Abstract
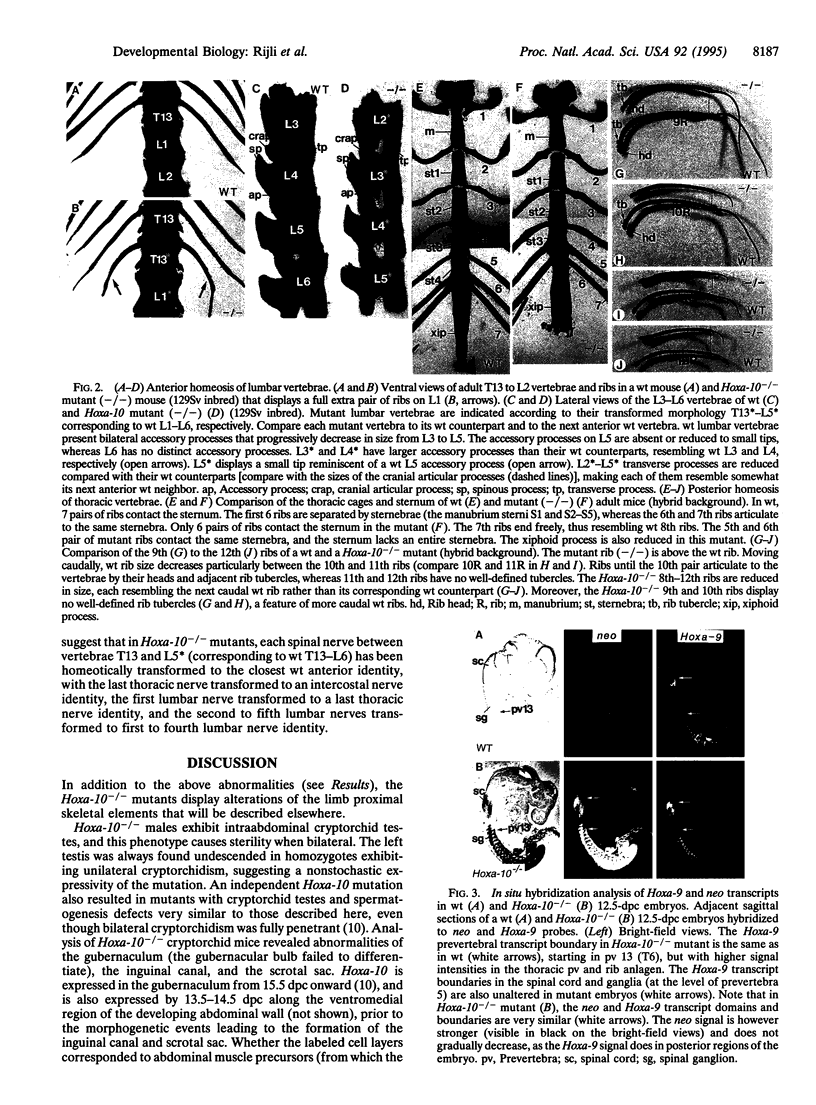
Homozygous mice mutated by homologous recombination for the AbdB-related Hoxa-10 gene are viable but display homeotic transformations of vertebrae and lumbar spinal nerves. Mutant males exhibit unilateral or bilateral criptorchidism due to developmental abnormalities of the gubernaculum, resulting in abnormal spermatogenesis and sterility. These results reveal an important role of Hoxa-10 in patterning posterior body regions and suggest that Hox genes are involved in specifying regional identity of both segmented and nonovertly segmented structures of the developing body.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Dollé P., Duboule D. Two gene members of the murine HOX-5 complex show regional and cell-type specific expression in developing limbs and gonads. EMBO J. 1989 May;8(5):1507–1515. doi: 10.1002/j.1460-2075.1989.tb03535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack H., Gruss P. The establishment of murine Hox-1 expression domains during patterning of the limb. Dev Biol. 1993 Jun;157(2):410–422. doi: 10.1006/dbio.1993.1145. [DOI] [PubMed] [Google Scholar]
- Horan G. S., Wu K., Wolgemuth D. J., Behringer R. R. Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12644–12648. doi: 10.1073/pnas.91.26.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson J. M., Beasley S. W. Embryological controversies in testicular descent. Semin Urol. 1988 May;6(2):68–73. [PubMed] [Google Scholar]
- Kostic D., Capecchi M. R. Targeted disruptions of the murine Hoxa-4 and Hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech Dev. 1994 Jun;46(3):231–247. doi: 10.1016/0925-4773(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Le Mouellic H., Lallemand Y., Brûlet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992 Apr 17;69(2):251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Lim T. M., Jaques K. F., Stern C. D., Keynes R. J. An evaluation of myelomeres and segmentation of the chick embryo spinal cord. Development. 1991 Sep;113(1):227–238. doi: 10.1242/dev.113.1.227. [DOI] [PubMed] [Google Scholar]
- Pollock R. A., Jay G., Bieberich C. J. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell. 1992 Dec 11;71(6):911–923. doi: 10.1016/0092-8674(92)90388-s. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Dollé P., Fraulob V., LeMeur M., Chambon P. Insertion of a targeting construct in a Hoxd-10 allele can influence the control of Hoxd-9 expression. Dev Dyn. 1994 Dec;201(4):366–377. doi: 10.1002/aja.1002010408. [DOI] [PubMed] [Google Scholar]
- Rijli F. M., Mark M., Lakkaraju S., Dierich A., Dollé P., Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993 Dec 31;75(7):1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Satokata I., Benson G., Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995 Mar 30;374(6521):460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Small K. M., Potter S. S. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1993 Dec;7(12A):2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Wensing C. J. The embryology of testicular descent. Horm Res. 1988;30(4-5):144–152. doi: 10.1159/000181051. [DOI] [PubMed] [Google Scholar]
- Wigston D. J., Sanes J. R. Selective reinnervation of intercostal muscles transplanted from different segmental levels to a common site. J Neurosci. 1985 May;5(5):1208–1221. doi: 10.1523/JNEUROSCI.05-05-01208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]