
Supplementary Table 1. Presentation of Shrinking Lung Syndrome in Subjects who did not Undergo Pulmonary Mechanics Evaluation
Supplementary Table 2. Total Lung Volume and Lung Densities