Abstract
Mouse embryonic stem cells (mESCs) are critical tools for genetic engineering, development of stem cell based therapies, and basic research on pluripotency and early lineage commitment. However, successful derivation of germline-competent embryonic stem cell lines has, until recently, been limited to a small number of inbred mouse strains. Recently, there have been significant advances in the field of embryonic stem cell biology, particularly in the area of pluripotency maintenance in the epiblast from which mESCs are derived. Here we describe a protocol for efficient derivation of germline competent mESCs from any mouse strain, including strains previously deemed non-permissive. We provide a primary method that is generally applicable to most inbred strains, as well as an alternative method for non-permissive strains. Using this protocol, mESCs can be derived in 3 weeks and fully characterized after an additional 12 weeks, at efficiencies as high as 90% and in any strain background.
Introduction
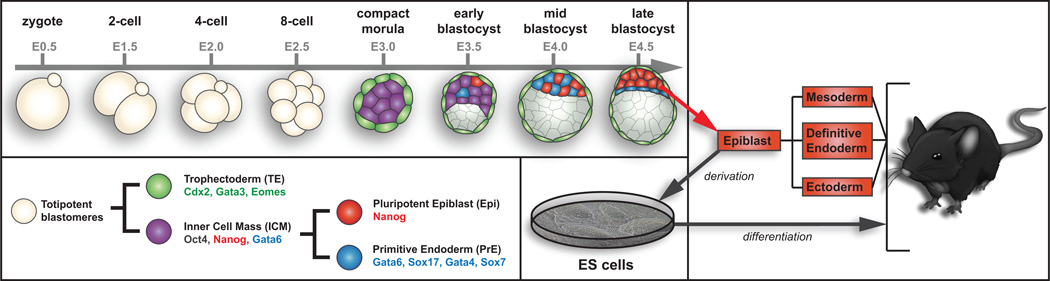
Embryonic stem cells (ESCs) are the ex vivo equivalent of the epiblast lineage of the blastocyst and therefore, share the same developmental potential to differentiate into any one of the three primary germ layers, mesoderm, definitive endoderm and ectoderm (Figure 1). This developmental pluripotency combined with a high capacity for self-renewal in vitro are defining features of ESCs. Mouse embryonic stem cells (mESCs) are derived from pre-implantation stage embryos 1,2. The progenitor cells that give rise to mESCs reside in the epiblast of the late blastocyst (~4 days post coitum) and express several pluripotency-associated factors, including Oct3/4 (Pou5f1) and Nanog 3. In addition to their capacity for self-renewal and stable pluripotency in vitro, mESCs have the defining capacity to populate the germline after microinjection into, or aggregation with, host embryos, making mESCs essential tools for genetic engineering 4. The Nobel prize-winning discovery that genes could be genetically modified in mice using mESCs was published over 30 years ago and since, nearly 50,000 genetically modified alleles have been created by individual investigators around the world and by the International Knockout Mouse Consortium (IKMC, www.knockoutmouse.org), which endeavors to create null and/or conditional null alleles for every gene in the mouse genome 5,6. mESCs are also used for basic research on pluripotency and for development of stem cell based therapies as the starting material for directed differentiation of enriched, defined cell types in vitro.
Figure 1. Overview of pre-implantation development in mice.
The pre-epiblast lineage in the early embryo is defined by lineage-restricted expression of the Oct3/4, Nanog and Gata6 genes. As early lineage specification proceeds, the pluripotent epiblast lineage is defined by Nanog expression. The epiblast lineage will give rise to all three definitive germ layers of the embryo-proper, namely all somatic cells and germ cells, and is the population from which mESCs are derived. mESCs cell lines retain the developmental potential of the epiblast lineage and as such, can contribute to all three germ layers and the germline of host blastocyst or morula stage embryos.
Derivation of mESCs
Despite knowledge of the basic requirements for mESCs to maintain pluripotency, derivation of mESCs remained inefficient and was limited to just a few mouse strains for many years 7. These, so-called permissive strains included 129 sub-strains, as well as the most commonly used inbred mouse strain, C57BL6. Early protocols demonstrated the requirement of leukemia inhibitory factor (LIF) to activate STAT3, bone morphogenic protein (BMP) (or serum), and mitotically inactivated feeder layers, preferably mouse embryonic fibroblasts (MEFs), to prevent differentiation of mESCs in vitro. However, derivation efficiency in permissive strains was at best 30%, as determined by the percentage of embryos giving rise to stable mESC lines 8,9. Moreover, in non-permissive mouse strains, like CBA, NOD, DBA and others derivation efficiency was either extraordinarily low or non-existent 7. Therefore, for mammalian geneticists who rely on specific inbred strain backgrounds for human disease modeling, genetically engineered alleles created in 129, C57BL6 or hybrid ES cells required 10 to 20 backcross generations (up to 2 years) to create the desired genetic background. Moreover, for mammalian species other than the mouse, genetic engineering was simply not possible due to an inability to derive legitimate ES cells despite considerable effort over many years. Recent advances in site-specific nuclease technologies (e.g ZFN, TALEN, CRISPR/Cas) are enabling direct, targeted deletion and targeted, sequential gene modifications via pronuclear injection of mouse embryos 10 and other species, including rat11,12. For genetic engineering, these technologies circumvent the need for ES cells. However, the applicability for multifaceted genomic modifications via homologous recombination with large inserts, across a variety of strains has not yet been demonstrated.
Derivation of mESCs from non-permissive strains
To overcome strain and species limitations to ES cell derivation, a variety of approaches have been used. For example, based on the premise that the presence of primitive endoderm caused loss of pluripotency in mES cell progenitors within the inner cell mass, careful excision of the epiblast by biopsy or immunosurgery was shown to improve derivation efficiency 3,13. In addition, for many years, delayed implantation or diapause induction by ovariectomy or tamoxifen injection was also used to promote derivation efficiency possibly via developmental stasis during which epiblast have an opportunity to expand. Finally, as knowledge of the genes required for early lineage specification and pluripotency has grown, protocols for efficient derivation of mES cells by promoting or inhibiting expression of specific genetic pathways were developed. Oct4 (Pou5f1) is a transcription factor that is essential for the maintenance of pluripotency in cells of the inner cell mass (ICM), the epiblast and in mES cell lines. Importantly, loss of Oct4 was shown to be a feature of cultured embryos that failed to give rise to stable ES cell lines 14. Based on this discovery, culture conditions that promote Oct4 expression, namely inhibition of the MAP kinase pathway, were introduced. However, successful derivation of mES cells from the recalcitrant strain background, CBA, still required a combination of diapause induction, epiblast excision and inhibition of MEK kinase via PD98059 14. In the context of these modifications to traditional ES cell derivation protocols, derivation efficiency in CBA was ~25%, a significant advance for a non-permissive strain 14.
The pluripotent ground state and overcoming barriers to mESC derivation
The discovery that self-renewal and pluripotency are intrinsic properties of mESCs was later demonstrated by Austin Smith and colleagues14, who showed that inhibition of MEK/ERK and glycogen synthase kinase-3 (GSK3) signaling (3i: PD184353, PD173074 / SU5402 and CHIR99021 respectively) were together sufficient, combined with activation of STAT3 by LIF (3i/LIF), to promote the pluripotent ground state of emergent ESCs from mice and from rats 15–17. These laboratories went on to show that inhibition of FGF receptor signaling is dispensible in the context of more potent inhibition of MEK signaling (2i: CHIR99021 to inhibit GSK3β and PD0325901 to inhibit MEK1/2)16. Both 3i/LIF and, subsequently, 2i/LIF culture conditions have since been successfully applied for efficient (50–70%) derivation of germline competent mESCs from recalcitrant strains like NOD, CBA and DBA 18–21. Moreover, these culture conditions have been used to successfully derive germline competent rESCs from rat embryos 16,17, an accomplishment that quickly led to the creation of the first rat gene knockout by homologous recombination in rESCs 22. Successful derivation of ESCs from recalcitrant strains and from rat using 2i/LIF culture conditions suggests that emergent ESCs from these strains / species are unable to maintain a pluripotent ground state under traditional ESC culture conditions (serum +LIF). In fact, it was later shown that unlike emergent ESCs from permissive strain background (e.g. 129), emergent ESCs from non-permissive strain backgrounds (e.g. NOD) are unstable and differentiate to a more advanced, EpiSC (post-implantation, epiblast stem cell) state, which has been termed a primed pluripotent state, in the absence of exogenously provided inhibitors of ERK signaling 23.
Although the basis of strain and species recalcitrance to ESC derivation is not yet fully understood, these results suggest that inhibition of the pathways responsible for differentiation of inner cell mass epiblast cells to post-implantation epiblast cells might be sufficient to overcome barriers to mESC derivation in all inbred strain backgrounds. This new model of the pluripotent, ground state of ESCs is an important advance in our understanding of early lineage commitment and has informed our mESC derivation protocol, which is highly efficient, regardless of strain background.
Experimental Design
We previously published efficient derivation of germ line competent mESC lines from the recalcitrant strain DBA/2J20. Crucial to the success of this protocol was the exclusion of serum during the outgrowth phase, combined with inhibition of MEK / ERK (1i: PD98059) signaling during the outgrowth phase and during subsequent culture of emergent ES cell lines (3i: CHIR99021, PD173074 and PD032901). Since published data later showed the FGF receptor inhibitor, PD173074, to be dispensible and the MEK inhibitor, PD98059, redundant, in the context of the more potent MEK inhibitor, PD032901 16, our current protocol utilizes the now standard 2i combination (CHIR99021 and PD032901) to achieve the same exogenous inhibition with simpler media formulae.
Our protocol begins with the harvest and culture of late blastocyst stage embryos, which can be generated by natural mating or by in vitro fertilization. These embryos are then cultured in derivation medium to allow for ICM outgrowth. Unlike traditional ESC derivation medium, which contains serum, our derivation medium utilizes serum replacement in the form of an artificial serum replacement or, in the case of non-permissive strain backgrounds, we use defined serum free medium 24. The exclusion of serum from the derivation medium was previously shown to promote mESC derivation efficiency 9,25 and we have found that it promotes NANOG expression in ICM outgrowths (Figure 2). NANOG, which is essential for acquisition and maintenance of pluripotency, is a biomarker of mESC progenitor cells and is a more reliable readout of cell potency than OCT3/4 14,26. Upon ICM disaggregation, incipient mESC lines are cultured either in traditional ESC medium +2i/LIF (variant A, step 10A) or in defined serum free media +2i/LIF (as described by Silva et al18, Ying and Smith24 and this protocol, variant B step 10B). We, and others, have found the latter culture conditions to be essential for robust derivation of ES cell lines from the recalcitrant NOD and it’s derivative strains (NSG and NRG) (Table 1)19. Although the original defined, serum free +2i/LIF conditions were created for feeder free derivation and culture, feeder layers improve derivation efficiency, promote colony attachment (which is favorable for sub-cloning, a key manipulation in gene targeting experiments) and may provide enhanced karyotypic stability. Therefore, we employ feeder layers of mitotically inactivated embryonic fibroblasts. Importantly, this protocol provides all of the steps necessary to derive and establish low passage (P3) ES cell lines from any inbred strain background, as well as detailed instructions for quality assurance and characterization. Regardless of the culture conditions, pluripotency and euploidy degrade with increasing passage number in mESCs (P20 and higher). Therefore, newly established mESC lines should be maintained with an eye towards maximizing and preserving low passage stocks.
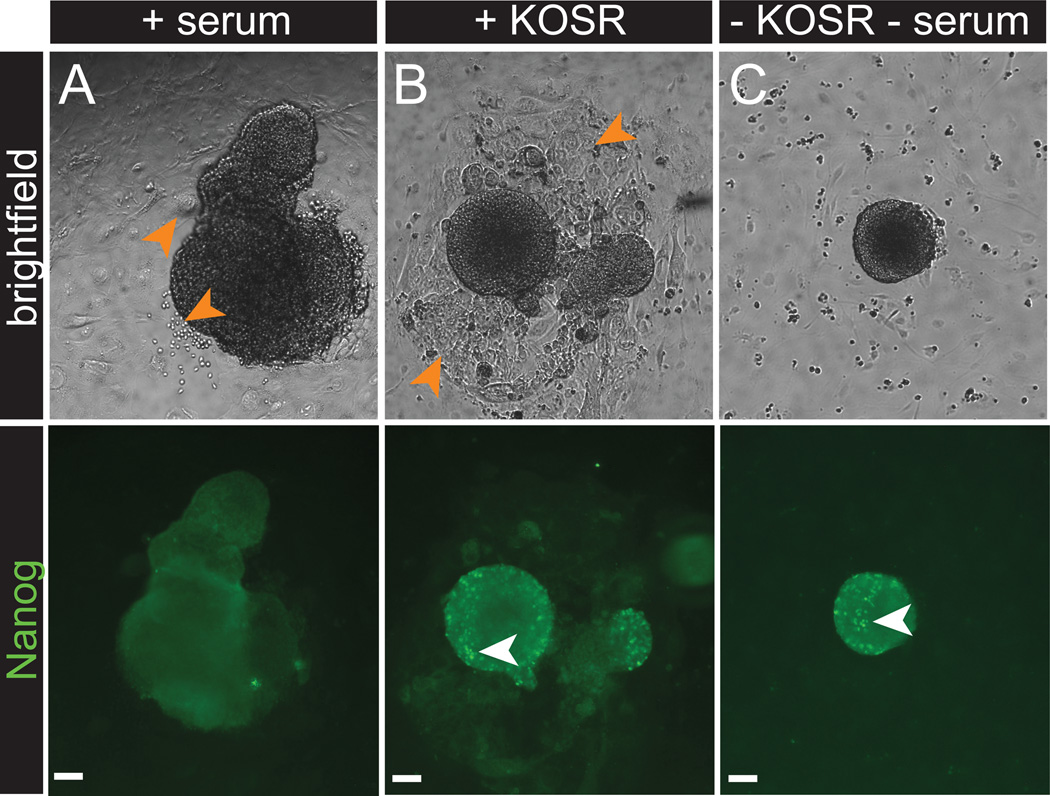
Figure 2.
NANOG immunolabeling in inner cell mass (ICM) outgrowths grown in the presence of 2i/LIF with or without serum or KOSR. ICM outgrowths grown in traditional derivation medium containing serum (A) have minimal NANOG expression (B) and exhibit differentiation (orange arrows). (B) Outgrowths grown in the presence of KOSR (knockout serum replacement) contain NANOG expressing cells (white arrows) but also exhibit differentiation (B, orange arrows). (C) Outgrowths grown in serum free media exhibit NANOG expressing cells (white arrows) with minimal differentiation. Scale bars, 50 µm. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
Table 1.
Derivation efficiencies in selected strain backgrounds using traditional derivation conditions and this protocol.
| Strain Background |
Reported efficiency in standard ES cell derivation conditions (serum, LIF31) |
Reference | Efficiency achieved using this protocol |
|---|---|---|---|
| DBA | 1% (DBA/1lacJ) | 32 | 60% (ref. 20) (DBA/2J) |
| BALB/c | 2.4% | 7 | 90%a (BALB/cJ) |
| C3H | 3% | 33 | 90%a (C3H/HeJ) |
| NOD | 1.5% | 34 | 75%a,b (NOD/ShiLtJ) |
| NSG | Unreported | 34 | 65% ab (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) |
| A/J | Unreported | NA | 90% a |
| PWD/J | Unreported | NA | 60% a |
| C57BL6 | 17-80% | 3,7,35 | 100% a (C57BL/6J) |
| CBA | 0% | 14 | Not attempted |
Sub-strains are provided if known / reported. Efficiency is the percentage of disaggregated embryos that gave rise to stable mESC lines, as defined by morphology and survival through passaging. In the literature, efficiency is defined by the number of stable mESCs generated from the number of embryos collected. With this protocol, the vast majority (90%) of embryos give rise to an outgrowth, but not every outgrowth is disaggregated due to budget constraints. Therefore an efficiency calculation based on number of embryos harvested would be a gross underestimate.
Our own unpublished data.
Required serum free culture conditions (as descried by Ying and Smith24 and this protocol, variant B).
Presumed similar permissiveness to NOD as NSG is a derivative strain.
MATERIALS
REAGENTS
Derivation of mES cells
Pregnant mouse, 3.5 days post coitum (d.p.c) CAUTION: Experiments involving rodents must conform to all relevant governmental and institutional (IACUC) standard operating procedures and regulations
Mouse embryonic fibroblasts (MEFs) (see reagent setup)
Mitomycin C (Sigma, M0503, prepare 1 mg/ml stock in PBS and store at 4°C for up to 6 months)
2-Mercaptoethanol 55mM (Invitrogen, cat. no. 21985-023) CAUTION: This reagent is a biohazard; adequate safety instructions should be taken when handling.
B-27 supplement (Invitrogen, cat. no. 17504-044)
CHIR99021 (Stemgent, cat. no. 04-0004) (see reagent setup)
Defined Trypsin Inhibitor (Invitrogen, cat. no. R-007–100)
Dulbecco’s Modified Eagle Medium (DMEM), high glucose, no glutamine, no sodium pyruvate (Invitrogen, cat. no. 11960069)
DMEM/F-12 medium (Invitrogen, cat. no. 11320-033)
Dimethyl sulfoxide, DMSO (Sigma-Aldrich, cat. no. D2650) CAUTION: This reagent is a biohazard; adequate safety instructions should be taken when handling
Ethanol, 70% (v/v) (Sigma Aldrich, cat. no. E7023) CAUTION: This reagent is a highly flammable and a skin and eye irritant.
Fetal bovine serum (FBS), ES grade (Lonza, cat. no. 14-501F) CRITICAL: FBS should be lot tested for maximum ES cell viability and growth. Filter through 0.45 µm filter and store 25 ml single-use aliquots at −20°C for up to 1 year.
Gelatin, 0.1% (STEMCELL Technologies, cat. no. 07903)
GlutaMAX (Invitrogen, cat. no. 35050061)
KOSR (Invitrogen, cat. no. 10828-028), store 25 ml single-use aliquots at −20°C for up to 1 year.
EmbryoMax KSOM Embryo Culture (1X) medium, powder (Millipore, cat. no. MR-020-P)
Leukemia inhibitory factor (LIF) 107 units (Millipore, cat. no. ESG1107)
EmbryoMax M2 Medium (1X), powder (Millipore, cat. no. MR-015-D)
MEM Non-Essential Amino Acid (NEAA) solution (Invitrogen, cat. no. 11140050)
N-2 supplement (Invitrogen, cat. no. 17502-048)
Neurobasal medium (Invitrogen, cat. no. 21103-049)
Phosphate buffered saline (PBS) without calcium and magnesium (Invitrogen, cat. no. 20012027)
PD0325901 (Stemgent, cat. no. 04-0006) (see reagent setup)
100X Penicillin Streptomycin (Pen Strep) (Invitogen, cat. no. 15140122)
Sodium Pyruvate, 100 mM (Invitrogen, cat. no. 11360070)
Trypsin-EDTA, 0.05% (Invitrogen, cat. no. 25300054)
Karyotyping
Colcemid, 10 µg/mL (Invitrogen, cat. no. 15212-012)
Glacial Acetic Acid (Sigma Aldrich, cat. no. A6283) CAUTION: This reagent is a biohazard and severe irritant; adequate safety instructions should be taken and all work with this chemical should be conducted in a fume hood.
KCL, 0.56% V/V (Sigma Aldrich, cat. no. P9541)
Methanol (Sigma Aldrich, cat. no. 494437) CAUTION: This reagent is a biohazard; adequate safety instructions should be taken when handling
Vectashield hard set mounting medium with DAPI (Vector Laboratories, cat. no. H-1500)
Immunolabeling
4',6-diamidino-2-phenylindole (DAPI) (Invitrogen, cat. no. D1306) (see reagent setup) or Hoechst (Invitrogen, cat. no. 33342).
FBS (Lonza, cat. no. 14-501F)
Goat anti-mouse IgG Alexa Fluor 488 (Invitrogen, cat. no. A11029)
Goat anti-rabbit IgG Alexa Fluor 488 (Invitrogen, cat. no. A11034)
-
Goat anti-mouse IgM Alexa Fluor 488 (Invitrogen, cat. no. A21042
CRITICAL: Secondary antibodies conjugated to fluorescent dyes are light sensitive. A broad array of Alexa fluorophores (Invitrogen) are available depending on the desired excitation / emission spectra. Moreover, alternatives to Alexa dyes are widely available, including FITC, Texas Red, rhodamine, etc.
Mouse IgG anti-Oct3/4 antibody (Santa Cruz Biologicals, cat. no sc-5279) (see reagent setup)
Mouse IgM anti-SSEA-1 antibody (Santa Cruz Biologicals, cat. no. sc-21702) (see reagent setup)
Rabbit IgG anti-NANOG antibody (Abcam, cat. no. ab21603)
PBS without calcium and magnesium (Invitrogen, cat. no. 20012027)
Paraformaldehyde, 4% (PFA) (Electron Microscopy Sciences, cat. no. 157-4) CAUTION: This reagent is a biohazard; adequate safety instructions should be taken when handling
Triton-X (Promega, cat. no, H5142)
SNP Panel/Genotyping
DNeasy blood and tissue kit (Qiagen, cat. no. 69506)
Tris, 1M pH 8.0 (Invitrogen, cat. no. AM9856)
Pathogen testing
Sabouraud Dextrose Agar deep fill plates (BD, cat. no. 221180)
Tryptose Phosphate Broth (Sigma Aldrich, cat. no. T8159)
EQUIPMENT
0.22 µM Filter unit such as Stericup-GV (Millipore, cat. no. SCGVU05RE)
Biosafety cabinet (e.g. Nuaire, model NU-425-400 or equivalent)
Calibrated, glass micropipets, 50 µl (Drummond Scientific, cat. no. 2-000-050) note: aspirator apparatus required for mouth-controlled embryo transfer pipet is supplied with the micropipets (see equipment setup). Alternatively, 9” glass Pasteur pipets, pulled over a flame can be assembled with a plastic mouthpiece and P1000 pipet tip as shown in Figure 3.
Cell counter, automatic (e.g Nexcelom Cellometer Auto T4) or manual haemocytometer (e.g. Reichert Bright-Line, Fisher Scientific, cat. no. 02-671-5)
Conical tubes, 15 mL (USA Scientific, cat. no. 1475-0511)
CoolCell LX (biocision, cat. no. BCS-405)
Cover glass, 24×60 mm (Fisher Scientific, cat. no. 12-548-5P)
Cryovials, 2 mL (USA Scientific, cat. no. 5612-2263)
- Dissection tools
-
○Micro-dissecting scissors (e.g. Roboz, cat. no. RS-5610)
-
○Forceps, 2 pairs (e.g. Roboz cat. no. RS-4984)
-
○
Microscope, inverted (e.g. Nikon, model TS100 or equivalent)
Microscope, stereo (e.g. Leica, model MZ12.5 or equivalent)
Microscope slides (Fisher Scientific, cat. no. 12-550-15)
Pasteur pipets, borosilicate, 9”, unplugged (Fisher Scientific, cat. No. 13-678-20C)
Serological pipettes, 5 mL (USA Scientific, cat. no. 1075-0810)
Serological pipettes, 10 mL (USA Scientific, cat. no. 1071-0810)
Syringes, 5 mL (BD, cat. no. 309646) with 27G needle (BD, cat. no. 305109)
Tissue culture treated plate, 4-well (Corning life sciences, cat. no. 353654)
Tissue culture treated plate, 6-well (USA Scientific, cat. no. 5665–7160)
Tissue culture treated dish, 35 mm (USA Scientific, cat. no. CC7682-3340)
Tissue culture treated dish, 60 mm (USA Scientific, cat. no. CC7682-3359)
Tissue culture treated dish, 100 mm (USA Scientific, cat. no. 5666-4160)
Vacuum Pump (Fisher Scientific, cat. no 13-878-40 or equivalent)
Water bath (Thermo Scientific, model 2864 or equivalent)
Water Jacketed CO2 incubator, 37°C, 5% CO2 with HEPA filtration and 95% humidity (Thermo Scientific, model 3120 or equivalent)
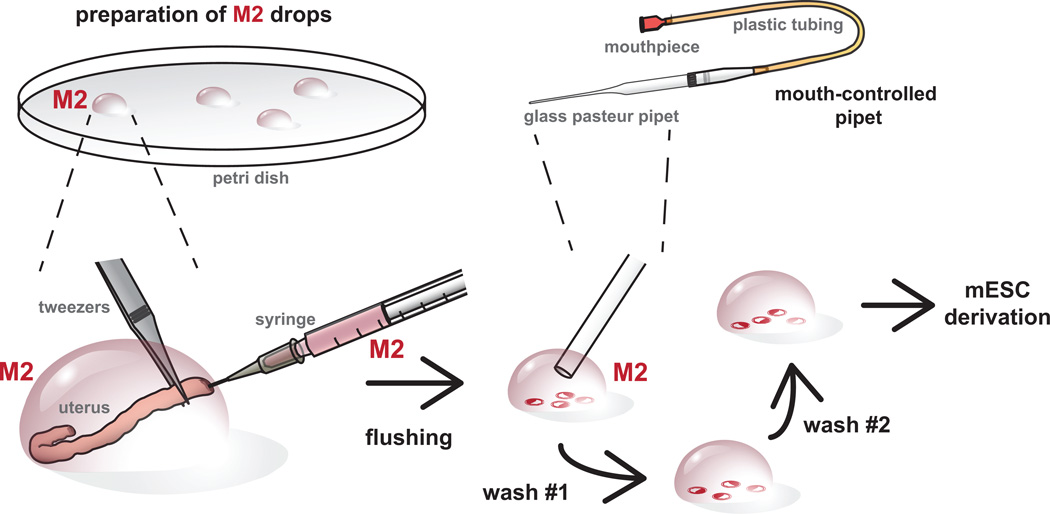
Figure 3.
Overview of blastocyst stage embryo harvest. Embryos are flushed from the uteri at 3.5 – 3.75 days post coitum (d.p.c.) using M2 media. Embryos are pooled and washed through through a series of M2 drops using a mouth-controlled pipet prior to plating for ES cell derivation. It is important to get rid of tissue debris prior to embryo plating. It also provides an opportunity to assess the stage and quality of embryos recovered for stem cells derivation. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
REAGENT SETUP
Cryopreservation medium is 80% culture medium (FBS or serum free ES cell medium, depending on the medium used for the mESC line), 10% (v/v) FBS and 10% (v/v) DMSO. Store medium at 4°C for up to one week.
EmbryoMax KSOM and M2 embryo culture media are made freshly according to the manufacturer’s instructions on the day of embryo harvest. CRITICAL Reagent must be freshly prepared.
KOSR ES medium is DMEM high glucose supplemented with either 15% (v/v) KOSR, 1X Pen Strep, 2 mM GlutaMAX, 1 mM sodium pyruvate, 0.1 mM MEM NEAA, 0.1 mM 2-Mercaptoethanol, 103 IU LIF, 1 µM PD0325901, and 3 µM CHIR99021. Sterilize medium through a 0.22 µM filter. Store at 4°C for up to one week, protected from light.
FBS ES medium is DMEM high glucose supplemented with 15% (v/v) FBS, 1X Pen Strep, 2 mM GlutaMAX, 1 mM sodium pyruvate, 0.1 mM MEM NEAA, 0.1 mM 2-Mercaptoethanol, 10^3 IU LIF, 1 µM PD0325901, and 3 µM CHIR99021. Sterilize medium through a 0.22 µM filter. Store at 4°C for up to one week, protected from light.
Serum Free ES medium is a 1:1 mixture of DMEM-F12/N2 (DMEM-F12 supplemented with N-2) and Neurobasal/B27 (Neurobasal supplemented with B27) with 1X (v/v) Pen Strep, 1 mM GlutaMAX, 0.5 mM sodium pyruvate, 0.1 mM MEM NEAA, 0.1 mM 2-Mercaptoethanol, 10^3 IU LIF, 1 µM PD0325901, and 3 µM CHIR99021. Sterilize medium through a 0.22 µM filter. Store at 4°C for up to one week, protected from light.
MEF medium is DMEM high glucose supplemented with 10% (v/v) FBS, 1% (v/v) Pen Strep, and 2 mM GlutaMAX. Sterilize medium through a 0.22 µM filter. Store at 4°C for up to one week, protected from light.
CHIR99021 Prepare a stock solution of 30 mM by resuspending 2 mg in 140 µl of DMSO. Aliquot single use volumes and store at −20°Cfor up to 6 months.
PD0325901 Prepare a stock solution of 10 mM by resuspending 2 mg in 414 µl of DMSO. Aliquot single use volumes and store at −20°C for up to 6 months.
3:1 Methanol:Glacial Acetic Acid In a fume hood, mix methanol and glacial acetic acid at a ratio of 3:1. Use 10 ml of fresh fixative per cell line. Keep on ice until ready to use. CRITICAL: Reagent must be freshly prepared.
Blocking Buffer is PBS with 5% FBS and 0.1% triton-X. Store at 4°C for up to one week.
DAPI stock solution Prepare a stock solution by dissolving 10 mg of DAPI in 2 ml of sterile molecular biology-grade water. Store this solution at 4°C for up to 1 year, protected from light. Use at a dilution of 1:2500.
Primary Antibody Solution Dilute primary antibodies 1:250 in PBS with 1% FBS. Working concentrations for antibodies can vary depending on the supplier and/or lot and therefore, may need to be determined empirically. Store diluted antibodies at 4°C for up to one month. To avoid repeated freeze thawing cycles, aliquot and store undiluted antibody at −20°C.
Secondary Antibody Solution Dilute secondary antibodies 1:1000 in PBS with 1% FBS. Working concentrations for antibodies can vary depending on the supplier and/or lot and therefore, may need to be determined empirically. Store diluted antibodies at 4°C for up to one month. To avoid repeated freeze thawing cycles, aliquot and store undiluted antibody at −20°C.
Mouse Embryonic Fibroblasts Derive mouse embryonic fibroblasts from embryonic day (E) 12.5–14.5 embryos that have been decapitated and eviscerated. Harvest embryos from a pregnant mouse 12.5–14.5 days post coitum (d.p.c). Wash decapitated and eviscerated embryos in PBS and place them in a clean 100mm culture dish containing 3–5 ml of 0.05% Trypsin-EDTA. Coarsely mince the embryos using sterile forceps and a razor blade. Draw the resulting fragments into a 10 ml syringe and pass through a 16-gauge needle 2–3 times to reduce the tissue to a slurry. Add the slurry to a 50 ml conical tube containing an equal volume of MEF medium and allow any remaining large fragments to settle. Transfer the contents (avoiding settled material) to a new 50 ml conical tube and centrifuge at 150 × g for 5 min. Resuspend the pellet in MEF medium, count cells and plate onto 100 mm, gelatinized dish (expect a yield of ~1 × 106 cells per embryo and plate at ~1×105 cells / cm2, which is approximately one 100 mm dish per embryo). When confluent (~2 days), passage 1:3 using 0.05% Trypsin-EDTA. After 3 passages, mitotically inactivate the MEFs using mitomycin-C or γ-irradiation (see below). CAUTION: Experiments involving rodents must conform to all relevant governmental and institutional (IACUC) standard operating procedures and regulations.
Mitotic inactivation of MEFs using mitomycin C Grow MEFs until confluent and then replace medium with medium containing 10 µg/ml mitomycin C. Return plates to the incubator for 2–3 hours. Wash extensively (2–3 times) with PBS and then harvest using 0.05% trypsin-EDTA and count. Resuspend cells in cryopreservation medium at a concentration of ~1×10^7 cells per ml, quickly aliquot into 1 ml cryovials and temporarily store at −80°C in CoolCell LX or equivalent container, which will allow for an optimum freeze rate of −1°C per minute. Transfer frozen cells to liquid nitrogen storage within 1 week. MEFs can be stored in liquid nitrogen indefinitely. To prepare feeder layers, thaw one vial and resuspend in ~100 ml MEF medium and plate onto gelatinized dishes at a density of 1 × 105 cells / cm2.
Mitotic inactivation of MEFs usingγ irradiation. Expose confluent MEFs to gamma rays and achieve an exposure of 5,000 – 10,000 rads. Harvest and resuspend in cryopreservation medium as described for mitomycin treated MEFs.
EQUIPMENT SETUP
Mouth Pipette Heat the center of a glass, calibrated micropipet over a gas microburner, stretch the pipet by hand and then break the pipet in the center. Assess the broken end under a stereomicroscope. Keep only those pipets that are thin, even, and have a diameter 1.5–2× that of a standard mouse blastocyst. Insert the pulled pipet into an aspirator apparatus (Figure 3). If mouth pipetting is not possible or desirable, micropipette aids are commercially available (e.g. Drummond, Captrol III, cat. 3-000-752) and can be used in place of the mouth controlled aspirator apparatus shown in Figure 3.
Gelatinized plates Gelatinize all tissue culture plates and dishes to promote cell attachment. Pipette a sufficient amount of 0.1% gelatin to cover the surface of a tissue culture treated plate. Incubate for at least 15 minutes at room temperature (20–25°C). Aspirate the gelatin and dry briefly.
MEF feeder cell plates Thaw and resuspend MEFs in MEF medium for a seeding density of ~1 × 105cells per ml and use the appropriate volume for~1 × 105 cells/cm2according to the growth area of the required plate / dishes. Prepare MEF feeder plates at least one day prior to use. MEF feeders may be used up to one week post plating. Prior to use, rinse MEF feeder plates with PBS. CRITICAL: MEF viability is highly variable in serum free culture media and generally, low passage (P2-P3) MEFs are more tolerant. Test higher passage (>P3) MEFs empirically for long-term survival (5–10 days) in serum free media.
Surface sterilization Sterilize all surfaces, bottles, racks, pipet aids, etc. and equipment by spraying or swabbing with 70% ethanol prior to all procedures in accordance with asceptic technique.
PROCEDURE
Preparation of MEF feeder plates Timing: 1 day
-
1.
One day prior to harvesting embryos, thaw and plate inactivated MEFs onto 4-well plates. The number of wells needed is dependent on the number of pregnant females used. The number of embryos received per female is highly variable but generally averages between 6–8. Blastocysts are plated in individual wells. Thus for one female 6–8 wells should be needed.
-
2.
The day of the embryo harvest, remove the MEF media and rinse the 4-well feeder plates with PBS. Replace the media with either KOSR ES medium (variant A, permissive strains) or serum free ES medium (variant B, non-permissive strains). Return plates to incubator until embryos are gathered. CRITICAL: For permissive strains (129, B6) and for most of the non-permissive strains listed in Table 1 use KOSR ES medium for derivation (variant A, step 10A). However, if you are deriving ES cells from a known non-permissive strain like NOD or a NOD derivative strain, use serum free ES cell medium (variant B, step 10B). If the strain has not been previously characterized as non-permissive or permissive, follow Step 10A initially and then repeat with Step 10B if the first attempt is unsatisfactory.
Collecting E3.5 blastocysts Timing: 15 minutes per pregnant female
-
3.
Remove the uteri of a 3.5 d.p.c. pregnant mouse. Trim away excess fat.
-
4.
With a 5 mL syringe, collect 4 ml of M2 medium and attach a 27-gauge needle. While securing the uterine horn with forceps, insert the needle and flush the uterine horn with 2 mL of M2 medium into a petri dish. Repeat this process with the second uterine horn (Figure 3).
-
5.
Using a mouth pipette, collect the flushed embryos and wash through several drops of M2 before placing into a final drop of M2 medium (Figure 3).
-
6.
If embryos are not fully expanded blastocysts with clearly discernable blastocoels (see Figure 1, E4.0), culture embryos overnight in a 35 mm dish of KSOM at 37°C, 5% CO2. If blastocysts are fully expanded at harvest, continue to step 5. CRITICAL: If the blastocysts are not fully expanded when proceeding to step 5, the percentage of embryos that hatch and attach to the feeders layers post plating may be very low (<50%). Embryos may also be harvested at 3.75 dpc to ensure a higher percentage of expanded blastocysts at harvest. CRITICAL STEP: Traditional mESC derivation protocols recommend removal of the zona pellucida prior to plating. While this step is unnecessary (viable embryos will hatch naturally under appropriate culture conditions) and can lead to embryo loss, there are some scenarios that may warrant zona removal. For a detailed protocol on zona removal using acid Tyrode’s solution, see Manipulating the Mouse Embryo, chapter 11, protocol 8 32.
Plating and early culture Timing: 7–9 days
-
7.
Using a mouth pipette (or alternative micropipette aid), plate blastocysts in either KOSR ES medium (variant A, see CRITICAL note in step 2) or serum free ES medium (variant B, see CRITICAL note in step 2), 1 blastocyst per well of a 4-well MEF feeder plate (Figure 4, day 1), containing the appropriate medium for derivation.
-
8.
Incubate the plates at 37°C, 5% CO2. Do not disturb the plates for 48 hours to allow for the blastocysts to hatch and attach to the feeder layer. The majority (80–90%) of blastocysts should hatch and attach to the feeder layer. On day 3 post plating, replace ½ the medium with fresh medium, using either KOSR ES medium or serum free ES medium depending on the desired variant (A or B, see step 2 CRITICAL note). Continue to feed in this manner every other day until disaggregation. After the initial 48 hours of incubation, monitor the growth of the outgrowth under a microscope daily (Figure 4, days 2–3). If outgrowths are prominent one week post plating, proceed to the next step. If not, wait one or two more days before proceeding to step 9. Do not allow outgrowths to grow so large that the center becomes dark (Note dark areas in outgrowths shown in Figure 2 are also depleted of NANOG). TROUBLESHOOTING
-
9.
Disaggregate outgrowths mechanically (option A) or enzymatically (option B).
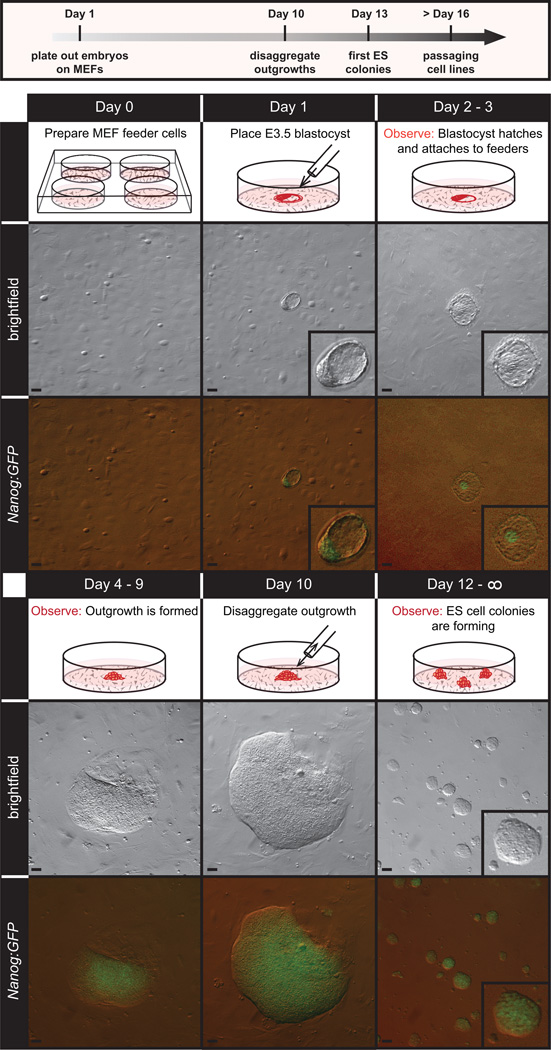
Figure 4.
Overview of ES cell derivation process. Blastocyst stage embryos are plated on mitotically inactivated MEFs and allowed to hatch from the zona pelludica. Shown here are transgenic embryos carrying a GFP reporter cassette driven by the NANOG promoter (NANOG:GFP). By day 2–3, hatched embryos attach to the feeder layer and over the next several days (4–9) the NANOG positive inner cell mass forms a large NANOG positive outgrowth from which mESCs are extracted by mechanical disaggregation and/or trypsinization. Scale bars, 50 µm. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
A. Mechanical disaggregation
Using a mouth pipette, transfer the outgrowth onto a new feeder-covered 4-well in either FBS ES medium (variant A; Step 10A) or serum free ES medium (variant B; step 10B). CRITICAL: At this step, if following variant A, outgrowths are now moved from KOSR ES medium to FBS ES cell medium. If following variant B, continue culture in serum free ES medium.
Using tweezers that have been sterilized with 70% ethanol, break up the outgrowth into small fragments.
Pipette fragments several times with the pulled pipette to achieve further disaggregation
Replace medium with the appropriate media as described in step 9.a.i above and observe wells daily. Once colonies have emerged (2–3 days later), enzymatically passage 1:1. The goal is not to reach confluency, but rather to dissociate cells before large colonies become dark. The incipient ES cell line is now at P1 (passage 1). Increase passage number by 1 with each subsequent exposure to trypsin and carefully track passage number by labeling plates and tubes. See step 10 for details on ES cell passage.
B. Enzymatic disaggregation
Carefully wash the wells with 500ul of PBS.
Aspirate the PBS and add 100ul of 0.05% trypsin to each well. Incubate at 37°C for 5 min.
Using a mouth-controlled pipette, disaggregate the outgrowth by pipetting up and down vigorously several times.
If following variant B, add 100ul of defined trypsin inhibitor to inactivate the trypsin. Pipette by gentle trituration. CRITICAL STEP This is not necessary if following variant A since the FBS in the medium inactivates the trypsin.
-
Add 500ul of either FBS ES medium (variant A) or serum free ES medium (variant B). Transfer onto a new feeder-covered 4-well.
CRITICAL: At this step, if following variant A, outgrowths are now moved from KOSR ES medium to FBS ES cell medium. If following variant B, continue culture in serum free ES medium.
The incipient ES cell line is now at P1 (passage 1). Increase passage number by 1 with each subsequent exposure to trypsin and carefully track passage number by labeling plates and tubes. See step 10 for details on ES cell passage. vii. Replace medium with appropriate medium as described in step 9.b.v. and observe the daily. ES cell colonies will form and should be visible 3–4 days after disaggregation.
ES cell maintenance Timing: 9 days
-
10.
Replace media and observe the cells daily. Continue to use FBS ES cell medium if following variant A or serum free medium if following variant B. Monitor growth and morphology and take careful notes. Mixed differentiation within the first 1–2 passages is not unusual, however continued culture should promote typical ES cell morphology (Figure 5). If poor morphology persists after the first 2 passages, the incipient ES cell line may be unstable. Expect variable growth rates among emergent lines and be aware that unusually rapid growth could be indicative of karyotypic instability (for example, trisomy for chromosome 8 often results in unusually rapid growth in mESCs). Also, be alert for signs of deterioration, which include vacuolated cytoplasm, detachment of cells from colonies, and cellular debris in the medium. If deterioration continues through passages, discard the cell line. Finally, be aware of signs of contamination including sudden change in pH, turbidity, small, round particles (yeast), and filaments (fungi). Discard contaminated cell lines and quarantine the remaining lines if possible. After disaggregation, passage the cells 1:4 (seeding density is ~1–4 × 105 cells/cm2 or ~3–5 × 105 cells per well), onto a 6-well MEF feeder plate (or 35mm dish) following option A if following variant A and following option B if following variant B. TROUBLESHOOTING
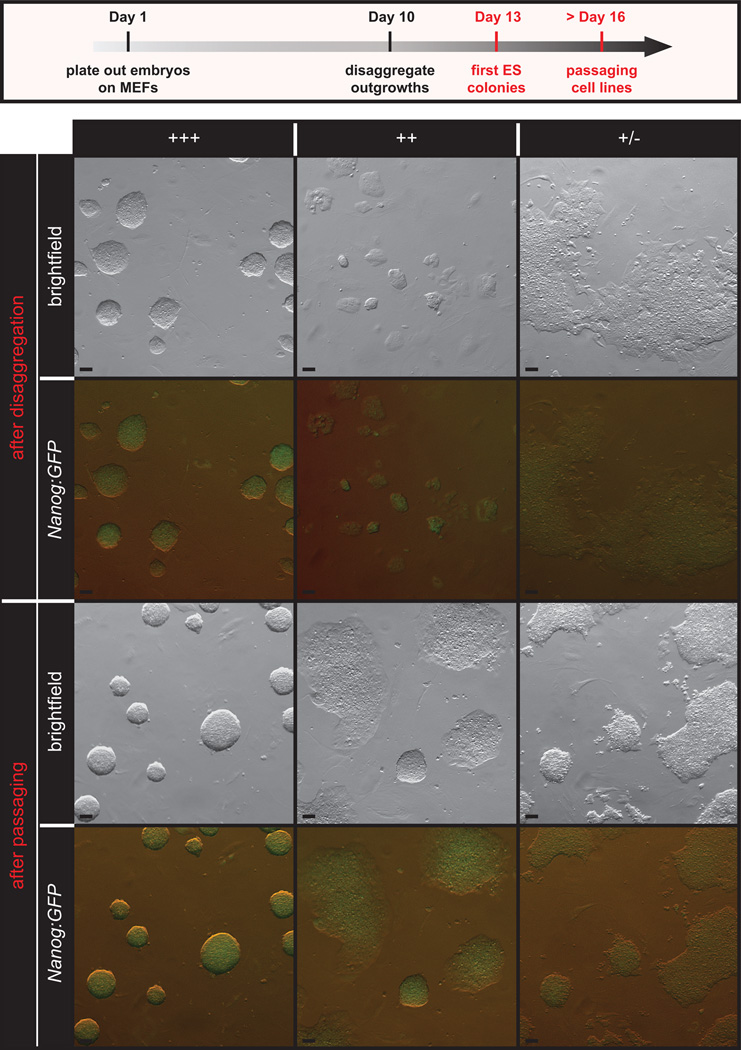
Figure 5.
Emergent mESC morphology. Healthy, emergent mESC lines grow as tightly formed, refractile colonies throughout passaging (+++) and have robust NANOG expression. Lines that show persistent differentiation, flat colony morphology or slow growth through early passaging (+ and +/−) are less desirable and are not likely to emerge as robust, germline competent mESC lines. Scale bars, 50 µm. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
A. Passaging cells, variant A, FBS ES cell medium
Rinse the cells with room temperature PBS
Add a quantity of 0.05% trypsin to cover the cell layer, usually 1/3 the amount used to culture the cells.
Return the cells to the incubator for 4–6 minutes. Observe cell dissociation with an inverted microscope, with occasional swirling.
As soon as colonies begin to dissociate, inactivate the trypsin by adding an equal amount of ES cell medium.
Collect the cells and then remove any remaining cells by rinsing the culture dish with additional ES cell medium. Collect both aliquots (trypsinized cells and the rinse) in one tube and create a single cell suspension by gentle trituration with a pipette.
Centrifuge for 5 minutes at 150 × g. Resuspend the pellet in 2.5ml of ES medium (for 35 mm plate), creating a single cell suspension through gentle trituration.
B. Passaging cells, variant B, serum free ES cell medium
Rinse the cells with room temperature PBS
Add a quantity of 0.05% trypsin to cover the layer of cells, usually 1/5-1/3 the amount used to culture the cells.
Return the cells to the incubator for 3–5 minutes. CRITICAL: cells cultured in serum free culture media will dissociate more quickly in the presence of trypsin. Avoid unnecessary exposure to trypsin by continually observing cell dissociation with an inverted microscope.
As soon as colonies begin to dissociate, inactivate the trypsin by adding an equal amount of defined trypsin inhibitor.
Collect the cells and then remove any remaining cells by rinsing the culture dish with serum free ES medium. Collect both aliquots (trypsinized cells and the rinse) in one tube and create a single cell suspension by gentle trituration with a pipette.
Centrifuge for 5 minutes at 150 × g. Resuspend the pellet in 2.5ml of serum free ES medium, creating a single cell suspension through gentle trituration.
-
11.
Continue to culture cells, replacing media with the appropriate medium (FBS ES medium for variant A and serum free medium for variant B) and observe the cells daily. Once 70% confluency is reached (2–3 days later), passage 1:4, onto a 100mm feeder-covered dish. Alternatively, if genotyping is required, cells can instead by passaged onto 2 60mm dishes, setting aside one for genotyping (see option C of step 19). TROUBLESHOOTING
-
12.
Freeze down cells at 70% confluency. Change media the morning before a freeze to minimize stress on cells. Follow the passaging protocol in step 10 above and resuspend in 1 mL of the appropriate medium (FBS ES medium for variant A and serum free medium for variant B). Count cells using either a manual or automatic cell counter. Centrifuge cells for 5 mins at 150 × g. Resuspend in freeze medium at a concentration of 3×106 cells per ml. Aliquot 1 ml per cryovial. Transfer to a CoolCell (or other container that will allow for a cool rate of −1°C/minute) and place at −80°C. 24 hours later, transfer cells to liquid nitrogen. The frozen cells are now at P3-P4 (see 9.b.vi. regarding passage number assignment), depending on whether the initial disaggregation (step 9) was enzymatic (P4) or mechanical (P3). TROUBLESHOOTING PAUSEPOINT Cells can now be stored in liquid nitrogen indefinitely. Carefully maintain these stocks and plan subsequent thaws and expansions to preserve low passage vials.
ESC Characterization Timing 3 weeks
-
13.
One day before seeding ES cells, thaw and plate mitotically inactivated MEFs. For the full characterization of one cell line, 2 60mm dishes and 6 wells on a 12-well plate are needed. In addition, for a full characterization, 2 gelatinized 60mms without feeders are needed. Incubate MEF feeder plates /dishes at 37°C, 5% CO2.
-
14.
The day after MEF plating, remove a vial of frozen ESCs from liquid nitrogen. Place the vial in a 37°C water bath until the cells begin to thaw.
-
15.
Sterilize the vial with 70% ethanol and then transfer cells to 10 ml of pre-warmed (37°C) media in a 15ml conical tube.
-
16.
Centrifuge the cells for 5 minutes at 150 × g.
-
17.
Resuspend the pellet in 5 ml of media and create a single cell suspension by gentle trituration with a serological pipette. Plate the cells onto a 60mm MEF feeder plate.
-
18.
Once the cells have reached 70% confluency, trypsinize as detailed in step 10, resuspend in 4 ml of ES media and divide into four 1 ml aliquots in separate 15ml conical tubes. Re-centrifuge one of the 1 ml aliquots of cell suspension at 150 × g, wash the pellet in PBS and re-suspend in 5 ml antibiotic free ES media.
-
19.
Seed the cells according to the instructions below for immunolabeling for markers of pluripotency (option A), mitotic chromosome counting (option B), DNA extraction for genotyping (option C) and pathogen testing (option D). We recommend all of these options for mESC characterization.
A. Immunolabeling for markers of pluripotency Timing: 5 hours
Add an additional 4 ml of ES media to one of the 1 ml aliquots of cell suspension. Seed 0.5 ml of the resulting suspension into each of 6 wells of a 12-well plate MEF feeder plate.
Incubate at 37°C, 5% CO2 for 1–2 days to allow cells to reach 50% confluency. Change the medium the morning of the prep.
Rinse the cells with room temperature PBS.
Fix cells in 4% PFA for 20 minutes at room temperature
Wash with PBS 3×5 minutes PAUSE POINT: fixed cells may be kept at 4°C in PBS for up to two weeks.
Incubate cells in all wells with blocking buffer for 15 minutes on a rocking platform at room temperature.
Incubate cells in primary antibody solution for at least 1 hour at room temperature on a rocking platform (one well for each primary anti-body, anti-OCT3/4, anti-NANOG and anti-SSEA1, and one well for each ‘no primary’ control option).
Wash with PBS 3 × 5 minutes.
Incubate cells in appropriate secondary antibody solution (e.g. goat anti-mouse IgG Alexa Fluor 488, goat anti-rabbit IgG Alexa Fluor 488 or goat anti-mouse IgM Alexa Fluor 488) for at least 1 hour at room temperature on a rocking platform.
Wash with PBS 3 ×5 minutes.
Incubate with DAPI for 10 minutes.
Wash with PBS 1×5 minutes.
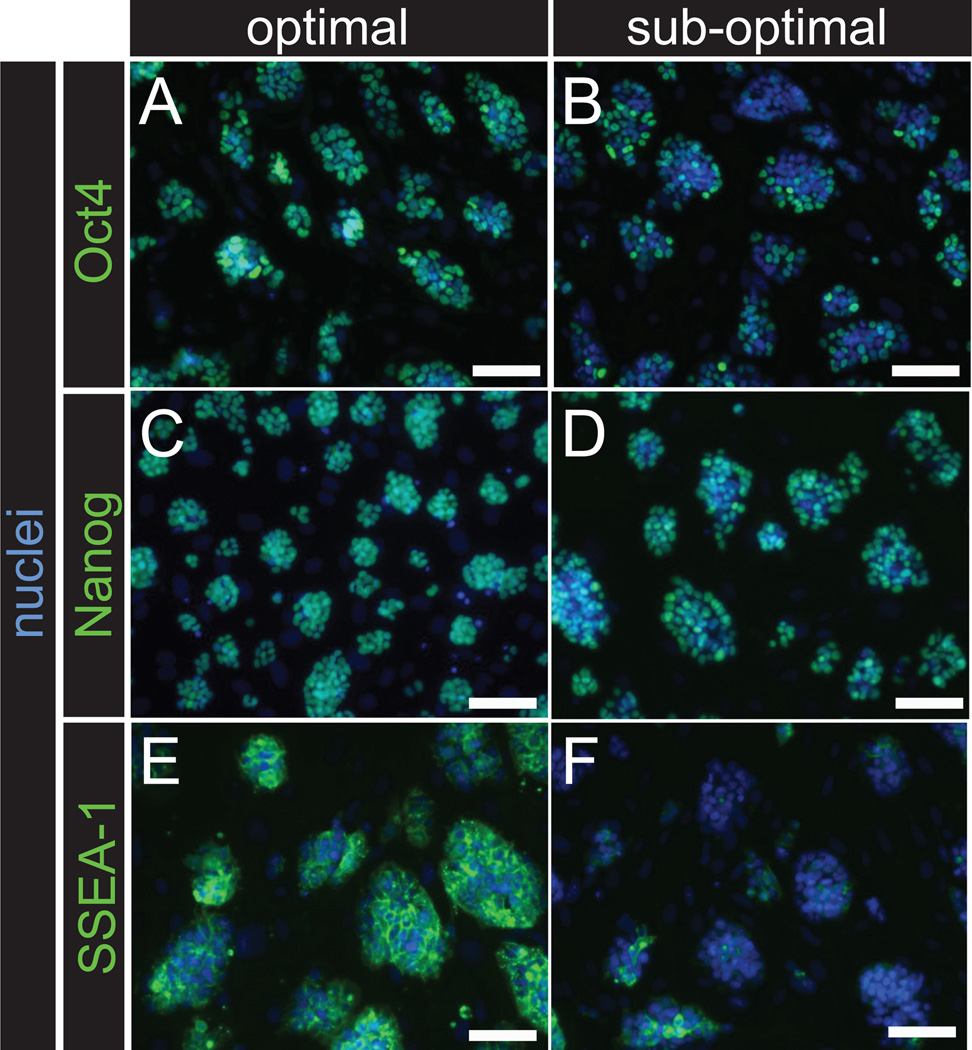
Image using inverted, epifluorescent microscope. NANOG and OCT3/4 antibody should label the nuclei of ES cells, while SSEA-1 antibody will label the surface of ES cells. Proceed with ES cell lines that show maximum distribution of labeling as shown in Figure 6 (optimal vs. sub-optimal). If desired, quantitative assessment of labeling may also be performed using flow cytometry (see 20). CRITICAL: Even robust, germline competent mESCs are heterogenous in their pluripotency marker expression and in poorly performing mESC lines, there can still be some pluripotency marker expression. Here, the goal of assessing pluripotency marker expression is to identify those lines with the most homogeneous / consistent expression of each maker, especially NANOG 26.
Figure 6.
mESC characterization by immunolabeling of pluripotency biomarkers. The pluripotency markers OCT3/4, NANOG and SSEA-1 can be used assess pluripotency in new mESC lines. Heterogeneous labeling (B, D, F) is associated with sub-optimal germline transmission when compared to lines that show optimal, consistent labeling (A, C, D) for these markers. Scale bars, 50 µm. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
B. Mitotic chromosome counting (Figure 7) Timing: 6 hours
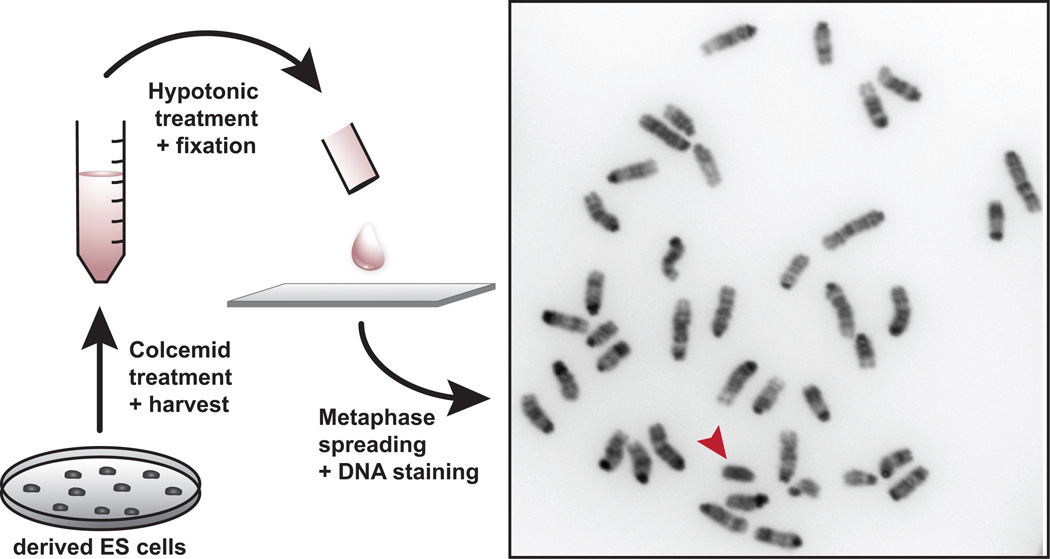
Figure 7.
Karyotypic analysis of ES cells. mESC lines are treated with colcemid to arrest cells in metaphase. Cells are fixed and ruptured by hypotonic treatment and nuclei are spread on glass slides. Mitotic chromosomes are stained with DAPI, which is a DNA intercalating dye that preferentially binds A-T rich DNA, resulting in a reverse G-banding pattern. For genetic engineering, male mESC lines are preferable for breeding efficiency. The Y chromosome (arrowhead) stains homogeneously with DAPI and is clearly distinguishable from the other chromosomes, which show discrete banding patterns. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
Take 1 mL of the cell suspension and add an additional 4ml of ES media. Seed onto a 60mm gelatinized dish.
Incubate at 37°C, 5% CO2 for 1–2 days to allow cells to reach 50%-70% confluency.
Add 10 µl of 10 µg/mL colcemid to 5 ml of fresh, warmed ES media.
Remove spent ES cell medium from one of the cultures growing on a gelatinized 60 mm plate and add the colcemid-containing medium to the culture.
Incubate the dish for three hours at 37°C.
Trypsinize the cells as detailed in step 10.
Aspirate and discard the supernatant, leaving about 500 µl of media in the tube. Gently pipet up and down to resuspend the pellet.
Steam clean slides over a water bath.
Using a P1000 pipette, add 5 ml KCL 0.56% to the cells slowly CRITICAL: KCL should be added in a drop-by-drop manner to prevent lysis. Carefully invert cells to mix.
Incubate for 60 minutes at room temperature.
Centrifuge for 5 minutes at 150×g.
Aspirate and discard the supernatant, leaving 1 mL of the hypotonic solution. Resuspend the pellet by gentle pipetting.
In a drop by drop manner, slowly add 1 mL of 3:1 methanol:glacial acetic acid. Swirl the tube as the fixative is added.
Centrifuge for 5 minutes at 150×g.
Aspirate and discard the supernatant leaving 1 mL of fixative. Resuspend the pellet by gentle pipetting.
Slowly add 3 mL of fixative, swirling to mix.
Centrifuge for 5 minutes at 150 × g.
Repeat steps xiv-xvi twice.
Resuspend the pellet in 1mL of fixative. PAUSE POINT: cells may be stored in fixative at −20°C for several years.
Steam a freshly cleaned slide over a water bath.
Immediately pipette 75 µl of the cell suspension onto the steamed slide from a height of 6 inches. Tilt the slide and blow gently to disperse the cells.
Place the slide on a hot plate to dry.
Store for 24–48 hours on a slide warmer at 37°C.
Apply a drop of Vectashield hard set mounting medium with DAPI to the slide and then apply a coverslip.
Acquire images of metaphase spreads at 60× magnification. Analyze at least 20 spreads per cell line by counting the total chromosome number and assessing sex chromosomes (XX or XY) as shown in Figure 7. Determine the percentage of spreads with 40 chromosomes. For example, if 19 of 20 spreads have 40 chromosomes and a Y chromosome is observed in every spread, the cell lines is 95% euploid, 40 XY. To select for mESC lines that will be used for genetic engineering, male lines are preferable for maximum breeding efficiency.
C. DNA extraction for SNP panel and/or genotyping Timing: 1 hour
Take 1 mL of the cell suspension and add an additional 4 ml of ES media. Seed onto a 60mm gelatinized dish. CRITICAL: For optimal and accurate mESC specific SNP genotyping, it’s important to exclude feeders.
Incubate at 37°C, 5% CO2 for 1–2 days to allow cells to reach 50%-70% confluency.
Extract DNA from a confluent culture using a Qiagen DNeasy kit following the manufacturer’s instructions and perform PCR as needed for genotyping.
To confirm the genetic background of the established cell line, submit DNA to a SNP panel, e.g. KBioscience mouse SNP panel28.
D. Pathogen Testing Timing: 2 weeks
Take the 5 ml of cells resuspended in antibiotic free ES medium and seed a 60 mm MEF feeder dish. Continue to culture in the absence of antibiotics for the remainder of the pathogen test.
Incubate the cells at 37°C, 5% CO2 for 1–2 days until fully confluent. Remove and reserve the 5 ml of used media CRITICAL: Contaminants can be harbored in the media so it is critical that used media, in addition to the cells, be tested.
Trypsinize the cells as detailed in step 10.
Resuspend the cells in the used medium, dividing into three tubes: 0.5 mL for bacterial testing, 2 ml for fungal and 2.5 ml for Mycoplasma testing.
For bacterial testing: Add the 0.5 ml to 4.5 ml of Tryptose Phosphate Broth. Incubate at 37°C for 48 hours. Check for growth.
For fungal testing: Inoculate a Saboraud plate with the 2 ml of culture. Culture for 14 days at room temperature. Check for growth.
For Mycoplasma testing: Spin down the 2.5 ml of culture and aspirate until 200 ul of media remains. Extract DNA using a DNeasy kit following the manufacturer’s instructions. Test for the presence of Mycoplasma using a PCR-based detection method (e.g., van Kuppeveld etal. 29).
If desired, submit a sample cells to a molecular Mouse Antibody Production (MAP) test to screen for mouse pathogens in accordance with institutional policies.
Germline transmission of parental lines Timing: 8 weeks
-
20.
Thaw a well-characterized ES cell line onto a 60mm MEF plate as detailed in steps 14–17 CRITICAL: To maximize the success of germline transmission the mES cell line should robustly express pluripotency markers and have a stable euploid karyotype of at least 70% 40, XY.
-
21.
Allow cells to reach 70% confluency
-
22.
Dissociate cells with trypsin.
-
23.
Inactivate trypsin and then add an additional 3 ml of media to the dish.
-
24.
Return to the incubator for 20 minutes to allow feeder cells to settle to the bottom of the dish.
-
25.
Carefully remove the supernatant to a 15 ml conical tube.
-
26.
Centrifuge for 5 minutes at 150× g.
-
27.
Wash with PBS.
-
28.
Centrifuge for 5 minutes at 150 × g.
-
29.
Resuspend in 1 ml of media buffered with 10 mM HEPES.
-
30.
Deliver cells to microinjection facility. Request host embryos from a strain that will allow for determination of mESC contribution by coat color as depicted in Figure 8. For a review of mouse coat color loci see Jackson30. CRITICAL: Microinjection of mESCs into host blastocyst embryos requires specialized equipment and expertise and is usually provided by a core facility. For laboratories interested establishing in-house microinjection / chimera production capability, detailed protocols are available from a variety of sources, including Nagy et al.27.
-
31.
Collect relevant data for 50–100 injections as shown in Table 2. Generate approximately 20 offspring per chimera and assess germline transmission using coat color as a guide (see Figure 8 and legend) and reference the data shown in Table 2. Up to 60% of the viable offspring from microinjection of a robust, parental male mES cell line should be chimeric and 80–100% of these should be male. Moreover, at least 50% of the chimeric males should have germ line transmission (GLT) rates of 50% or more.
TROUBLESHOOTING
See Table 3 for Troubleshooting guidance.
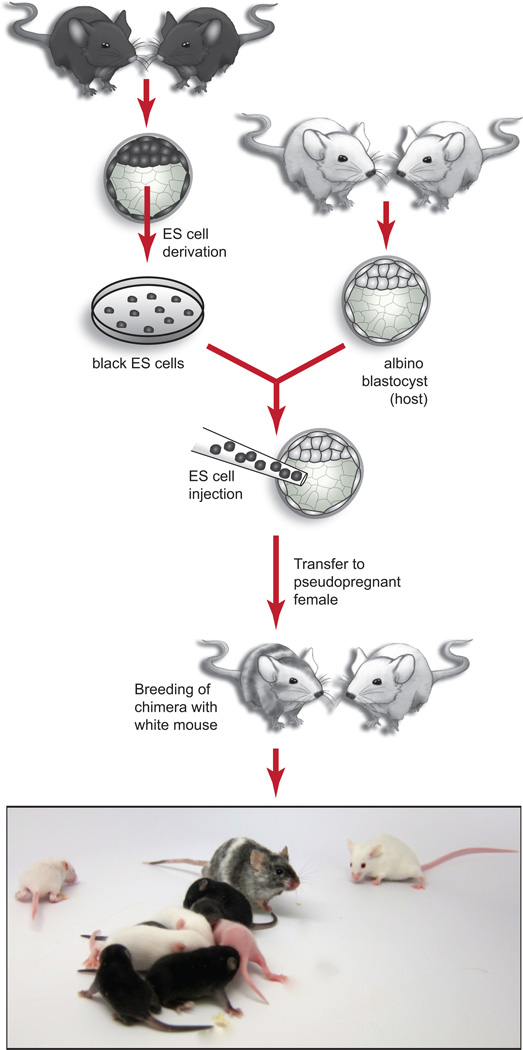
Figure 8.
Germline testing of mESC lines. mESC lines are injected into host blastocyst embryos and mESC contribution to resulting chimeras is assessed by coat color. In this example, mESCs derived from a non-albino strain (e.g. C57BL/6J, genotype a/a, Tyr+/Tyr+) are injected into host blastocysts from an albino strain (e.g. C57BL-Tyrc2J/J, genotype a/a, Tyrc/Tyrc). The resulting chimeras will have patches of fur that are albino (contributed by host blast) and non-albino (in this example, black (a/a) pigmentation is contributed by ES cells). Contribution from the mESC line is evidenced by the overall percentage of non-albino coat color. Generally, high contribution by a male ES cell line will also produce predominantly male chimeras. Female chimeras are possible, but are less productive. The male chimeras are then bred with an albino strain (in this case, a/a, Tyrc/Tyrc) to ensure that germ line transmission can be assessed by coat color. In this example, pigmentation is only possible in offspring that are a/a, Tyr+/Tyrc (black pups in image) where the wild type Tyr and an a allele are contributed by sperm derived from the ES cell line. Albino offspring are a/a, Tyrc/Tyrc where the Tyrc allele and an a allele are contributed by sperm derived from the host blastocyst. Pups shown are from 2 litters, 7 and 11 days post partum. All procedures involving mice were approved by The Jackson Laboratorys and Sloan-Kettering Institutes Institutional Animal Care and Use Committees and were performed in accordance with the National Institutes of Health guidelines for the care and use of animals in research.
Table 2.
Data collected to assess mESC suitability for chimera contribution and germ line transmission (GLT).
| Development | |
| No. of embryos transferred | 50 |
| # of pups born | 9–25 |
| % born | 18-50% |
| Chimeras | |
| # of chimeras | 3–14 |
| % chimeras | 30-60% |
| # male chimeras | 2–14 |
| % male chimeras | 80-100% |
| Coat Color Contribution | |
| 100% | 0–8 |
| >80% | 0–5 |
| 40-80% | 0-7 |
| <40% | 0–8 |
| Fertility | |
| #set up | 2–14 |
| # productive | 1–10 |
| % productive | 30-70% |
| Germline transmission | |
| #chim. w/GLT | 1–10 |
| % GLT | 50-100% |
| Rates of germline transmission | |
| 100% | 0–10 |
| >50% | 0–10 |
| 10-50% | 0 |
| <10% | 0 |
Acceptable ranges are provided based on our accumulated data from microinjection of 10, low passage (P6-P11), parental (mESCs that have not been genetically engineered) mESCs from a variety of strains, including BALB/cByJ, C3H/HeJ, PWD/PhJ, NOD/ShiLtJ and C57BL/6J. Each ES cell line was injected into 50 blastocyst stage host embryos. The % born is the number of viable offspring born / number of embryos transferred and can vary significantly depending on the microinjection facility as well as mESC quality. The % chimeras is the number of chimeras born / total number born. The % male chimeras is the number of chimeric males / total males born. Coat color contribution is based on a subjective assessment of mESC contribution to the host embryo based on coat color in wean age animals. This assessment could vary based on microinjection facility and operator. The % GLT is the number of animals demonstrating germ line transmission / the number of productive animals. To assess fertility, chimeras are housed with proven breeders (typically as trios) at the age of 8–12 weeks and are kept for up to 12 weeks (to generate 20 offspring per chimera). Chimeras that do not produce offspring within this timeframe are considered non-productive. Germline transmission rate is the number of ES cell derived offspring (by coat color) / total offspring (~20).
Table 3.
Troubleshooting
| Steps | Problem | Possible reasons | Possible solution |
|---|---|---|---|
| 8 | Blastocyst failure to Attach or hatch |
Blastocyst not fully developed |
Plate only fully expanded blastocysts |
| Unhealthy MEF feeder layer |
Verify that MEFs are seeded at correct density and test for ability to support ES cell viability and growth with an established mESC line |
||
| Embryos are unable to hatch from the zona pellucida |
Dissolve the zona pellucida using acid Tyrode’s before plating blastocyst onto a feeder layer |
||
| 10–12 | Differentiation | Improper composition Of culture media |
Verify that media were made correctly and check dates on all media components |
| Blastocyst attachment to edge of feeder layer / well |
Blastocysts should be placed in the center of the well to avoid attachment to the sides of the well. Dishes can be rotated clockwise and/or counterclockwise to ‘swirl’ the embryos into the center of each circular well. |
||
| Overgrowth of cultures | Outgrowths and colonies should be dissociated before the center of the outgrowth becomes dark |
||
| Strain is non-permissive | Repeat the derivation according to variant B |
||
| 10–12 | Poor growth, and/or sudden change in media color, cloudiness in medium, presence of fungi, yeast, bacteria |
Contamination | Clean and sterilize the cell culture incubator and review proper asceptic technique technique. Discard all contaminated cultures and quarantine any cultures growing in the same incubator. Discard and replace all media. |
| 10–12 | Excessive cell death, poor survival after passage |
Improper composition of culture media |
Ensure that media were prepared correctly and check all expiration dates. All culture media should be stored at 4°C and should be protected from light. Media should be discarded and replaced after 1- 2 weeks. |
| Excessive exposure to trypsin |
Inhibit trypsin as soon as cells begin to disassociate. |
||
| 11 | Poor post-thaw viability |
Improper storage conditions |
Cells should be initially frozen at – 80°C in a container that allows for a controlled freeze (1C per minute) and then rapidly transferred to liquid N2 after 1 – 7 days. Also, be sure to check integrity of cryovials |
| Improper media composition |
Verify that freeze media were made correctly |
||
| Poor handling | Always minimize the exposure of thawed cells to the cryopreservation medium, which contains DMSO. DMSO is an effective adjuvant but is also toxic to cells at ambient temperatures. Cells require a slow freeze and a rapid thaw. To thaw rapidly, use a 37°C water or bead bath. Sterilize the outside of the vial and immediately transfer thawed contents to media immediately after thawing. |
||
| 35 | Germline transmission is not detected |
Selected breeding scheme doesn’t allow for assessment of germ line transmission by coat color |
Verify that the coat color genotype of the ES cell line, the host blastocyst and breeder strain will allow for assessment of germ line transmission by coat color. If the wrong strains have been selected, it may be possible to genotype offspring using strain specific SNPs. Otherwise, select a different strain to breed chimeras or repeat with a different host blastocycst. |
| Cells are karyotypically abnormal |
Verify the karyotype of the cell line, sub clone or re-derive if necessary |
||
| Pluripotency is limited, cells have poor viability or poor proliferative potential |
If a high passage vial was used, choose a lower passage for testing. Alternatively (and more likely), this result may indicate that the mESC line is inadequate. In this case, test a different line from the same derivation, or repeat the derivation according to variant B. |
||
ANTICIPATED RESULTS
Using this protocol, we have produced germline competent ES cell lines from a variety of mouse strains; including, those thought to be non-permissive, such as DBA 20, PWD and NOD (our own unpublished data). Careful selection of healthy, blastocyst stage embryos, combined with minimal manipulations prior to plating promotes a high yield of ICM outgrowths from embryos that hatch naturally from the zona pellucida and adhere to MEF feeders. Under the derivation conditions described here, up to 90% of ICM outgrowths will yield incipient ES cell lines, with some variation depending on the strain background and the experience of the technicians. During the outgrowth phase, exclusion of serum from the derivation media minimizes trophectoderm differentiation and 2i/LIF promote expansion of the epiblast.
In incipient ES cell lines, we have found that serum-free 2i/LIF media (variant B) promotes robust NANOG expression and ultimately, higher yield of coat color chimeras through germline testing 20. While serum-free culture conditions are typically employed in feeder-free culture systems, we have found that the use of feeders promotes more efficient derivation (# of embryos giving rise to ES cell lines / # embryos plated) and also yields a higher percentage of adherent ES cell lines, which are more favorable for gene targeting experiments. However, not surprisingly, MEF viability in the absence of serum is highly variable. This can be overcome with careful selection of low passage feeder MEFs that have been previously tested for survival in serum free conditions and careful monitoring of feeder layers during culture. In addition, in the context of a gene targeting experiment, serum can be temporarily introduced during drug selection to promote MEF survival post-electroporation. Given these complexities, if a particular mouse strain has not been previously characterized as non-permissive (see Table 1), derivation of ES cells in the presence of serum (variant A) should be attempted first. If robust pluripotent, karyotypically stable ES cell lines are not achieved with variant A, then serum free derivation should follow.
It is important to closely monitor the growth of incipient mES cell lines with careful observation of morphology, density, and to replenish media daily. The timing of passages is dictated by the confluency of the culture (and not convenience) and therefore will vary from once incipient cell line to the next. Cell lines should be cryopreserved at P3-P4 and subsequent culturing and freezing should be carefully planned to ensure ample frozen stocks of low passage cells. Generally, as passage number increases, genomic stability and pluripotency decline. Moreover, there is emerging evidence (though still anecdotal) that while 2i/LIF conditions promote pluripotency, prolonged culture may promote aneuploidy; therefore, it is important to consider weaning newly derived, characterized mESC lines from 2i. With the exception of lines created by variant B, we maintain low passage stocks in 2i/LIF and then create higher passage, working stocks that are weaned from 2i. To do this, cells are essentially brought through several passages with decreasing doses of 2i (e.g. dividing the dose by two at each passage) and then working stocks vials are cryopreserved.
Before proceeding with germline testing or in vitro differentiation, newly derived embryonic stem cells lines should be extensively characterized. mES cell lines should be free of Mycoplasma, bacteria, fungi, and mouse pathogens. SNP paneling is recommended to confirm the strain of origin of mES cells and karyotypic assessment should be used to determine sex and % euploidy. For gene targeting, male ES cell lines that are 70% euploid (40, XY) or higher are acceptable, but >90% is ideal. Karyotypic stability is stochastic in cultured cells and should be reassessed after extensive passaging, as in a gene targeting experiment. Cell lines that have developed an unstable karyotype may be sub-cloned, however given the efficiency with which ES cells can be derived, re-derivation is preferable. Finally, to ensure maximum developmental potential reflected in robust chimera contribution, highly euploid mESCs should robustly express well-accepted markers of the pluripotent state such as NANOG, OCT4, SOX2 and SSEA-1.
Acknowledgements
This work was supported in part by National Institutes of Health, Office of Research Infrastructure Programs (U42-OD011102, U42-OD010921). Work in AKH’s laboratory is supported by the Human Frontiers Sciences Program and National Institutes of Health (R01-HD052115 and RO1-DK084391). We grateful for the excellent microinjection services provided by the Cell Biology and Microinjection core at The Jackson Laboratory.
Footnotes
Author contributions. A.C. and L.G.R. developed the protocol and wrote the manuscript. C.B., I.G., and L.R.D. contributed to and / or supported the development of the protocol. N.S. and A.-K.H. contributed to and expanded the protocol and contributed to the writing manuscript.
Competing Financial Interests. The authors declare no competing financial interest.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley A, Hasty P, Davis A, Ramirez-Solis R. Modifying the mouse: design and desire. Bio/technology. 1992;10:534–539. doi: 10.1038/nbt0592-534. [DOI] [PubMed] [Google Scholar]
- 5.Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. The Mouse Genome Database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic acids research. 2012;40:D881–D886. doi: 10.1093/nar/gkr974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley A, et al. The mammalian gene function resource: the International Knockout Mouse Consortium. Mammalian genome: official journal of the International Mammalian Genome Society. 2012;23:580–586. doi: 10.1007/s00335-012-9422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawase E, et al. Strain difference in establishment of mouse embryonic stem (ES) cell lines. Int J Dev Biol. 1994;38:385–390. [PubMed] [Google Scholar]
- 8.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buehr M, et al. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biology of reproduction. 2003;68:222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, et al. One-Step Generation of Mice Carrying Reporter and Conditional Alleles by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zschemisch NH, et al. Zinc-finger nuclease mediated disruption of Rag1 in the LEW/Ztm rat. BMC immunology. 2012;13:60. doi: 10.1186/1471-2172-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauschild J, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12013–10217. doi: 10.1073/pnas.1106422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handyside AH, et al. Biopsy of human preimplantation embryos and sexing by DNA amplification. Lancet. 1989;1:347–349. doi: 10.1016/s0140-6736(89)91723-6. [DOI] [PubMed] [Google Scholar]
- 14.Buehr M, Smith A. Genesis of embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2003;358:1397–1402. doi: 10.1098/rstb.2003.1327. discussion 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buehr M, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Li P, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell stem cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinholdt LG, et al. Generating embryonic stem cells from the inbred mouse strain DBA/2J, a model of glaucoma and other complex diseases. PloS one. 2012;7:e50081. doi: 10.1371/journal.pone.0050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols J, et al. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med. 2009;15:814–818. doi: 10.1038/nm.1996. [DOI] [PubMed] [Google Scholar]
- 22.Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying QL, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 25.Markoulaki S, Meissner A, Jaenisch R. Somatic cell nuclear transfer and derivation of embryonic stem cells in the mouse. Methods. 2008;45:101–114. doi: 10.1016/j.ymeth.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Silva J, et al. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: a Laboratory Manual. Cold Spring Harbor Laboratory Press; 2003. Production of chimeras; pp. 453–506. [Google Scholar]
- 28.Petkov PM, et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kuppeveld FJ, et al. 16S rRNA-based polymerase chain reaction compared with culture and serological methods for diagnosis of Mycoplasma pneumoniae infection. Eur. J. Clin. Microbiol. Infect. Dis. 1994;13:401–405. doi: 10.1007/BF01971997. [DOI] [PubMed] [Google Scholar]
- 30.Jackson IJ. Molecular and developmental genetics of mouse coat color. Annu. Rev. Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- 31.Roach ML, McNeish JD. Methods for the isolation and maintenance of murine embryonic stem cells. Methods Mol. Biol. 2002;185:1–16. doi: 10.1385/1-59259-241-4:1. [DOI] [PubMed] [Google Scholar]
- 32.Roach ML, Stock JL, Byrum R, Koller BH, McNeish JD. A new embryonic stem cell line from DBA/1lacJ mice allows genetic modification in a murine model of human inflammation. Exp. Cell Res. 1995;221:520–525. doi: 10.1006/excr.1995.1403. [DOI] [PubMed] [Google Scholar]
- 33.Brown DG, Willington MA, Findlay I, Muggleton-Harris AL. Criteria that optimize the potential of murine embryonic stem cells for in vitro and in vivo developmental studies. In Vitro Cell. Dev. Biol. 1992;28A:773–778. doi: 10.1007/BF02631066. [DOI] [PubMed] [Google Scholar]
- 34.Nagafuchi S, et al. Establishment of an embryonic stem (ES) cell line derived from a non-obese diabetic (NOD) mouse: in vivo differentiation into lymphocytes and potential for germ line transmission. FEBS Lett. 1999;455:101–104. doi: 10.1016/s0014-5793(99)00801-7. [DOI] [PubMed] [Google Scholar]
- 35.Gardner RL, Brook FA. Reflections on the biology of embryonic stem (ES) cells. Int. J. Dev. Biol. 1997;41:235–243. [PubMed] [Google Scholar]