Abstract
On July 31, 2013, the Prostate Cancer Foundation assembled a working committee on the molecular biology and pathologic classification of neuroendocrine differentiation in prostate cancer. The committee consisted of genitourinary oncologists, urologists, urological surgical pathologists, basic scientists, and translational researchers, with expertise in this field. It was concluded that the proceedings of the meeting should be reported in 2 manuscripts appealing to different target audiences, one to focus on surgical pathology and the other to review the molecular aspects of this disease. New clinical and molecular data emerging from prostate cancers treated by contemporary androgen deprivation therapies, as well as primary lesions, have highlighted the need for refinement of diagnostic terminology to encompass the full spectrum of neuroendocrine differentiation. It is envisioned that specific criteria associated with the refined diagnostic terminology will lead to clinically relevant pathologic diagnoses that will stimulate further clinical and molecular investigation and identification of appropriate targeted therapies.
Keywords: small cell carcinoma, Paneth cell like, large cell neuroendocrine carcinoma, carcinoid, prostate adenocarcinonma
On July 31, 2013, the Prostate Cancer Foundation assembled a working committee on the molecular biology and pathologic classification of neuroendocrine (NE) differentiation in prostate cancer. The committee consisted of genitourinary oncologists, urologists, urological surgical pathologists, basic scientists, and translational researchers, with expertise in this field. It was concluded that the proceedings of the meeting should be reported in 2 manuscripts appealing to different target audiences, one to focus on surgical pathology and the other to review the molecular aspects of this disease. New clinical and molecular data emerging from prostate cancers treated by contemporary androgen deprivation therapies, as well as primary lesions, have highlighted the need for refinement of diagnostic terminology to encompass the full spectrum of NE differentiation. It is envisioned that specific criteria associated with the refined diagnostic terminology will lead to clinically relevant pathologic diagnoses that will stimulate further clinical and molecular investigation and identification of appropriate targeted therapies.
NE CELLS IN NORMAL PROSTATE HISTOLOGY
The NE component of the normal prostate consists of a small subset of cells, randomly scattered within the epithelium of the prostate glands in all anatomic zones. These cells contain a variety of peptide hormones, such as serotonin, histamine, chromogranin A, calcitonin and other members of the calcitonin gene family, neuropeptide Y, vasoactive intestinal peptide, bombesin/gastrin- releasing peptide, parathyroid hormone–related protein, neuron-specific enolase (NSE), thyroid-stimulating hormone–like peptide, somatostatin, vascular endothelial growth factor, and others.1–3 These substances affect target cells by endocrine, paracrine, and autocrine mechanisms. By light microscopy, these cells rest on the basal cell layer between the secretory cells. They typically do not extend to the lumen but often have narrow apical and lateral dendritic extensions. They are not reliably recognizable by hematoxylin and eosin examination but may contain granular eosinophilic cytoplasm distinct from Paneth cell–like change.4,5 NE cells are more commonly present in the prostate than in any other organ within the genitourinary tract.
NE CELLS AND DIFFERENTIATION IN PROSTATE CANCER
NE cells are defined in current practice by immunohistochemical (IHC) positivity for either synaptophysin, chromogranin, or CD56. NSE immunoreactivity is, despite its name, not sufficiently specific for the diagnosis of NE differentiation. NE cells have also been noted in neoplasms of the prostate, in which they have generated recent interest in their relation to castrationresistant disease. NE cells lack androgen receptors (ARs), and NE differentiation increases after androgen deprivation and in castration-resistant prostate cancer (CRPC).6 As a result of their secretory products, NE cells could stimulate the proliferation of prostate carcinoma cells and increase their aggressiveness through the inhibition of apoptosis and stimulation of neoangiogenesis.1,7–10 The amount of NE differentiation of prostate adenocarcinoma increases with disease progression and in response to androgen deprivation therapy (ADT).6,11,12 There is also emerging evidence suggesting that transformation to a predominantly AR-negative prostate cancer with increasing NE differentiation by IHC may be an important resistance mechanism in castration-resistant disease and is likely more common than previously recognized. This may be related to patients living longer, more potent AR signaling inhibition with new approved therapies (ie, abiraterone, enzalutamide), and/or because of increased awareness due to more common metastatic biopsy protocols in the setting of CRPC.13,14
The current World Health Organization (WHO) histologic classification of NE tumors of the prostate includes: (1) focal NE differentiation in conventional prostate adenocarcinoma; (2) carcinoid tumor (WHO well-differentiated NE tumor); and (3) small cell NE carcinoma (WHO classification, poorly differentiated NE carcinoma).15 Although this NE classification is analogous to other organs, it does not account for the unique aspects of NE differentiation in prostate cancer. The newly proposed classification of NE prostate carcinoma is outlined in Table 1.
TABLE 1.
Pathologic Classification of NE Differentiation in Prostate Carcinoma
| Usual prostate adenocarcinoma with NE differentiation |
| Adenocarcinoma with Paneth cell NE differentiation |
| Carcinoid tumor |
| Small cell carcinoma |
| LCNEC |
| Mixed (small or large cell) NE carcinoma—acinar adenocarcinoma |
USUAL PROSTATE ADENOCARCINOMA WITH NE DIFFERENTIATION
Definition
Morphologically typical, usual acinar or ductal adenocarcinoma of the prostate in which NE differentiation is demonstrated by IHC alone (ie, synaptophysin, CD56, chromogranin).
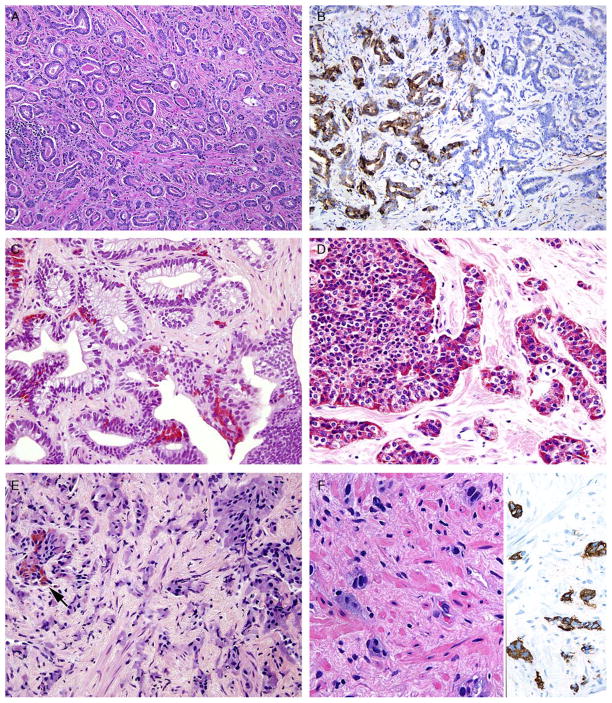
In the early 1970s, Azzopardi and Evans4 recognized the presence of argentaffin cells within normal prostatic adenocarcinoma. Immunohistochemically, usual adenocarcinoma of the prostate demonstrates scattered NE cells in 10% to 100% of cases, in part depending on the number of slides studied and the number of antibodies used (Table 2) (Figs. 1A, B).7,12,16,17
TABLE 2.
IHC of NE Differentiation in Prostate Tumors
| PSA | NE Markers | Ki67 | |
|---|---|---|---|
| PCa. | Positive | Scattered + cells | Not increased in NE cells |
| PCa. with Paneth cell NE differentiation | Variably positive | Diffuse positive in Paneth cells | Few cases studied—not increased |
| Carcinoid-like tumor | Usually* positive | Positive | Not studied |
| Carcinoid tumor | Negative | Diffusely positive | Usually low Rarely increased (typically <5%–20%) |
| SC carcinoma | Usually negative or scattered positive cells | Positive in ~90% of cases | > 50%, typically >80% |
| LC NE carcinoma | Usually negative but may be positive | Diffusely positive | Usually >50% |
| Mixed NE (SC/LC) usual PCa. | Same as above for each component | Same as above for each component | Same as above for each component |
Results refer to carcinoid-like areas. Tumors usually associated with usual prostatic adenocarcinoma.
PCa. indicates adenocarcinoma; SC, small cell; LC, large cell.
FIGURE 1.
A, Adenocarcinoma of the prostate Gleason score 3+3 = 6. B, Same case as (A) with areas showing positivity for synaptophysin (left) that are indistinguishable in their glandular morphology from areas that are synaptophysin negative (right). C, Gleason score 6 adenocarcinoma and adjacent high-grade prostatic intraepithelial neoplasia with Paneth cell–like NE granules. D, Sheets and cords of prostatic adenocarcinoma with Paneth cell–like NE granules. E, Cords of adenocarcinoma of the prostate with amphophilic cytoplasm and scattered cells with eosinophilic granules (arrow). F, Cords of adenocarcinoma of the prostate with amphophilic cytoplasm (left) staining diffusely for synaptophysin (right).
In these cases, prostate-specific antigen (PSA) is positive in the usual adenocarcinoma but variably positive in the NE cells.18 It is controversial whether NE differentiation in typical adenocarcinomas worsens prognosis. In some studies suggesting a correlation, the prognostic relationship was weak and not sufficient to be useful clinically.1,19–22 Most of the studies have shown no effect of NE differentiation on outcome, including 1 study each analyzing NE differentiation in prostate cancer on needle biopsy and transurethral resection of the prostate.23–34
Summary
Currently, as the clinical significance remains uncertain, it is not recommended to routinely use IHC stains to detect any NE differentiation in an otherwise morphologically typical primary adenocarcinoma of the prostate.
ADENOCARCINOMA WITH PANETH CELL–LIKE NE DIFFERENTIATION
Definition
Histologically, typical adenocarcinoma of the prostate containing varying proportions of cells with prominent eosinophilic cytoplasmic granules on routine light microscopy (Paneth cell–like change), which are chromogranin positive and contain neurosecretory granules by electron microscopy.
The term Paneth cell–like change has been used to describe distinctive eosinophilic NE cells.35 Paneth cell– like NE differentiation in prostatic adenocarcinoma can be seen as either patchy isolated cells or diffusely involving glands or nests (Fig. 1C).36,37 These Paneth cell– like cells may be present in well-formed glands of Gleason pattern 3 but also can be present in cords of cells with bland cytology, wherein on strictly applying the Gleason grading system one would assign a Gleason pattern 5 (Fig. 1D). Although by the Gleason system areas of Paneth cell–like NE differentiation may be graded as pattern 5, their bland cytology, typically limited nature, and frequent association with lower-grade conventional adenocarcinoma raises questions as to whether this unique histology should not be diagnosed as high grade. Of 16 radical prostatectomy specimens with Paneth cell– like NE cells lacking glandular differentiation, there was organ-confined cancer in 62.5% of cases, only 4 cases with seminal vesicle invasion, and none with pelvic lymph node metastases. The postoperative course was also favorable with a >90% actuarial PSA progression-free risk at 5 years. The prognosis seemed to be driven by conventional parameters independent of NE differentiation. The only 2 patients who progressed after radical prostatectomy had Gleason score 7 conventional cancer with extraprostatic extension and seminal vesicle invasion, with one also having ductal differentiation and positive margins. In cases in which the entire tumor is composed of Paneth cell–like cells and areas of the tumor lack glandular differentiation, it is questionable whether these tumors should be assigned a Gleason score. A comment could be provided as to the generally favorable prognosis of this morphologic variant of adenocarcinoma of the prostate on the basis of the limited data available.36 However, the data on the prognostic significance of Paneth cell–like differentiation are still limited, and we have seen anecdotal cases in which such a tumor progressed to metastatic disease with small cell carcinoma.
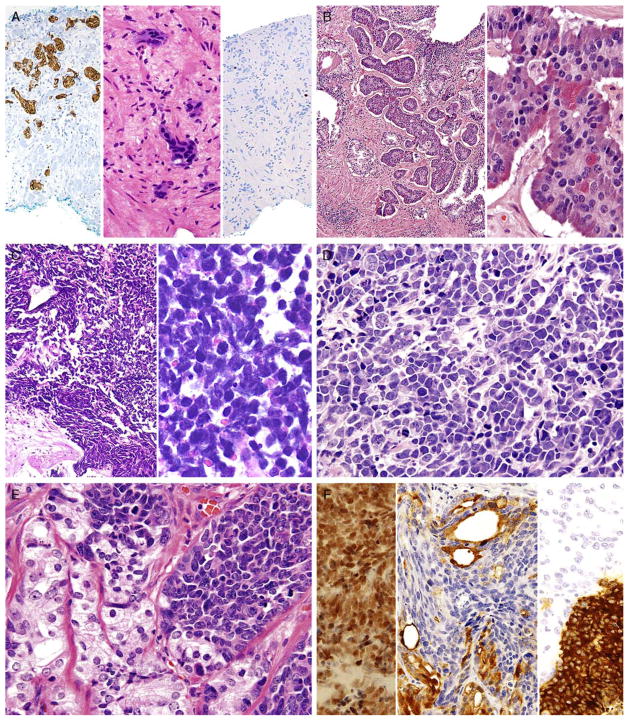
In some cases, one can see a spectrum of Paneth cell– like cells with eosinophilic granules adjacent to identical cells with deeply amphophilic cytoplasm lacking granules, with both cell types labeling diffusely with NE markers. Uncommonly, cancers may only consist of cords of cells with bland cytology and only amphophilic cytoplasm, with either rare or absence of the characteristic eosinophilic granules (Figs. 1E, F). These cells are also diffusely positive for NE markers (Fig. 1F) and, in the few cases studies, have a low Ki67 proliferation rate (Fig. 2A). These cells with amphophilic cytoplasm arranged in cords with bland cytology, typically in a very limited focus, are typically associated with other cells showing Paneth cell–like changes and should be considered a variant of Paneth cell–like change. Both the classic Paneth cell–like changes and this variant may not express prostate markers possibly given that their cytoplasm is replaced by NE granules (Table 2). The key to recognizing these cases is to first note the architectural pattern of nests and cords in a small focus. Secondly, these tumors have deeply amphophilic cytoplasm with careful search in most cases revealing rare Paneth cell– like eosinophilic granules. Finally, the above finding in combination with either no prominent nucleoli or rare visible nucleoli may prompt IHC staining for NE markers.
FIGURE 2.
A, Cords of adenocarcinoma of the prostate with amphophilic cytoplasm (center) staining diffusely for synaptophysin (left) with a low Ki67 proliferation rate (right). B, Carcinoid-like tumor with nests of cells (left). Higher magnification (right) shows uniform round nuclei with “salt and pepper” chromatin and scattered Paneth cell–like granules. The tumor was positive for NE markers and negative for PSA. Adjacent was usual adenocarcinoma (not shown). C, Low (left) and high (right) magnification of small cell carcinoma of the prostate. D, Intermediate cell type variant of small cell carcinoma of the prostate with slightly more open chromatin and occasional small nucleoli. Tumor was positive for NE markers (not shown). E, Mixed usual adenocarcinoma of the prostate (left) with small cell carcinoma (right). F, Same case as (E) with small cell carcinoma component positive for TTF-1 (left). Synaptophysin labeled the small cell carcinoma component (right, bottom) and not the adenocarcinoma component (right, top). PSA (center) was positive in the adenocarcinoma component but not in the small cell carcinoma.
Summary
Currently, as the clinical significance is incompletely understood, one may use IHC stains to confirm NE differentiation in the eosinophilic (Paneth cell–like) and amphophilic cells. The term adenocarcinoma with Paneth cell–like NE differentiation should be used. In addition, a comment may be made that in the absence of prior ADT, Gleason grading of areas showing Paneth cell–like or amphophilic NE change in areas without glandular differentiation may not be applicable.
CARCINOID TUMOR
Definition
A well-differentiated NE tumor occurring in the prostate gland, showing the classic morphology of carcinoid tumor at other sites such as the lung, but which is not closely associated with usual prostate carcinoma or which does not arise from the urethra or extend from the bladder.
True carcinoid tumors of the prostate are extremely rare. To diagnose a carcinoid of the prostate and distinguish it from a prostate adenocarcinoma with carcinoid-like features, the following features should be present: (1) not closely associated with concomitant adenocarcinoma of the prostate; (2) immunohistochemically positive for NE markers and negative for PSA; and (3) originating in the prostatic parenchyma. The rationale for excluding PSA expression is that usual prostate adenocarcinoma can express NE markers and can have a bland cytologic appearance with areas resembling a carcinoid tumor; in order for the diagnosis of “carcinoid tumor” to be a distinctive entity, it should be restricted to cases that are not usual prostate adenocarcinoma (ie, lacking PSA). Of the cases in the literature, there are only 5 cases that satisfy this definition.38–41 Some of the older reported cases of carcinoid tumor of the prostate predate the use of IHC and cannot be verified. One case based on the illustration provided is a urethral carcinoid as opposed to prostatic in origin.42 The reports by Tash and colleagues of a 38-year-old man and by Slater and colleagues of a 69-year-old individual may be carcinoids, yet IHC stains for PSA or any other prostatic marker were not reported.43,44 Similarly, in the study by Wasserstein et al,45 no IHC analyses were performed. Murali et al46 illustrates images of 2 prostatic carcinoids in their review article, yet no details are provided about the cases. Turbat-Herrera et al47 reported a “prostatic carcinoid” that was negative for PSA, yet in contrast to carcinoids only 2+ scattered synaptophysin-positive cells were present, and the tumor had diffuse prominent nucleoli. The “prostatic carcinoid” reported by Egan and colleagues had “intraductal carcinoid” and was admixed with usual prostate adenocarcinoma and most likely represents the recently described phenomenon of “small cell–like change in high-grade prostatic intraepithelial neoplasia, intraductal carcinoma, and invasive prostatic adenocarcinoma.”48,49 There are 5 bona fide cases of prostatic carcinoids. Two cases were in men in their 30s, younger than typically seen with adenocarcinoma of the prostate.38,39 The remaining 3 cases were in even younger male individuals with multiple endocrine neoplasia IIB syndrome.40,41 Patients were 7, 19, and 22 years of age. Although the data are limited, prostatic carcinoids tend to present with locally advanced disease, including some with regional lymph node metastases, yet still have a favorable prognosis. It is reasonable for these true carcinoids to be graded in an analogous manner to those of the gastrointestinal tract based on mitotic rates and Ki67 proliferation rates.
Several cases have been reported in which a “carcinoid-like” or “carcinoidal” appearance of the tumor with nested architecture and uniform nuclei has been present. These tumors may on occasion also exhibit IHC and/or ultrastructural evidence of NE differentiation (Fig. 2B). Some authors consider IHC staining with PSA a key discriminator, wherein true prostatic carcinoids are negative, and carcinoid-like carcinomas are positive. Most cases reported with carcinoid-like morphology have admixed usual prostate cancer or the carcinoid-like tumor expressed PSA (Table 2).50–56 Several carcinoid-like prostate cancers appear to be variants of Paneth cell–like NE differentiation with a paucity or absence of eosinophilic granules, in which PSA may be negative.57 Clinically, carcinoid-like adenocarcinomas have behaved like ordinary prostate carcinomas, and in none of these cases has a carcinoid syndrome been present. Prostate-specific acid phosphatase (PSAP) immunoreactivity is not discriminatory in the assessment of whether a tumor is a true carcinoid or adenocarcinoma with carcinoid-like features as even some nonprostatic carcinoid tumors express PSAP.58 Although most “carcinoid-like” tumors have not produced clinical symptoms, several cases have produced adrenocorticotropic hormone in sufficient quantity to result in Cushing syndrome.52
Summary
The diagnosis of carcinoid tumor should be made very rarely and strictly applying the criteria outlined above in the definition. In such cases, particularly in younger patients, investigation for stigmata of multiple endocrine neoplasia syndrome should be initiated. Tumors with PSA-negative nests and cords of cells that are admixed with usual prostate adenocarcinoma should not be diagnosed as carcinoid tumor, as such cases may represent adenocarcinoma with Paneth cell–like NE differentiation or its more subtle variant with deeply amphophilic cytoplasm.
SMALL CELL CARCINOMA
Definition
Small cell carcinoma is a high-grade tumor defined by characteristic nuclear features, including lack of prominent nucleoli, nuclear molding, fragility, and crush artifact (Fig. 2C). High nuclear to cytoplasmic ratio and indistinct cell borders are characteristic, as is a high mitotic rate and apoptotic bodies. In resection specimens, as opposed to needle biopsy cores, geographic necrosis may be frequent.
Approximately 40% to 50% of small cell carcinomas have a history of usual prostatic adenocarcinoma. The interval between the diagnosis of small cell carcinoma and prior usual prostatic cancer ranges from 1 to 300 months (median 25 mo).59 Historically, pure small cell carcinoma was seen at initial diagnosis in about 50% to 60% of cases with the remaining cases admixed with prostate adenocarcinoma (as discussed below). Clinical recognition of the emergence of small cell carcinoma during the progression of the disease is increasing and leading to more frequent biopsies of metastatic sites. Patients with this aggressive disease have frequent visceral metastases and less often paraneoplastic syndromes such as those associated with ectopic adrenocorticotropic hormone, hypercalcemia, or inappropriate antidiuretic hormone production. The diagnosis of small cell carcinoma of the prostate is reached on the basis of morphologic features similar to those found in small cell carcinomas of the lung as defined in the 1999 WHO classification criteria of pulmonary neoplasms.60–62 Morphologic variations of small cell carcinoma include: intermediate cell type with slightly more open chromatin and visible small nucleoli seen in about 30% to 40% of cases, which may be beyond that allowable in the strict diagnosis of small cell carcinoma of the lung (Fig. 2D).59 Less commonly, there is the presence of tumor giant cells and Indian filing.59 Neurosecretory granules have been demonstrated within several prostatic small cell carcinomas. Using IHC techniques, the small cell component is positive for 1 or more NE markers (synaptophysin, chromogranin, CD56) in almost 90% of cases.59,61 PSA and other prostatic markers such as P501s are positive in about 17% to 25% of cases, although often very focally (Table 2).59,61 In 24% and 35% of cases, positivity is noted for p63 and high–molecular weight cytokeratin, markers typically negative in prostatic carcinoma.61 Studies have demonstrated TTF-1 expression in >50% of small cell carcinomas of the prostate, limiting its utility in distinguishing primary small cell carcinoma of the prostate from a metastasis from the lung.59,61,63,64
Because of the rarity of primary small cell carcinoma of the prostate, an important diagnostic consideration is exclusion of metastasis or local extension from other sites such as the bladder. A technique that can distinguish small cell carcinoma of the prostate from other small cell carcinomas is documentation by fluorescence in situ hybridization (FISH) or reverse transcription polymerase chain reaction of a gene fusion between members of the ETS family of genes, in particular ERG (ETS-related gene) and TMPRSS2, found in approximately one half of the usual prostatic adenocarcinomas.65 In a similar percent of cases, small cell carcinoma of the prostate is positive for TMPRSS2-ERG gene fusion by FISH.66–71 Importantly, it should be noted that compared with usual acinar carcinoma harboring TMPRSS2-ERG rearrangements, small cell carcinoma with TMPRSS2-ERG rearrangement is not reliably positive for ERG protein by IHC, presumably because of lack of AR expression in small cell carcinoma.66 In addition, in the setting of standard treatment for CRPC, ERG protein expression may not be present by IHC requiring the use of FISH. According to one study, there is strong and diffuse membrane staining for CD44 in all prostatic NE small cell carcinomas, whereas in usual prostatic adenocarcinomas only rare positive scattered tumor cells are CD44 positive.43 However, current work by one of the authors has not substantiated this finding and has concluded that this antibody is not useful in the distinction of high-grade adenocarcinoma of the prostate from small cell carcinoma.
The median cancer-specific survival of patients with small cell carcinoma of the prostate in 191 men according to the SEER database from 1973 to 2004 was 19 months; 60.5% of men presented with metastatic disease with a decreased survival related to stage; 2- and 5-year survival rates were 27.5% and 14.3%, respectively.72 Given the high rate of occult metastases, clinically localized small cell prostate cancer is typically treated aggressively, often with multimodality therapy, with chemotherapy and radiation similar to limited-stage small cell lung cancer. Metastatic small cell carcinoma of the prostate is treated with platinum-based combination chemotherapy with regimens similar to those used to treat small cell lung carcinoma.73–76 Some experts treat pure small cell carcinoma with chemotherapy alone, whereas others add ADT.
Summary
Small cell carcinoma of the prostate is an aggressive malignancy recognized by relatively typical morphologic features, although cases occurring in the prostate may exhibit a slightly wider spectrum of cytologic features than would be allowable at other tumor sites. In tumors showing classic morphology, IHC may not be necessary, although may be frequently useful for confirmation of the diagnosis in view of its important prognostic and therapeutic ramifications.
MIXED NE CARCINOMA—ACINAR ADENOCARCINOMA
Definition
Biphasic carcinoma with distinct, recognizable, admixed components of NE (small cell or large cell) carcinoma and usual conventional acinar adenocarcinoma; rarely, the adenocarcinoma component may have ductal or other variant features. Most cases that are encountered in clinical practice are mixed small cell carcinoma—acinar carcinoma.
It is not infrequent that tumors are mixed small cell carcinoma and adenocarcinoma of the prostate (Table 2) (Figs. 2E, F).59 In mixed cases, the transition between the small cell and acinar components is abrupt and each readily identifiable as distinctive. As with other unusual subtypes of prostate cancer, we do not assign a Gleason score to small cell carcinoma but only to the conventional adenocarcinoma component if untreated. In reported mixed cases, small cell carcinoma predominated (median: 80% of the tumor); the Gleason score of the adenocarcinoma was ≥8 in 85% of these cases.59 According to the SEER database, in a study of 191 men with prostatic small cell carcinoma, the presence of concomitant high-grade adenocarcinoma as opposed to lower-grade adenocarcinoma was an independent predictor of worse cancer-specific mortality.72 In this study, the relative amount of small cell carcinoma was not recorded. Most patients with mixed small cell carcinoma and adenocarcinoma present with metastatic castration-resistant disease. In these cases, whether mixed small cell and adenocarcinoma are treated differently compared with pure small cell carcinoma depends on the clinical scenario. Patients with metastatic mixed tumors that are clinically aggressive are often treated with both ADT and chemotherapy (platinum + etoposide or platinum + taxane).
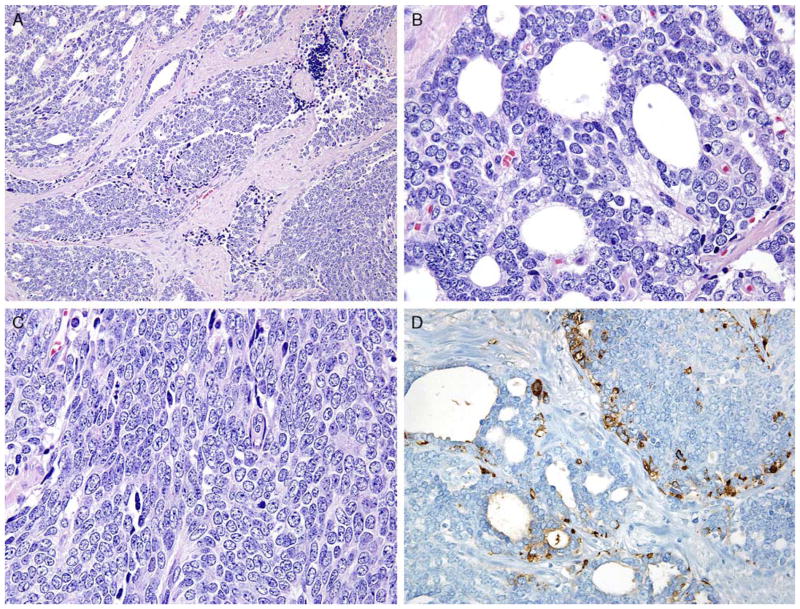
Uncommonly, a significant component or the entire prostatic tumor shows overlap between small cell carcinoma and usual prostate adenocarcinoma without discrete classic small cell carcinoma or usual prostate adenocarcinoma components (Fig. 3). It should not be surprising that these cases exist, as small cell carcinoma is currently thought to represent transdifferentiation from usual prostate adenocarcinoma.77,78 In these overlap cases, it is particularly difficult to determine whether they should be diagnosed as small cell carcinoma or Gleason pattern 5 adenocarcinoma. Tumor is typically arranged in sheets, but lumen formation can be seen without the apical cytoplasm seen in gland-forming adenocarcinoma. Cells typically have scant cytoplasm with smaller nucleoli than seen in Gleason pattern 5 adenocarcinoma yet more prominent than seen in small cell carcinoma. Mitotic figures are common. Typically, within the tumor there is a continuum of morphologies present, with some areas showing more features of small cell carcinoma and others that more closely resemble high-grade adenocarcinoma. IHC for NE markers shows positivity as is the staining for prostate markers (Table 2). These tumors are best recognized when there is morphologic concern for NE carcinoma (sheet-like architecture and scant cytoplasm) but in which the morphologic features, particularly the cytology, is not typical for either small cell carcinoma or conventional acinar carcinoma. As there has not been a specific category for these lesions, there are no data on their prognosis and optimal treatment, and further studies are encouraged. Consequently, the issue of appropriateness of Gleason grading for these cases has not been addressed. We recommend the following terminology for these cases: Prostate carcinoma with overlapping features of small cell and acinar adenocarcinoma.
FIGURE 3.
A, Case with overlapping features between small cell—usual adenocarcinoma with gland formation (upper left) and sheets of cells (lower right). B, Higher magnification of Figure 4A. Despite showing lumen formation, there is a lack of apical cytoplasm typical of usual adenocarcinoma of the prostate. C, Higher magnification of Figure 4A with solid sheets of cells. Cells have a high nuclear to cytoplasmic ratio like small cell carcinoma yet lack its high mitotic/apoptotic rate. Nucleoli are intermediate between small cell and usual adenocarcinoma of the prostate. D, Same case as Figure 4A with positivity for synaptophysin in both solid and glandular areas.
Summary
Mixed NE carcinoma—acinar adenocarcinoma is a high-grade, aggressive tumor. In tumors showing classic morphology, IHC may not be necessary, although it may be frequently useful for confirmation of the diagnosis in view of its important prognostic and therapeutic ramifications. As the role of potent AR-targeted therapies such as abiraterone acetate and enzalutamide in cases of metastatic castration-resistant mixed NE-adenocarcinoma is uncertain, it is recommended that the percentage and grade of the acinar component be provided. This information may be valuable for individual case management and as data for further studies.
LARGE CELL NE CARCINOMA
Definition
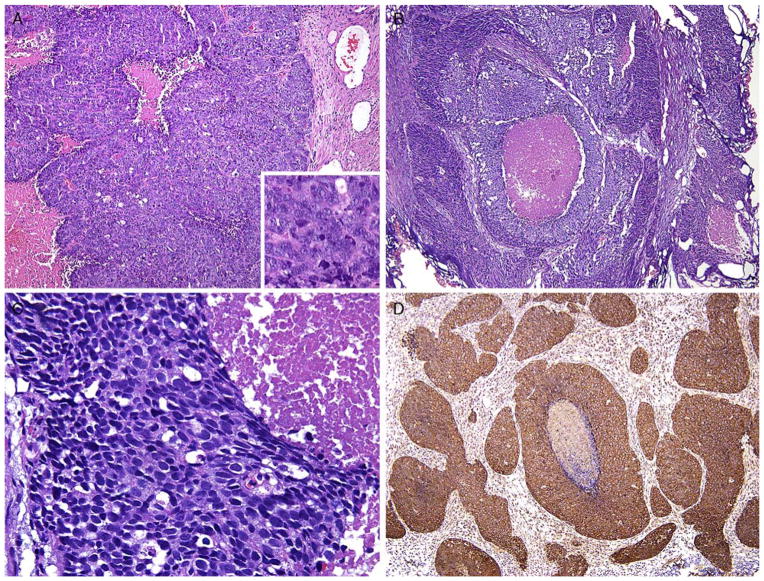
A high-grade tumor that shows NE differentiation its morphology consisting of large nests with peripheral palisading and often geographic necrosis, in which the cytology is that of non–small cell carcinoma (prominent nucleoli, vesicular clumpy chromatin, and/or large cell size and abundant cytoplasm) accompanied by a high mitotic rate (Fig. 4). NE differentiation is supported immunohistochemically with extensive staining with at least 1 NE marker (synaptophysin, chromogranin, CD56).
FIGURE 4.
A, LCNEC sheets of cells and geographic necrosis. Inset shows numerous prominent nucleoli. Tumor was diffusely positive for chromogranin. B, Another example of LCNEC with large nests of cells and geographic necrosis. C, Higher magnification of Figure 4A with cells having abundant cytoplasm. D, LCNEC (same case as Fig. 4A) with diffuse synaptophysin immunoreactivity.
Large cell NE carcinoma (LCNEC) of prostate is exceptionally rare, particularly its pure form. The largest series by Evans et al79 describes 7 cases of LCNEC, only 1 pure and apparently de novo. Six other cases represented progression from prior typical prostate adenocarcinoma, after long-standing hormonal therapy. According to the authors, the large cell NE component was composed of sheets and ribbons of amphophilic cells with large nuclei, coarse chromatin, and prominent nucleoli. Mitotic activity was high, and foci of necrosis were present. The LCNEC component was strongly positive for CD56, CD57, chromogranin A, synaptophysin, and P504S. Ki67 proliferative index was >50%. LCNEC has also been described in association with small cell carcinoma and adenocarcinoma.80
Importantly, in this series, a minor (<10%) component of conventional prostate adenocarcinoma showing hormonal deprivation effect was identified in all but the single de novo case. In the remainder of cases, the authors describe “hybrid features of both LCNEC and conventional-type prostatic adenocarcinoma” with treatment effect. They describe PSA and PSAP expression in the conventional component that was focal or absent in the LCNEC areas. Although prostate markers are usually negative in LCNEC, we have noted cases with all the hematoxylin and eosin and IHC features of LCNEC with PSA staining. Given that adenocarcinoma with Gleason score 5+5=10 may on occasion diffusely express NE markers immunohistochemically, it is the consensus that the definition of LCNEC should be more restrictive than what was reported by Evans and colleagues. In addition to IHC expression of NE markers, there should also be evidence of morphologic NE differentiation consisting of large nests of cells with peripheral palisading. Diagnosed accordingly, LCNEC is extremely rare. Given its rarity and that usual high-grade prostate adenocarcinoma with IHC expression of NE markers have incorrectly been included in the past as LCNEC, additional studies are needed to categorize the treatment and prognosis of LCNEC. In the study by Evans and colleagues cases were associated with rapid dissemination and death with metastatic disease at a mean of 7 months.
Summary
LCNEC is a rare aggressive malignancy in the prostate on the basis of very limited data. In the cases described to date, many such cases have been associated with usual adenocarcinoma and usually in a setting of prior therapy. Further study using standardized criteria and terminology is needed.
CRPC WITH SMALL CELL CARCINOMA–LIKE CLINICAL PRESENTATION
Genitourinary oncologists have noted certain clinical features characteristic of small cell carcinoma of the prostate. These clinical features are distinct from what is typically seen in usual prostate adenocarcinoma and are present in a significant proportion of morphologically heterogenous CRPCs. Some experts have hypothesized that prostate cancers that share clinical features with small cell prostate cancer (Table 3) also share its responsiveness to chemotherapy and underlying biology.75 These cases have been termed “anaplastic prostate cancer” by clinicians. The term “anaplastic” is unsatisfactory because it has a more specific meaning for surgical pathologists denoting pleomorphic cytology and could lead to additional confusion. Morphologically, cases with these clinical findings could be pure or mixed small cell carcinoma, yet could also consist of the typical histology of high-grade usual prostatic adenocarcinoma or LCNEC.81 As there is greater understanding, acceptance, and refinement of CRPC, it is anticipated that tumors within this clinical category will be further classified into molecularly defined pathologic subsets. The understanding of their biology will facilitate the establishment of an optical nomenclature that encompasses the clinical and pathologic spectrum of these tumors. The molecular paper emanating from this workshop will deal with genetic alterations that might help better define these tumors. With the introduction of new potent hormonal agents into the clinic, its incidence is anticipated to escalate.
TABLE 3.
Clinical Manifestations Associated With Small Cell Carcinoma
| Visceral metastases |
| Radiographically predominant lytic bone metastases by plain x-ray or CT scan |
| Bulky (5 cm) lymphadenopathy or bulky (≥5 cm) high-grade (Gleason ≥8) tumor mass in prostate/pelvis |
| Low PSA at initial presentation (before ADT or at symptomatic progression in the castrate setting) plus high-volume tumor burden. High level of serum chromogranin can be detectable |
| Short interval (≤6 mo) to androgen-independent progression after the initiation of hormonal therapy with or without the presence of NE markers |
| Any of the following in the absence of other causes: (A) elevated serum LDH (≥ 2× IULN); (B) malignant hypercalcemia; (C) elevated serum CEA (≥ 2× IULN) |
Adapted and modified from Aparicio et al.75 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
CT indicates computed tomography; LDH, lactate dehydrogenase.
IHC AND FISH IN THE DIAGNOSIS AND CLASSIFICATION OF NE DIFFERENTIATION IN PROSTATE CANCER
IHC plays a vital role and should be approached at 2 levels. For the issue of confirming NE differentiation, markers for NE differentiation include synaptophysin, chromogranin, and CD56. CD57 (Leu7) is expressed in a high percentage of acinar adenocarcinomas with and without NE differentiation and, along with NSE, are not recommended.
If there is any uncertainty about the histogenesis, that is, whether a tumor is primary to the prostate, markers for prostatic lineage—PSA, PSAP, PSMA, prostein (p501s), NKX3.1, ERG (by IHC or FISH)—may be used. In our opinion, PSA and ERG FISH detection would be the first line of approach.
Additional considerations for the role of IHC include diagnosis, prognosis, and predictive purposes. The formal utility of Ki67/MIB-1 IHC is not established; however, generally observed ranges are outlined in Table 2. Molecular studies of CRPC (which include cases showing NE differentiation) show alterations of AR signaling and loss of PSA expression by IHC. The IHC expression of AR across the proposed subtypes of NE carcinoma needs to be systematically evaluated such that its role in classification of these tumors may be determined. Promising new molecular targets that may be amenable to future IHC-based or FISH-based classification and predictive strategies include Aurora A kinase and N-Myc; however, these markers are not yet validated for clinical use.80,81
One final issue regarding the use of IHC in workup of these cases concerns the type of pathologic sample that is best for this analysis. In patients with multiple samples, including needle biopsies, radical prostatectomy, and sampling of a metastasis, the metastatic site and/or the histology of the sample most suspicious for NE differentiation should be evaluated.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocr Relat Cancer. 1999;6:503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsson PA, Wadstrom LB, Alumets J, et al. Peptidehormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract. 1987;182:298–307. doi: 10.1016/S0344-0338(87)80065-1. [DOI] [PubMed] [Google Scholar]
- 3.Bonkhoff H, Stein U, Remberger K. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol. 1993;423:291–294. doi: 10.1007/BF01606893. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi JG, Evans DJ. Argentaffin cells in prostatic carcinoma: differentiation from lipofuscin and melanin in prostatic epithelium. J Pathol. 1971;104:247–251. doi: 10.1002/path.1711040406. [DOI] [PubMed] [Google Scholar]
- 5.Kazzaz BA. Argentaffin and argyrophil cells in the prostate. J Pathol. 1974;112:189–193. doi: 10.1002/path.1711120310. [DOI] [PubMed] [Google Scholar]
- 6.Hirano D, Okada Y, Minei S, et al. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–592. doi: 10.1016/j.eururo.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39:135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: Morphogenesis, proliferation, and androgen receptor status. Prostate Suppl. 1998;8:18–22. [PubMed] [Google Scholar]
- 9.Bonkhoff H, Wernert N, Dhom G, et al. Relation of endocrineparacrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991;19:91–98. doi: 10.1002/pros.2990190202. [DOI] [PubMed] [Google Scholar]
- 10.Xing N, Qian J, Bostwick D, et al. Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate. 2001;48:7–15. doi: 10.1002/pros.1076. [DOI] [PubMed] [Google Scholar]
- 11.Berruti A, Mosca A, Porpiglia F, et al. Chromogranin A expression in patients with hormone naive prostate cancer predicts the development of hormone refractory disease. J Urol. 2007;178:838–843. doi: 10.1016/j.juro.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Mucci NR, Akdas G, Manely S, et al. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Hum Pathol. 2000;31:406–414. doi: 10.1053/hp.2000.7295. [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl JMed. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 15.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. WHO Classification of Tumors. Lyon, France: IARC Press; 2004. Pathology and Genetics: Tumors of the Urinary System and Male Genital Organs. [Google Scholar]
- 16.Komiya A, Suzuki H, Imamoto T, et al. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol. 2009;16:37–44. doi: 10.1111/j.1442-2042.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 17.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: Implications for new treatment modalities. Eur Urol. 2005;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Cohen RJ, Glezerson G, Haffejee Z. Prostate-specific antigen and prostate-specific acid phosphatase in neuroendocrine cells of prostate cancer. Arch Pathol Lab Med. 1992;116:65–66. [PubMed] [Google Scholar]
- 19.Berruti A, Mosca A, Tucci M, et al. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer. 2005;12:109–117. doi: 10.1677/erc.1.00876. [DOI] [PubMed] [Google Scholar]
- 20.Bostwick DG, Qian J, Pacelli A, et al. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol. 2002;168:1204–1211. doi: 10.1016/S0022-5347(05)64626-5. [DOI] [PubMed] [Google Scholar]
- 21.Theodorescu D, Broder SR, Boyd JC, et al. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80:2109–2119. [PubMed] [Google Scholar]
- 22.Weinstein MH, Partin AW, Veltri RW, et al. Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol. 1996;27:683–687. doi: 10.1016/s0046-8177(96)90398-6. [DOI] [PubMed] [Google Scholar]
- 23.Cohen RJ, Glezerson G, Haffejee Z. Neuro-endocrine cells—a new prognostic parameter in prostate cancer. Br J Urol. 1991;68:258–262. doi: 10.1111/j.1464-410x.1991.tb15318.x. [DOI] [PubMed] [Google Scholar]
- 24.Shariff AH, Ather MH. Neuroendocrine differentiation in prostate cancer. Urology. 2006;68:2–8. doi: 10.1016/j.urology.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Casella R, Bubendorf L, Sauter G, et al. Focal neuroendocrine differentiation lacks prognostic significance in prostate core needle biopsies. J Urol. 1998;160:406–410. [PubMed] [Google Scholar]
- 26.Abrahamsson PA, Cockett AT, di Sant’Agnese PA. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. Prostate Suppl. 1998;8:37–42. [PubMed] [Google Scholar]
- 27.Allen FJ, Van Velden DJ, Heyns CF. Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol. 1995;75:751–754. doi: 10.1111/j.1464-410x.1995.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 28.Ahlgren G, Pedersen K, Lundberg S, et al. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate. 2000;42:274–279. doi: 10.1002/(sici)1097-0045(20000301)42:4<274::aid-pros4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Tan MO, Karaoglan U, Celik B, et al. Prostate cancer and neuroendocrine differentiation. Int Urol Nephrol. 1999;31:75–82. doi: 10.1023/a:1007175924082. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MK, Arber DA, Coffield KS, et al. Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer. 1994;74:1899–1903. doi: 10.1002/1097-0142(19941001)74:7<1899::aid-cncr2820740712>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Ishida E, Nakamura M, Shimada K, et al. Immunohistochemical analysis of neuroendocrine differentiation in prostate cancer. Pathobiology. 2009;76:30–38. doi: 10.1159/000178153. [DOI] [PubMed] [Google Scholar]
- 32.Jeetle SS, Fisher G, Yang ZH, et al. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch. 2012;461:103–107. doi: 10.1007/s00428-012-1259-2. [DOI] [PubMed] [Google Scholar]
- 33.Aprikian AG, Cordon-Cardo C, Fair WR, et al. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J Urol. 1994;151:914–919. doi: 10.1016/s0022-5347(17)35121-2. [DOI] [PubMed] [Google Scholar]
- 34.Aprikian AG, Cordon-Cardo C, Fair WR, et al. Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer. 1993;71:3952–3965. doi: 10.1002/1097-0142(19930615)71:12<3952::aid-cncr2820711226>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 35.Weaver MG, Abdul-Karim FW, Srigley J, et al. Paneth cell-like change of the prostate gland. A histological, immunohistochemical, and electron microscopic study. Am J Surg Pathol. 1992;16:62–68. doi: 10.1097/00000478-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Tamas EF, Epstein JI. Prognostic significance of paneth cell-like neuroendocrine differentiation in adenocarcinoma of the prostate. Am J Surg Pathol. 2006;30:980–985. doi: 10.1097/00000478-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Adlakha H, Bostwick DG. Paneth cell-like change in prostatic adenocarcinoma represents neuroendocrine differentiation: Report of 30 cases. Hum Pathol. 1994;25:135–139. doi: 10.1016/0046-8177(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 38.Freschi M, Colombo R, Naspro R, et al. Primary and pure neuroendocrine tumor of the prostate. Eur Urol. 2004;45:166–169. doi: 10.1016/j.eururo.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 39.Giordano S, Tolonen T, Tolonen T, et al. A pure primary low-grade neuroendocrine carcinoma (carcinoid tumor) of the prostate. Int Urol Nephrol. 2010;42:683–687. doi: 10.1007/s11255-009-9660-8. [DOI] [PubMed] [Google Scholar]
- 40.Goulet-Salmon B, Berthe E, Franc S, et al. Prostatic neuroendocrine tumor in multiple endocrine neoplasia type 2B. J Endocrinol Invest. 2004;27:570–573. doi: 10.1007/BF03347481. [DOI] [PubMed] [Google Scholar]
- 41.Whelan T, Gatfield CT, Robertson S, et al. Primary carcinoid of the prostate in conjunction with multiple endocrine neoplasia IIb in a child. J Urol. 1995;153:1080–1082. [PubMed] [Google Scholar]
- 42.Zarkovic A, Masters J, Carpenter L. Primary carcinoid tumour of the prostate. Pathology. 2005;37:184–186. doi: 10.1080/14767050500058903. [DOI] [PubMed] [Google Scholar]
- 43.Slater D. Carcinoid tumour of the prostate associated with inappropriate ACTH secretion. Br J Urol. 1985;57:591–592. doi: 10.1111/j.1464-410x.1985.tb05878.x. [DOI] [PubMed] [Google Scholar]
- 44.Tash JA, Reuter V, Russo P. Metastatic carcinoid tumor of the prostate. J Urol. 2002;167:2526–2527. [PubMed] [Google Scholar]
- 45.Wasserstein PW, Goldman RL. Primary carcinoid of prostate. Urology. 1979;13:318–320. doi: 10.1016/0090-4295(79)90435-7. [DOI] [PubMed] [Google Scholar]
- 46.Murali R, Kneale K, Lalak N, et al. Carcinoid tumors of the urinary tract and prostate. Arch Pathol Lab Med. 2006;130:1693–1706. doi: 10.5858/2006-130-1693-CTOTUT. [DOI] [PubMed] [Google Scholar]
- 47.Turbat-Herrera EA, Herrera GA, Gore I, et al. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch Pathol Lab Med. 1988;112:1100–1105. [PubMed] [Google Scholar]
- 48.Egan AJM, Youngskin TP, Bostwick DG. Mixed carcinoidadenocarcinoma of the prostate with spindle cell carcinoid: the spectrum of neuroendocrine differentiation in prostatic neoplasia. Pathol Case Rev. 1996;1:65–69. [Google Scholar]
- 49.Lee S, Han JS, Chang A, et al. Small cell-like change in prostatic intraepithelial neoplasia, intraductal carcinoma, and invasive prostatic carcinoma: a study of 7 cases. Hum Pathol. 2013;44:427–431. doi: 10.1016/j.humpath.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Almagro UA. Argyrophilic prostatic carcinoma. case report with literature review on prostatic carcinoid and “carcinoid-like” prostatic carcinoma. Cancer. 1985;55:608–614. doi: 10.1002/1097-0142(19850201)55:3<608::aid-cncr2820550322>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 51.Azumi N, Shibuya H, Ishikura M. Primary prostatic carcinoid tumor with intracytoplasmic prostatic acid phosphatase and prostate-specific antigen. Am J Surg Pathol. 1984;8:545–550. doi: 10.1097/00000478-198407000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Ghali VS, Garcia RL. Prostatic adenocarcinoma with carcinoidal features producing adrenocorticotropic syndrome. immunohistochemical study and review of the literature. Cancer. 1984;54:1043–1048. doi: 10.1002/1097-0142(19840915)54:6<1043::aid-cncr2820540619>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 53.Ghannoum JE, DeLellis RA, Shin SJ. Primary carcinoid tumor of the prostate with concurrent adenocarcinoma: a case report. Int J Surg Pathol. 2004;12:167–170. doi: 10.1177/106689690401200214. [DOI] [PubMed] [Google Scholar]
- 54.Stratton M, Evans DJ, Lampert IA. Prostatic adenocarcinoma evolving into carcinoid: selective effect of hormonal treatment? J Clin Pathol. 1986;39:750–756. doi: 10.1136/jcp.39.7.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojas-Corona RR, Chen LZ, Mahadevia PS. Prostatic carcinoma with endocrine features. A report of a neoplasm containing multiple immunoreactive hormonal substances. Am J Clin Pathol. 1987;88:759–762. doi: 10.1093/ajcp/88.6.759. [DOI] [PubMed] [Google Scholar]
- 56.Montasser AY, Ong MG, Mehta VT. Carcinoid tumor of the prostate associated with adenocarcinoma. Cancer. 1979;44:307–310. doi: 10.1002/1097-0142(197907)44:1<307::aid-cncr2820440152>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Reyes A, Moran CA. Low-grade neuroendocrine carcinoma (carcinoid tumor) of the prostate. Arch Pathol Lab Med. 2004;128:e166–e168. doi: 10.5858/2004-128-e166-LNCCTO. [DOI] [PubMed] [Google Scholar]
- 58.Sobin LH, Hjermstad BM, Sesterhenn IA, et al. Prostatic acid phosphatase activity in carcinoid tumors. Cancer. 1986;58:136–138. doi: 10.1002/1097-0142(19860701)58:1<136::aid-cncr2820580124>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 60.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Yao JL, Madeb R, Bourne P, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30:705–712. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Ro JY, Tetu B, Ayala AG, et al. Small cell carcinoma of the prostate. II. immunohistochemical and electron microscopic studies of 18 cases. Cancer. 1987;59:977–982. doi: 10.1002/1097-0142(19870301)59:5<977::aid-cncr2820590521>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 63.Agoff SN, Lamps LW, Philip AT, et al. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13:238–242. doi: 10.1038/modpathol.3880044. [DOI] [PubMed] [Google Scholar]
- 64.Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24:1217–1223. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 66.Lotan TL, Gupta NS, Wang W, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol. 2011;24:820–828. doi: 10.1038/modpathol.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo CC, Dancer JY, Wang Y, et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum Pathol. 2011;42:11–17. doi: 10.1016/j.humpath.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williamson SR, Zhang S, Yao JL, et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: Evidence supporting monoclonal origin. Mod Pathol. 2011;24:1120–1127. doi: 10.1038/modpathol.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheble VJ, Braun M, Wilbertz T, et al. ERG rearrangement in small cell prostatic and lung cancer. Histopathology. 2010;56:937–943. doi: 10.1111/j.1365-2559.2010.03564.x. [DOI] [PubMed] [Google Scholar]
- 71.Schelling LA, Williamson SR, Zhang S, et al. Frequent TMPRSS2-ERG rearrangement in prostatic small cell carcinoma detected by fluorescence in situ hybridization: the superiority of fluorescence in situ hybridization over ERG immunohistochemistry. Hum Pathol. 2013 doi: 10.1016/j.humpath.2013.05.005. ▪:▪. [DOI] [PubMed] [Google Scholar]
- 72.Deorah S, Rao MB, Raman R, et al. Survival of patients with small cell carcinoma of the prostate during 1973–2003: A populationbased study. BJU Int. 2012;109:824–830. doi: 10.1111/j.1464-410X.2011.10523.x. [DOI] [PubMed] [Google Scholar]
- 73.Amato RJ, Logothetis CJ, Hallinan R, et al. Chemotherapy for small cell carcinoma of prostatic origin. J Urol. 1992;147:935–937. doi: 10.1016/s0022-5347(17)37427-x. [DOI] [PubMed] [Google Scholar]
- 74.Rubenstein JH, Katin MJ, Mangano MM, et al. Small cell anaplastic carcinoma of the prostate: seven new cases, review of the literature, and discussion of a therapeutic strategy. Am J Clin Oncol. 1997;20:376–380. doi: 10.1097/00000421-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–3080. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 77.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 78.Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evans AJ, Humphrey PA, Belani J, et al. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–693. doi: 10.1097/00000478-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Aparicio A, Tzelepi V, Araujo JC, et al. Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: morphological, immunohistochemical, and gene expression profiles. Prostate. 2011;71:846–856. doi: 10.1002/pros.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatmentrelated neuroendocrine prostate cancer. Neoplasia. 2013;15:1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]