Abstract
Objective
Longitudinal studies have begun to clarify the phenotypic characteristics of adolescents and young adults at clinical high risk for psychosis. This 8-site randomized trial examined whether a 6-month program of family psychoeducation was effective in reducing the severity of attenuated positive and negative psychotic symptoms and enhancing functioning among individuals at high risk.
Method
Adolescents and young adults (mean 17.4±4.1 years) with attenuated positive psychotic symptoms, brief and intermittent psychosis, or genetic risk with functional deterioration were randomly assigned to 18 sessions of family-focused therapy for individuals at clinical high risk (FFT-CHR) in 6 months or 3 sessions of family psychoeducation (enhanced care, or EC). FFT-CHR included psychoeducation about early signs of psychosis, stress management, communication training, and problem-solving skills training, whereas EC focused on symptom prevention. Independent evaluators assessed participants at baseline and 6 months on positive and negative symptoms and social-role functioning.
Results
Of 129 participants, 102 (79.1%) were followed at 6 months. Participants in FFT-CHR showed greater improvements in attenuated positive symptoms over 6 months than participants in EC (F[1,97]=5.49, P=.02). Negative symptoms improved independently of psychosocial treatments. Changes in psychosocial functioning depended on age: participants over 19 years showed more role improvement in FFT-CHR, whereas participants between 16 and 19 years showed more role improvement in EC. The results were independent of concurrent pharmacotherapy.
Conclusion
Interventions that focus on improving family relationships may have prophylactic efficacy in individuals at high risk for psychosis. Future studies should examine the specificity of effects of family intervention compared to individual therapy of the same duration and frequency.
Keywords: attenuated psychotic symptoms, schizophrenia, early warning signs, psychoeducation, family therapy
INTRODUCTION
There has been increasing interest in the role of psychosocial interventions for adolescents and young adults who are at clinical high risk (CHR) for psychosis. Longitudinal studies have identified phenotypic precursors to psychosis that may narrow the populations for whom early interventions are applied, although false positive prediction rates are high. About 35% of individuals with attenuated or intermittent psychosis symptoms, schizotypal personality disorder, or a family history of psychosis with recent functional deterioration develop an episode of psychosis over 2–3 years.1 A meta-analysis of 2,500 persons at clinical high risk in 27 studies found a conversion rate of 18% in 6 months, 22% in 1 year, and 36% after 3 years.2
Intervening during the high-risk period may reduce subthreshold psychotic symptoms, enhance social and role functioning, and, over the long-term, prevent or delay conversion to episodes of psychosis. Results from early intervention trials indicate that targeted medication strategies have short-term benefits on attenuated positive symptoms in individuals at clinical high risk.3–7 A meta-analysis that included 4 randomized psychotherapy trials with participants at high risk concluded that cognitive behavioral therapy (CBT) was associated with a greater reduction in positive symptoms (standardized mean difference = −.27) over 6–12 months compared to supportive therapy.6 The effects of CBT and other psychosocial interventions on negative symptoms, functioning, and quality of life were nonsignificant across studies. 6
We hypothesized that early psychosocial intervention would be strengthened by involving family members in treatment. First, individuals at high risk for psychosis are often adolescents living with their parents, and parental involvement may enhance the young person’s access to mental health services. Second, the evolution of attenuated psychotic symptoms may be affected by family contextual variables. For example, levels of expressed emotion (i.e., criticism, hostility, or overprotectiveness) in parents were associated with the severity of attenuated psychotic symptoms in youth at clinical high risk over 6 months.8 Levels of parental expressed emotion (EE) may escalate in reaction to the functional deterioration of an offspring with emerging psychosis but may also become a stressor for the offspring.9,10
This article reports results of the first multisite randomized trial of a family intervention for youth at high risk for psychosis. We tested an adaptation of family-focused therapy (FFT), a psychoeducational treatment found to be effective in stabilizing symptoms and delaying recurrences among adults with bipolar I or II disorder and youth with or at high risk for bipolar spectrum disorders.11–13 The adaptation of FFT for individuals at clinical high risk for psychosis (FFT-CHR) emphasizes coping with stressors that may contribute to psychotic symptoms, behavioral activation to reduce negative symptoms and increase social engagement, and skills training to enhance interpersonal communication and problem-solving.
This trial was conducted within the 8-site North American Prodrome Longitudinal Study, 2 (NAPLS-2).14 FFT-CHR was administered in weekly and biweekly sessions over 6 months and compared to a brief (1 month) family educational intervention (enhanced care, or EC). We examined two hypotheses: (1) compared to EC, FFT-CHR would be associated with decreases in subthreshold positive symptoms (primary outcome) and negative symptoms; and (2) FFT-CHR would be associated with greater gains in psychosocial functioning (secondary outcome) over 6 months.
METHOD
Participants
All 8 research centers of the NAPLS-2 consortium (Emory University, Harvard University, University of Calgary, University of California Los Angeles, University of California San Diego, University of North Carolina, Yale University, and Zucker Hillside Hospital) contributed participants to the study. The study was approved by the human research review boards of all centers.
Between January 2010 and February 2012, participants who had consented for the NAPLS-2 naturalistic study and were living with or in frequent contact with significant others (parents, grandparents, spouses or partners) were approached by a NAPLS-2 research assistant and invited to be evaluated for a randomized study of family intervention. Participants and at least one relative gave written informed assent or consent to participate following a full explanation of the procedures. Participants under age 18 signed an assent form that also required the permission signature of their parent.
Participants met the following eligibility criteria: (1) age between 12 and 35 years; (2) speaks and writes English, (3) meets criteria for one of three prodromal syndromes as assessed by the Structured Interview for Prodromal Syndromes (SIPS)15 and the Scale of Prodromal Symptoms (SOPS)16: attenuated positive symptoms with worsening in the past year; brief intermittent psychosis; or genetic risk and deterioration, defined as a ≥ 30% decline in Global Assessment of Functioning (GAF)17 scores in the past year, plus either a diagnosis of schizotypal personality disorder or having a first-degree relative with psychosis. Participants were excluded if they met current DSM-IV-TR criteria for schizophrenia or schizoaffective disorder, pervasive developmental disorders, substance use disorders, or neurological disorders based on the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (SCID-P). 18,19
Study participants who were geographically dispersed were given the opportunity to participate in FFT via secure videoconference; two families accepted this option. Further information regarding recruitment strategies in NAPLS-2 can be found elsewhere. 14,20
Procedures: Outcome Assessments
Prior to the random assignments (baseline) and at 6-month follow-up, independent evaluators (IEs) who were unaware of therapy conditions administered the SIPS interview and rated the SOPS positive and negative symptom scales covering the prior month. IEs also administered the SIPS/SOPS whenever a conversion event was suspected. IEs instructed participants not to reveal their treatment assignment. The five SOPS positive symptom scales ranged from 0 (absent) to 6 (severe and psychotic) and included unusual thought content, suspiciousness, perceptual disturbances, grandiosity, and disorganized communication. The six negative symptom scales included social anhedonia, avolition, decreased emotional expressiveness, decreased experience of emotions and self, ideational richness, and diminished role functioning. A change in one or more positive symptom items to a score of 6 for a minimum duration (≥ 1 hr for ≥ 4 days per week in the past month) was rated as a psychotic conversion.
Study entry and conversion criteria were established through team consensus diagnoses from case vignettes (see 14). Prior to the study, IEs at the 8 NAPLS-2 sites were able to reliably distinguish subthreshold from psychotic levels of positive symptoms (K range, .80–1.0).20 During the trial, annual cross-site comparisons of total SOPS ratings with “gold standard” SOPS ratings (intraclass correlations) ranged from 0.82–.93 among IEs across sites; for attenuated positive symptoms, the range was .92–.96.14
At each follow-up, IEs made 100-point GAF ratings covering the prior month. They also rated the 10-point Global Functioning-Role (GF-Role) adjustment (i.e., work or school) scale and the Global Functioning-Social (GF-Social: i.e., romantic or peer relationship) scale. 21
Interventions
A lead study investigator who was neither involved in the provision of treatments nor the follow-up evaluations conducted the random assignments to FFT-CHR or EC, with 50% of participants allocated to each condition. Allocations, performed using Efron’s biased coin toss,22 were stratified by site and whether or not the participant was prescribed an antipsychotic medication at baseline. Allocation results were sent by email to each site’s PI.
Drug treatment was not a requirement of the study. When participants were taking medications (i.e., antipsychotics, antidepressants, or anxiolytics), their pharmacotherapy was managed by a study psychiatrist, unless they wished to consult a community provider. Psychiatrists were not told which psychosocial treatment the participant was receiving. Physicians could adjust medication regimens or add rescue medications (e.g., antipsychotics) as needed during the trial, and visit frequency was allowed to vary by physician/patient agreement.
FFT-CHR was administered to participants and parents (and when possible, siblings) in 18 1-hour family sessions (12 weekly and 6 biweekly sessions over 6 months). The objectives of sessions 1–6 (psychoeducation) were to assist the individual at high risk and family members to develop a personalized prevention plan summarizing stressors associated with positive or negative symptoms, and potential coping strategies (e.g., pleasant event scheduling, relaxation exercises). In sessions 7–12 (communication enhancement training), participants rehearsed skills for expressing positive feelings, active listening, requesting changes in another person’s behavior, communication clarity, and expressing negative feelings. In sessions 13–18 (problem-solving), participants learned to break down larger problems (e.g., “We have to stop fighting”) into smaller ones (e.g., “We need to use lower tones of voice”), generate and evaluate solutions, and develop a solution implementation plan. Between sessions, families were given homework assignments to encourage generalization of the skills. (The clinicians’ manual is accessible at http://www.semel.ucla.edu/champ/downloads-clinicians). The EC treatment consisted of 3 weekly psychoeducational sessions in which clinicians assisted the individual at high risk and family members to develop a personalized prevention plan.
When crises arose during the course of participation in either of the treatments, family members or therapists scheduled individual crisis management sessions and/or the family was referred for emergency services. If the individual at high risk converted to psychosis, s/he was evaluated by assessment team members and transitioned to first episode psychosis services or local inpatient services. The participants were not withdrawn from the trial due to conversion events.
As part of informed consent, patients were informed about alternative treatments they could consider for prodromal symptoms and/or comorbid diagnoses (e.g., major depressive disorder). They were allowed to participate in additional individual and/or substance abuse treatment without being withdrawn from the trial. The independent evaluator tracked all non-protocol treatments obtained by the participant during the 6-month trial, including any contacts with non-study psychotherapists, counselors, or school-based personnel.
Clinician Training and Monitoring
A total of 24 clinicians from the NAPLS sites (minimum: master’s degree-level) were trained in FFT-CHR and EC during a 2-day workshop in December 2009. During the trial, two expert clinicians (DJM, MPO) listened to tapes of FFT-CHR or EC sessions and offered supervision to clinicians at least once every 2 weeks. Tapes of sessions were rated for fidelity using an adapted version of the 13-item Therapy Competence and Adherence Scales (TCAS).23 Interrater reliability on the 13 scale items ranged from .61-.86. Supervisors classified 90% of the FFT-CHR and EC sessions (122 ratings), drawn from all phases of therapy, as above acceptable fidelity thresholds (ratings ≥ 5 on a 7-point scale). Clinicians were found to be equally skillful in providing psychoeducation in both conditions, with no differences in TCAS ratings of rapport-building, pacing of sessions, or session command. As expected, clinicians were significantly more likely to provide communication enhancement training (χ2(3)=17.36, p=.001) and problem solving skills training (χ2(3)=7.27, p=.03) in the FFT-CHR than the EC sessions.24
Data Analysis
Participants in FFT-CHR and EC were compared at baseline (t-tests and X2) on sex, age, and baseline SOPS scores (Table 1). These variables were also examined as predictors of early study termination. The primary and secondary hypotheses were that participants in FFT-CHR would show greater improvements in SOPS positive and negative symptoms, respectively, from pretreatment to the 6-month reassessment than participants in EC. These hypotheses were tested using mixed-effects regression models with a repeated measures structure and a random subject-level intercept (PROC MIXED in SAS/STAT). 25 These models examined the effects of time (baseline, 6 months), treatment group, and the treatment by time interaction on scores for the 5 SOPS positive and 6 negative symptom items. Antipsychotic treatment (prescribed or not at time of randomization) and site were included as covariates in each model. This approach allowed subscales to be included as a within-subject factor, to determine whether hypothesized treatment effects were general across all positive and/or negative symptoms or specific to individual symptoms. All analyses were by intent-to-treat. Power for the study’s repeated measure design, calculated prior to the trial based on an expectation of 120 participants and 20% attrition, was 80% to detect a medium-sized (0.50 SD) group difference in symptoms (alpha=.05, two-tailed).
Table 1.
Sample Characteristics
| Variable | Enhanced Care (n = 63) | FFT-CHR (n = 66) | Total (N = 129) |
|---|---|---|---|
| Age, mean yrs. (SD) | 17.4 (3.9) | 17.3 (4.2) | 17.4 (4.07) |
| Female sex, n (%) | 28 (44.4) | 27 (40.1) | 55 (42.6) |
| Years of education | 10.3 (2.6) | 10.3 (2.8) | 10.3 (2.7) |
| Hispanic ethnicity, n (%) | 15 (23.8) | 13 (19.7) | 28 (21.7) |
| Race, n (%) | |||
| Caucasian | 44 (69.8) | 45 (68.2) | 89 (69.0) |
| Asian American | 4 (6.3) | 3 (45.5) | 7 (5.4) |
| African American | 10 (15.9) | 10 (15.2) | 20 (15.5) |
| Native American | 1 (1.6) | 2 (3.0) | 3 (2.3) |
| Other | 4 (4.8) | 6 (7.6) | 10 (6.2) |
| Living with parents, n (%) | 56 (88.9) | 61 (92.4) | 117 (90.7) |
| Prodromal Syndrome Category | |||
| Attenuated positive symptoms | 58 (92.1) | 60 (90.9) | 118 (91.5) |
| Genetic risk and deterioration | 1 (1.6) | 2 (3.0) | 3 (2.3) |
| Brief Intermittent Psychosis | 3 (4.8) | 0 (0.0) | 3 (2.3) |
| Schizotypal personality disorder | 1 (1.6) | 4 (6.1) | 5 (3.9) |
| Lifetime axis I disorder, n (%) | |||
| Anxiety | 35 (55.6) | 32 (48.5) | 67 (51.9) |
| Mood | 18 (28.6) | 27 (40.9) | 45 (34.9) |
| Attention-deficit/hyperactivity | 7 (11.1) | 15 (11.6) | 22 (17.1) |
| Alcohol abuse/dependence | 4 (6.3) | 4 (6.1) | 8 (6.2) |
| Cannabis abuse | 6 (9.5) | 7 (10.6) | 13 (10.1) |
| Global Assess. Functioning, prior mo., M (SD) | 46.7 (9.2) | 47.3 (9.3) | 47.0 (9.2) |
| Protocol sessions, M (SD) | 2.1 (1.2) | 11.0 (7.1) | 6.4 (6.6) |
| Received Extra-Protocol Therapy, n (%) | 19 (34.5%) | 17 (36.2%) | 36 (35.3%) |
Note: FFT-CHR = family-focused treatment for individuals at clinical high risk.
Exploratory mixed models were used to examine group, time, and group by time effects on changes in psychosocial functioning (pretreatment to 6 month changes in GAF, GF-Role, and GF-Social scores). The potentially moderating effects of sex and age were examined in mixed models that included two-way and three-way interactions between treatment, sex/age, and time on changes in symptoms or functioning. Because of a high degree of positive skew, age was treated as a categorical variable: early/middle adolescence (ages 12–15 yrs), late adolescence (ages 16–19), and young adulthood (ages 20 and older). Our study design had 95% power to detect a three-way interaction between treatment, age group, and time with a medium effect size (f=.25) (p < .05).
RESULTS
Sample Composition
Participants were 129 adolescents and young adults (mean 17.4 ± 4.1 yrs., range 12–32; 42.6% females). Of the 129, 27 (20.9%) were taking antipsychotic medications at randomization. None of the variables listed in Table 1 differed across the treatment groups.
Treatment and Study Completion
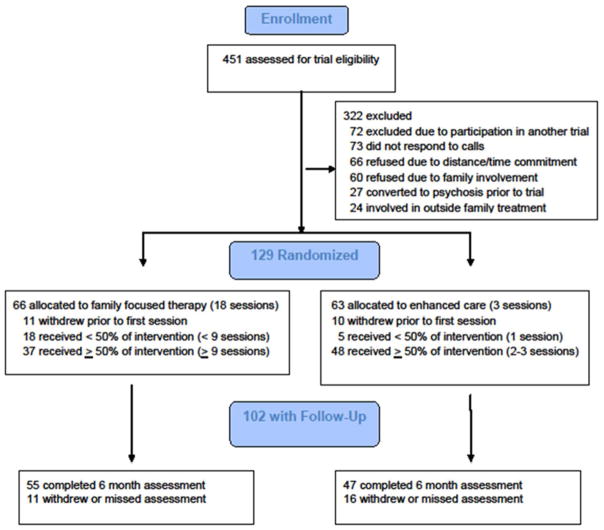
Rates of study withdrawal did not differ across treatments (Figure 1): 55 of 66 FFT-CHR participants (83.3%) and 47 of 63 (74.6%) EC participants completed the baseline and 6-month assessments (χ2(1)=1.49, p=.22). Because of its shorter duration, the mean interval between the final therapy session and the 6-month outcome interview was longer in EC (21.8±15.9 weeks) than in FFT-CHR (10.7±12.6 weeks).
Figure 1.
CONsolidated Standards of Reporting Trials (CONSORT) Diagram
The average number of FFT-CHR sessions was 11.0±7.1 (range 0–19), and EC sessions, 2.4±1.2 sessions (range 0–4; Table 1). Of 66 FFT-CHR participants, 42 (63.6%) were exposed to at least one session of communication or problem-solving skills training. Of 63 EC participants, 50 (79.4%) were exposed to most or all (2–3 sessions) of the psychoeducational content. Participants in FFT-HR were equally likely to obtain extra-protocol individual or group therapy sessions (34.5%) as participants in EC (36.2%; χ2(1)=.03, p=.86; Table 1). Sex, age, ethnicity, baseline GAF score, or use of antipsychotics was not associated with completion of the study or completion of either treatment.
Positive and Negative Symptoms
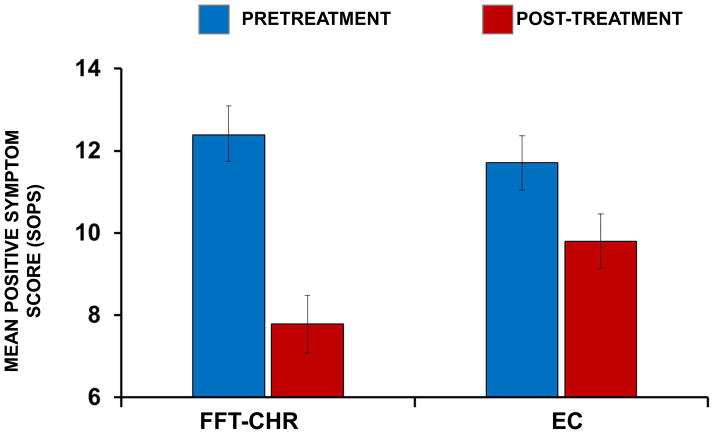
There was a significant main effect of time (F[1, 97]=31.95, p<.0001) and no main effect of treatment group (F[1,97]=.96, p=.33) on changes in total SOPS positive symptoms. There was, however, a treatment group by time interaction on positive symptoms (F[1,97]=5.49, p=.02), indicating greater improvement from baseline to 6 months in FFT-CHR compared to EC (d=.56; Figure 2). Participants who were prescribed antipsychotics at baseline showed greater improvement in attenuated positive symptoms over 6 months than those who were not prescribed antipsychotics (F[1,97]=5.96, p=.02). There was no interaction between psychosocial treatment, baseline antipsychotics, and time on attenuated positive symptoms (F[1,97]=1.58, p=.21), nor were there main or moderating effects of site, sex or age (p >.10).
Figure 2.
Effects of family-focused treatment for individuals at clinical high risk (FFT-CHR) and enhanced care (EC) on positive symptoms over 6 months. SOPS = Scale of Prodromal Symptoms. The values are adjusted means and standard errors from intent-to-treat mixed-effect regression models. The treatment by time interaction was significant (F[1, 97] = 5.49, p = .02; n=102).
A repeated measure mixed model that included the 5 SOPS items (considered simultaneously) as within-subject variables revealed a treatment by time interaction on changes in positive SOPS scores (F[1,986]=4.17, p=.04), but no psychosocial treatment by SOPS item by time interaction (F[4,986]=.43, p=.79). Thus, FFT-CHR was associated with equivalently greater improvements across all positive symptoms compared with EC.
There was a main effect of time on negative symptoms (F[1,95]=15.14, p<.0002), no effect of psychosocial treatments (F[1,95]=.47, p=.50) and no treatment group by time interaction (F[1,95])=.93, p=.34). However, participants who entered the trial on antipsychotics showed greater improvement in total negative symptom scores than those not on antipsychotics (F[1,95]=4.96, p=.03). The improvements in total negative symptom scores were not attributable to changes in specific negative symptom items (F(5,1198)=.33, p=.90).
Inclusion in the mixed models of site, sex, age, or number of weeks between last therapy session and the 6-month outcome assessment did not alter these results. There were no within-group associations in either treatment condition between number of sessions attended and improvements over 6 months in positive or negative symptoms.
Conversion to Psychosis
Of the 102 participants with baseline and 6-month SOPS data, conversions to psychosis occurred in 6 (5.9%). None of the 6 participants who converted were taking antipsychotic medications at entry. Conversions were observed in 5 of 47 EC participants (10.6%; mean days to conversion = 54.6±61.1) and in 1 of 55 (1.8%) FFT-CHR participants (adjusted OR=4.7). This participant converted within 30 days of randomization.
Psychosocial Functioning
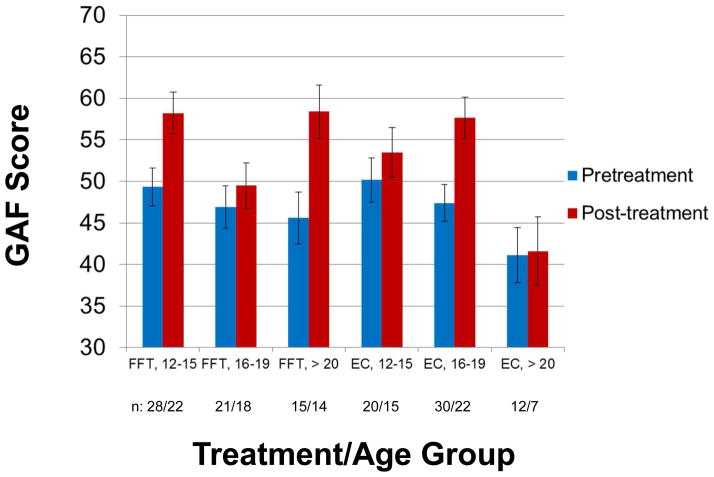
There was a main effect of time on changes in GAF scores from baseline to 6 months (F[1,93] =25.99, p<.0001), indicating that functioning was improving. However, there was no interaction between treatment group (or antipsychotic group) and time on GAF scores. A secondary analysis revealed an interaction between psychosocial treatment and time that depended on participants’ ages (F[2,92]=5.72, p=.005) (Figure 3). Among participants who were young adults (ages 20 or older; n=27), FFT-CHR was associated with larger pre/post increases in GAF scores than EC (p=.04; d=1.24). However, among older adolescents (16–19 yrs., n=51), EC was associated with greater increases in GAF scores than FFT-CHR (p< .05; d= −.67). Younger adolescents (ages 12–15; n = 50) improved by an average of 8.9 scale points in FFT-CHR and 3.3 points in EC, a nonsignificant difference (p=.13; d=.43).
Figure 3.
Effects of family-focused treatment for individuals at clinical high risk (FFT-CHR) and enhanced care (EC) on Global Assessment of Functioning (GAF) scores over 6 months. The values are adjusted means and standard errors from intent-to-treat mixed-effect regression models. The three-way interaction between treatment group, age group, and time was significant (F[2,92]=5.72, p=.005, n=102).
There was a three-way interaction between treatment, age group, and time on changes in GF–Role scores (F[2,92]=8.65, p=.0004), indicating greater improvements in role functioning in young adults (≥ 20 yrs.) who received FFT-CHR (p=.02; d=.70). In contrast, improvements in role functioning in adolescents (age 16–19 yrs) were greater in EC than in FFT-CHR (p=.004; d= −.94), but there was no treatment group difference among younger adolescents (12–15 yrs.; p=.35). GF-Social scores improved with time across all age groups (F[1,92]=11.82, p< .001), but there was no interaction between treatment group, age, and time (p=.52). There were no within-group associations in either treatment condition between number of sessions and changes in GAF or GF-Role or –Social scores.
Changes in Pharmacological Treatments
Data on medications prescribed at baseline and 6-months were available for 111 of the 129 participants (Table 2). A log-linear regression analysis indicated that participants in both psychosocial treatments were more likely to be prescribed antipsychotics during the trial (35.2%) than at time of randomization (20.9%) (χ2(1)=6.34, p=.01). There were no interactions between treatment condition and time (baseline, 6 months) on prescriptions for antipsychotics, antidepressants, anxiolytics, psychostimulants, or mood stabilizers (Table 2).
Table 2.
Medication Regimens at Time of Randomization and 6 Month Reassessment
| Medication Class | Assessment Point | Enhanced Care (n =63) | FFT-CHR (n=66) |
|---|---|---|---|
| Antipsychotic | |||
| Baseline | 13 (20.6) | 14 (21.2) | |
| 6 months | 17 (32.7) | 21 (35.6) | |
| Antidepressant | |||
| Baseline | 20 (31.8) | 18 (27.3) | |
| 6 months | 20 (38.5) | 25 (42.4) | |
| Psychostimulant | |||
| Baseline | 5 (7.9) | 7 (10.6) | |
| 6 months | 7 (13.5) | 9 (15.3) | |
| Anxiolytic | |||
| Baseline | 7 (11.1) | 5 (7.6) | |
| 6 months | 8 (15.4) | 7 (11.9) | |
| Mood stabilizer | |||
| Baseline | 3 (4.8) | 4 (6.1) | |
| 6 months | 3 (5.8) | 7 (11.9) | |
Note: Frequencies of medication class indicate the number of participants who were prescribed each type of medication, followed by percentages in each treatment condition. At 6 months, medication data were available for 52 of 63 (82.5%) participants in enhanced care and 59 of 66 (89.4%) participants in family-focused treatment for individuals at clinical high risk for psychosis (FFT-CHR).
DISCUSSION
To our knowledge, this is the first randomized trial of a family intervention for individuals at high risk for psychosis. Modern psychoeducational approaches emphasize building the vulnerable person’s skills for managing symptoms, with family members as allies in this process.26 FFT-CHR was associated with greater improvement in attenuated positive symptoms over 6 months than a brief treatment (EC) oriented toward symptom prevention. The effects did not extend to negative symptoms. Our results are similar to those of randomized trials of CBT in individuals at high clinical risk, trials that have demonstrated significant effects on positive but not negative symptoms.6
Prior intervention studies of populations with attenuated psychotic symptoms followed over 6-month to 1-year periods have observed low rates of conversion to psychosis (range 0% to 22%).4,27–30 In this trial, 6 of 102 (5.9%) participants developed a psychotic episode over 6 months, 5 in EC (10.6%) and one in FFT-CHR (1.8%). Future trials with longer follow-up will be necessary to clarify whether structured family interventions (without adjunctive medications) can affect conversion rates in individuals at clinical high risk.
The results of this trial for the primary outcome of attenuated positive symptoms can be compared to results of a recent trial of FFT vs. brief psychoeducation on stabilizing mood symptoms among children and adolescents at high risk for bipolar disorder.13 Over 1 year, children and adolescents with major depression and/or subthreshold mania - all of whom had a first-degree relative with bipolar I or II disorder - had more rapid recoveries from depression symptoms, greater reductions in hypomanic symptoms, and more time in remission if they received FFT than brief psychoeducation. The coping skills emphasized in FFT, including early recognition of illness episodes, broadening skills for coping with stress, and effective family communication and problem-solving, may be equally applicable to emerging psychotic or bipolar disorders.
In the present study, participants in both psychosocial treatments showed significant improvements in negative symptoms over 6 months. Although changes in negative symptoms may be attributable to the passage of time, it is also conceivable that the behavioral activation component of FFT-CHR and EC, in which individuals at high risk and their family members are encouraged to increase their engagement in rewarding events, was responsible for a portion of these changes. Adapting and testing behavioral activation treatments for depression in young populations at high risk for psychosis would more directly test this hypothesis. 31
In a prior study involving a subset of participants from the current trial, individuals at high risk and parents who received FFT-CHR showed greater increases from a pretreatment to a 6-month family interactional assessment in positive communication behaviors (e.g., active listening) and greater decreases in conflictual behaviors (e.g., criticisms) than individuals in EC. 32 One mechanism by which family interventions may exert their effects is through increasing the ratio of positively- to negatively- valenced communication behaviors in families, which may help buffer the person at high risk from other forms of environmental stress.33
A secondary finding was that both groups of participants improved in social and role functioning over 6 months. Participants over age 20 showed greater functional improvement in FFT-CHR compared to EC, whereas participants between the ages of 16–19 showed greater functional improvement in EC. The key difference in session content between the two treatments is the emphasis on communication and problem-solving skills in FFT-CHR.24 Increases in the individual’s use of positive communication strategies with parents or siblings may lead to reciprocal increases in these family members’ use of constructive communication, which may in turn increase the individual’s self-efficacy regarding managing peer and work relationships. Young adult participants may be better able to generalize the use of skill training to their school and work settings than adolescent participants. In contrast, older adolescents may respond best to a brief self-management approach that emphasizes coping with symptoms in stressful interpersonal circumstances. A prior study showed that adolescents with bipolar disorder who reported higher levels of stress in peer relationships showed less improvement in intensive family treatment over 12 months than those who reported low levels of peer stress.34 These findings, if replicated in other psychosocial treatment trials of youth at clinical high risk, suggest the importance of considering the effects of age on skill acquisition and functional recovery.
A possible interpretation of this study is that antipsychotic medications are more effective in treating attenuated positive and negative symptoms than psychosocial treatments. However, only a small subsample of participants (20.9%) entered the study on antipsychotic agents. Antipsychotics were not assigned randomly; thus, participants who had previously responded to antipsychotics may have been overrepresented in this subsample. Conducting psychosocial treatment trials in individuals at high risk who are ineligible for or refuse antipsychotic agents may lead to sampling biases. Instead, standardized algorithms for drug selection or augmentation, monitoring participants’ adherence to regimens, and keeping physicians blind to psychotherapy conditions are necessary to rule out the inter-current effects of pharmacotherapy.
As is true of the majority of randomized trials of psychotherapy for individuals at risk for psychosis,6 the experimental and control treatments in this trial differed in the number of protocol sessions (18 versus 3). Thus, we cannot determine whether the greater benefits of FFT-CHR on positive symptoms can be attributed to specific factors (i.e., its broader array of skill training modules), differences in the length of the interval between the last treatment session and the 6-month assessment, or the greater frequency of therapeutic contact in FFT-CHR and increased opportunities for clinicians to intervene early with symptom changes. Nonetheless, studies that compare a new treatment to usual care – which usually means comparing treatments that are not matched on number of sessions – are a critical first step in determining whether future trials of the treatment should be pursued. Differences between treatments in duration and therapist attention are often consistent with the way treatments are given in practice.35 For example, families of young patients with subthreshold psychosis would be unlikely to attend a lengthy educational program if a shorter, equally effective alternative were available.
Another study limitation is that the 129 participants represent a small subset of the 451 NAPLS-2 participants assessed for the trial (Figure 1). Although there were a variety of reasons that participants in NAPLS-2 did not participate in this trial, the majority of those excluded refused or had family members who refused because of distance and time commitments. This rate of exclusion is comparable to rates found in other studies of psychosocial intervention for individuals at high risk for psychosis. 7,27 Nonetheless, it is incumbent upon researchers and clinicians to develop psychosocial interventions that have a broader reach for the intended high-risk populations. Future trials should consider the cost-effectiveness of psychosocial interventions in individuals at high risk, especially when requiring the involvement of family members who may incur economic or practical costs. Briefer treatments that address immediate symptoms (e.g., anxiety reduction), perhaps followed by booster sessions focused on preventative maintenance strategies, may decrease the net costs of psychosocial care. Alternatives to in-person treatment, such as telehealth or internet-based approaches, deserve further exploration in these populations.36
Despite these limitations, this study contributes to a growing body of evidence suggesting that early psychosocial interventions benefit individuals at risk for psychosis. Contrary to concerns regarding the possible negative impact of early intervention37, a broad range of treatments (individual, family, group, CBT, supportive) are associated with symptomatic improvement,6 and in this study more treatment was of greater benefit than less. Future studies that compare family interventions to equally intensive psychosocial interventions that have a track record in populations at risk for psychosis, such as CBT, would allow control over therapist attention variables. Moreover, studies testing family and individual modalities would allow the comparison of mediational pathways (e.g., improvements in family communication versus changes in behavioral activation or attributional styles) in stabilizing attenuated positive symptoms among vulnerable individuals.
Clinical Guidance.
This randomized trial examined an 18-session, 6-month family-focused therapy for 129 individuals at clinical high risk (FFT-CHR) for psychosis. Participants were between the ages of 12 and 35 years old and had attenuated positive symptoms with recent functional deterioration.
FFT-CHR included psychoeducation regarding stress management and prevention of psychosis symptoms, communication training, and problem-solving skills training. The comparison treatment, enhanced care (EC), involved three sessions of family psychoeducation focusing on symptom management.
The participants in FFT-CHR had greater reductions in positive symptoms than participants in EC over 6 months.
The participants who were 20 years or older showed greater improvements in psychosocial functioning (e.g., work or school performance) if they received FFT-CHR than if they received EC, whereas those between ages 16 and 19 years old showed greater functional improvements in EC than in FFT-CHR.
Young adults with subthreshold psychotic symptoms may benefit from family interventions that emphasize psychoeducation and skills training.
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) grants 1RC1MH088546 (TDC, DJM), and R01MH093676 (DJM), and a grant from the Staglin Family Foundation (TDC). Catherine Sugar, PhD, of the Department of Biostatistics, UCLA served as the statistical expert for this research.
The authors thank Serine Uguryan, BA, of the Department of Psychology, UCLA for her assistance in data management.
Footnotes
Clinical trial registration information--Prevention Trial of Family Focused Treatment in Youth at Risk for Psychosis; http://clinicaltrials.gov/; NCT01907282.
Disclosure: Dr. Miklowitz has received grant funding from NIMH, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Attias Family Foundation, the Danny Alberts Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, and the Knapp Foundation; and book royalties from Guilford Press and John Wiley and Sons. Dr. Cannon has received grant funding from NIMH. Drs. O’Brien, Schlosser, Addington, Candan, Domingues, Walsh, De Silva, Friedman-Yakoobian, and Mss. Marshall and Ms. Zinberg report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. David J. Miklowitz, University of California, Los Angles (UCLA) School of Medicine, Los Angeles, CA.
Dr. Mary P. O’Brien, Yale University, New Haven, CT.
Dr. Danielle A. Schlosser, University of California, San Francisco, CA.
Dr. Jean Addington, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta.
Dr. Kristin A. Candan, Zucker Hillside Hospital, NY.
Ms. Catherine Marshall, Hotchkiss Brain Institute, University of Calgary, Calgary, Alberta.
Dr. Isabel Domingues, University of California, San Diego.
Dr. Barbara C. Walsh, Yale University, New Haven, CT.
Ms. Jamie L. Zinberg, University of California, Los Angles (UCLA) School of Medicine, Los Angeles, CA.
Dr. Sandra D. De Silva, University of California, Los Angles (UCLA) School of Medicine, Los Angeles, CA.
Dr. Michelle Friedman-Yakoobian, Mental Health Center Division of Public Psychiatry, Beth Israel Deaconess Medical Center, and Harvard Medical School, Boston, MA.
Dr. Tyrone D. Cannon, Yale University, New Haven, CT.
References
- 1.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 3.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized double-blind clinical trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 4.McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 5.Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 6.Stafford MR, Jackson H, Mayo-Wilson E, Morridson AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. doi: 10.1136/bmj.f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233. doi: 10.1136/bmj.e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlosser DA, Zinberg JL, Loewy RL, et al. Predicting the longitudinal effects of the family environment on prodromal symptoms and functioning in patients at-risk for psychosis. Schizophr Res. 2010;118(1–3):69–75. doi: 10.1016/j.schres.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarlane WR, Cook WL. Family expressed emotion prior to onset of psychosis. Fam Process. 2007;46(2):185–197. doi: 10.1111/j.1545-5300.2007.00203.x. [DOI] [PubMed] [Google Scholar]
- 10.Miklowitz DJ. The role of family systems in severe and recurrent psychiatric disorders: a developmental psychopathology view. Dev Psychopathol. 2004;16:667–688. doi: 10.1017/s0954579404004729. [DOI] [PubMed] [Google Scholar]
- 11.Miklowitz DJ, Scott J. Psychosocial treatments for bipolar disorder: Cost-effectiveness, mediating mechanisms, and future directions. Bipolar Disord. 2009;11:110–22. doi: 10.1111/j.1399-5618.2009.00715.x. [DOI] [PubMed] [Google Scholar]
- 12.Miklowitz DJ, Axelson DA, Birmaher B, et al. Family-focused treatment for adolescents with bipolar disorder: results of a 2-year randomized trial. Arch Gen Psychiatry. 2008;65(9):1053–1061. doi: 10.1001/archpsyc.65.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miklowitz DJ, Schneck CD, Singh MK, et al. Early intervention for symptomatic youth at risk for bipolar disorder: a randomized trial of family-focused therapy. J Am Acad Child Adol Psychiatry. 2013;52(2):121–131. doi: 10.1016/j.jaac.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addington J, Cadenhead KS, Cornblatt BA, et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142(1–3):77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of inter-rater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins KA, McGlashan TH, Quinlan D, et al. Factorial structure of the Scale of Prodromal Symptoms. Schizophr Res. 2004;68(2):339–347. doi: 10.1016/S0920-9964(03)00053-7. [DOI] [PubMed] [Google Scholar]
- 17.Hall R. Global Assessment of Functioning: A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D. C: American Psychiatric Press; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 20.Addington J, Cadenhead KS, Cannon TD, et al. North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33(3):665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begg CB, Iglewicz B. A treatment allocation procedure for sequential clinical trials. Biometrics. 1980;36:81–90. [PubMed] [Google Scholar]
- 23.Weisman AG, Okazaki S, Gregory J, et al. Evaluating therapist competency and adherence to behavioral family management with bipolar patients. Fam Process. 1998;37:107–121. doi: 10.1111/j.1545-5300.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- 24.Marvin SE, Miklowitz DJ, O’Brien MP, Cannon TD. Family-focused therapy for individuals at clinical high risk of psychosis: treatment fidelity within a multisite randomized trial. Early Interv Psychiatry. 2014 Apr 11; doi: 10.1111/eip.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ger D, Everitt BS. Handbook of Statistical Analyses Using SAS. 2. London: CRC Press; 2001. [Google Scholar]
- 26.Liberman RP. Recovery from Disability: Manual for Psychiatric Rehabilitation. Washington, D. C: American Psychiatric Publishing, Inc; 2008. [Google Scholar]
- 27.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophr Res. 2011;125(1):54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 28.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 29.Morrison AP, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomised controlled trial. Br J Psychiatry. 2004;185:291–297. doi: 10.1192/bjp.185.4.291. [DOI] [PubMed] [Google Scholar]
- 30.Yung AR, Phillips LJ, Nelson B, et al. Randomized controlled trial of interventions for young people at ultra high risk for psychosis: 6-month analysis. J Clin Psychiatry. 2011;72(4):430–440. doi: 10.4088/JCP.08m04979ora. [DOI] [PubMed] [Google Scholar]
- 31.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74(4):658–670. doi: 10.1037/0022-006X.74.4.658. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MP, Miklowitz DJ, Cannon TD. A randomized trial of family focused therapy with youth at clinical high risk for psychosis: effects on interactional behavior. J Consult Clin Psychol. 2014;82(1):90–101. doi: 10.1037/a0034667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miklowitz DJ, Johnson SL. Social and familial risk factors in bipolar disorder: basic processes and relevant interventions. Clin Psychol Sci Pract. 2009;16(2):281–296. doi: 10.1111/j.1468-2850.2009.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EY, Miklowitz DJ, Biuckians A, Mullen K. Life stress and the course of early-onset bipolar disorder. J Affect Disord. 2007;99:37–44. doi: 10.1016/j.jad.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chambless DL, Hollon SD. Treatment validity for intervention studies. In: Cooper H, Sher K, Camic P, Gonzalez R, Long D, Panter A, editors. APA Handbook of Research Methods in Psychology. Washington, D.C: Am Psychol Assoc; 2012. pp. 1–24. [Google Scholar]
- 36.Hollon SD, Muñoz RF, Barlow DH, et al. Psychosocial intervention development for the prevention and treatment of depression: Promoting innovation and increasing access. Biol Psychiatry. 2002;52:610–630. doi: 10.1016/s0006-3223(02)01384-7. [DOI] [PubMed] [Google Scholar]
- 37.Warner R. The prevention of schizophrenia: what interventions are safe and effective? Schizophr Bull. 2001;27(4):551–562. doi: 10.1093/oxfordjournals.schbul.a006895. [DOI] [PubMed] [Google Scholar]