Abstract
Infrared neural stimulation (INS) describes a method, by which an infrared laser is used to stimulate neurons. The major benefit of INS over stimulating neurons with electrical current is its spatial selectivity. To translate the technique into a clinical application it is important to know the energy required to stimulate the neural structure. With this study we provide measurements of the radiant exposure, at the target structure that is required to stimulate the auditory neurons. Flat polished fibers were inserted into scala tympani so that the spiral ganglion was in front of the optical fiber. Angle polished fibers were inserted along scala tympani, and rotating the beveled surface of the fiber allowed the radiation beam to be directed perpendicular to the spiral ganglion. The radiant exposure for stimulation at the modiolus for flat and angle polished fibers averaged 6.78±2.15 mJ/cm2. With the angle polished fibers, a 90° change in the orientation of the optical beam from an orientation that resulted in an INS-evoked maximum response, resulted in a 50% drop in the response amplitude. When the orientation of the beam was changed by 180°, such that it was directed opposite to the orientation with the maxima, minimum response amplitude was observed.
Keywords: infrared neural stimulation, hearing, cochlea, laser, optical stimulation
1. INTRODUCTION
Infrared radiation (IR) can be used to directly stimulate nerves, neurons, cardiomyocytes and vestibular hair cells [for a review see 1, 2]. While the safety and efficacy of infrared neural stimulation (INS) has been demonstrated in various studies, the typical thresholds values for INS vary drastically [3–17]. Factors that contribute to the variation include not only the structural and physiological differences of the system investigated but include the criteria used to determine a positive response, and the method by which the strength of the laser stimulus is reported. Threshold for INS has been defined as the radiant exposure required for a detectable response, including recordings of nerve potentials, electromyograms, muscle twitches, or other organotypic functional observations. In some publications, the stimulus levels for thresholds are reported in radiant energies; in other studies radiant exposures are provided. The radiant energy is typically measured at the tip of the optical fiber in air and is an objective measure. However, this value does not reflect the radiant energy at the target structure because fluids and tissue between the tip of the optical fiber and the target can absorb and scatter the photons and reduce the incident radiation. To calculate the corresponding energy values at the target for stimulation, the exact distance between the tip of the optical fiber and the target structure and the extinction coefficient are required. The reporting of radiant exposure is even more complicated. The radiant exposure is, by definition, the ratio of the radiant energy and the corresponding irradiated area [e.g. 18]. In addition to challenges in reporting the energy at the target, challenges occur for the determination of the irradiated area or the spot size. The spot size has been either reported as the core diameter of the optical fiber [19], calculated from the aperture of the optical fiber [9, 20–22], determined in air and in fluids using the knife-edge technique [19, 23, 24], from images taken with a thermal camera [25], or by temperature changes that were determined using thermochromic ink dissolved in agar [26]. Like the approximations for the radiant energy, matter between the tip of the optical fiber and the target tissue can scatter the photons and can increase the spot size.
In the present study, we have utilized flat and angle-polished optical fibers to deliver the radiation to cochlear spiral ganglion. In vivo experiments were used to estimate the energy on target and to determine the effect of the orientation of the beam path to the target structure. The radiant energy was measured at the tip of the optical sources in air. The distances between the optical source and the possible target structures were determined from microComputed Tomography (microCT) performed at the conclusion of the experiments. The spot size was calculated from the numerical aperture of the fiber and from direct measurements.
2. METHODS
Pigmented guinea pigs (200–800 g) of either sex were used in the experiments. Care and use of animals were carried out within guidelines of the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Northwestern University.
2.1 Experimental approach
After the animals were anesthetized with urethane (1.3 g/kg i.p.), which was supplemented by ketamine (44 mg/kg) and xylazine (5 mg/kg), a c-shaped skin incision was made behind the left ear lobe and the cervicoauricular muscles were removed. The cartilaginous outer ear canal was exposed and cut to insert an ear bar into the ear canal. During the experiments the left ear was stimulated acoustically with the Beyer DT770Pro speaker. The left bulla was exposed and opened approximately 2×3 mm with a motorized drill (World Precision Instruments, Sarasota, FL). The basal turn of the cochlea was identified and a cochleostomy was created with a 0.5 mm Buckingham footplate hand drill (Richards Manufacturing Co., Memphis, TN). A flat-polished or angle-polished optical fiber was inserted through the opening of the cochlear wall. For the present experiments, the flat polished fibers were 200 μm in core diameter, the numerical aperture was 0.22, and the acceptance angle was 25.4° in air and about 19° in fluids (P200-5-VIS-NIR, Ocean Optics, Dunedin, FL). Angle-polished side-firing optical fibers were obtained from Lockheed Martin Aculight (Bothell, WA) and were inserted along the cochlea. Rotating of the fiber allowed the determination of the pointing accuracy. The individual optical fibers were mounted to a micromanipulator (MHW103, Narishige, Tokyo, Japan) to ensure consistent orientation during stimulation. To measure compound action potentials (CAPs), a silver ball electrode was placed on the round window with a reference in the tissue near the neck. To damage the cochlea during the experiments, about 50μL neomycin (20 mM in Ringer’s Lactate) were injected into scala tympani through the cochleostomy. After cochlear damage was confirmed by the elevation of CAP thresholds, flat polished and angle polished fibers were mounted to a goniometer attached to a 3D manipulator, and were inserted into scala tympani. The flat polished fibers were inserted perpendicular to the modiolus and the angle-polished fibers were inserted along the cochlear turn until contact was made with the bony cochlear wall. For the angle polished fiber, threshold energy and response amplitude for a given radiant energy was determined for different placements of the optical fiber. At a fixed radiant energy, the resulting CAP amplitude was determined for the selected position of the optical fiber. Next, the fiber was rotated by 45° and the cochlea was stimulated for the new fiber position and orientation. The procedure was repeated for every 45° until a full rotation (360°) was accomplished. For the measurements with a fixed radiant energy, typically 40μJ/pulse was selected for stimulation. The optical fiber was than rotated to the pointing angle at which the largest CAP amplitude was measured. At this angle, an energy-versus-CAP amplitude contour was recorded for at least 10 different radiant energy levels. Next, the optical fiber was retracted by 100–250 μm and the procedure was repeated. One to 25 locations along the cochlear turn were measured. After completion of the data acquisition the fiber was moved back to the location and the pointing angle at which the maximum response was observed. The initial measurement was repeated. At the conclusion of the measurements, the fluids were wicked away and were replaced by a viscous solution of dental acrylic. After the acrylic had cured the animal was euthanized, the bulla was harvested with the fiber in place and the cochlea and the optical fiber were imaged using microCT. The distance between the tip of the optical fiber and the modiolar wall could be measured and was used to calculate the energy at the bone, which was covering the spiral ganglion.
2.2 Reconstruction of the beam path with the microCT
The cochleae were imaged with the microCT operated at 45 kV tube voltage, 88 μA tube current and 300 ms integration time for each projection. A complete description of the scanner can be found elsewhere (Scanco Medical Web site, www.scanco.ch). Each of the 120–300 contiguous slices was reconstructed on 1,024 × 1,024 grid with 30 μm isotropic volume elements (voxels). The reconstructed slices were imported into ImageJ and were converted into a stack of TIFF files. Reorienting the reconstructed cochlea in ImageJ provided the images for the optical fibers and allowed to determine the distance from the optical stimulation source (end of the optical fiber) to the target structure (spiral ganglion neurons in Rosenthal’s canal). A detailed description of the Methods is provided for example in Stock [27].
2.3 Infrared neural stimulation
Cochlear stimulation was achieved with a diode laser (Lockheed Martin Aculight Corp., Bothell, WA). The laser was coupled to the angle-polished or flat-polished optical fibers (Ocean Optics Inc., Dunedin, FL) with a core diameter of 200 μm. The radiation wavelength was 1862 nm and the pulse duration was 50 or 100 μs. The pulse energy of the laser was controlled directly by varying the current to the laser diode and was between 0 and 127 μJ/pulse at the tip of the optical fiber in air. The energy E at the modiolus was calculated with the following equation: , where E denotes the energy per pulse at the modiolus, E0 the energy per pulse at the tip of the optical fiber, μ the extinction coefficient, and d the distance between the optical fiber and the modiolus that was determined from microCT. The corresponding radiant exposures at the modiolus were calculated by dividing the estimated energy value obtained at the modiolus (E0) by the spot size. The spot size was calculated by using the core diameter of the optical fiber, the spread of the radiation in water, and the distance of the tip of the optical fiber from the target structure, the modiolus. The radius r for the spot was calculated as r = d/tan(α), where α is the angel by which the radiation beams spreads with distance from the fiber. Pulses were presented at 250 Hz repetition rate in pulse trains of 300 ms duration. For each data point 10–20 pulse trains were presented at a repetition rate of 2 Hz.
2.4 Data analysis and statistics
For data analysis, the peak-to-peak amplitude of the CAP was measured either after the first infrared pulse was delivered or for the average amplitude of the CAPs which were recorded between 100 and 300 ms. Average ± standard deviations are provided.
3. RESULTS
The placement of the optical fiber relative to the modiolar wall was determined from microCT (Figure 1). From the tomographic reconstructions two different slices that contained the optical fiber were randomly selected. For each of the planes the distance between the tip of the optical fiber and the bony modiolus were measured along the beam path using ImageJ (NIH). The two values obtained for each cochlea were averaged. The distance between the tip of the optical fiber and the modiolus was between 150 and 1712 μm with and averaged 692±356 μm (mean±sd).
Figure 1.
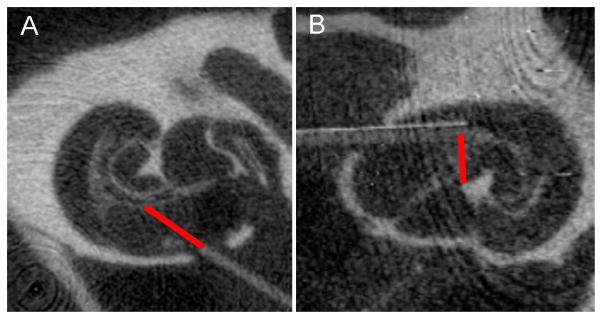
MicroCT images showing examples of the two different types of fibers, a flat polished fiber (A) and an angle polished fiber (B). The fibers were inserted into scala tympani of a guinea pig. The orientation of the flat polished fiber was towards the modiolus. The angle polished fiber was inserted along scala tympani. The red bar indicates the beam path.
3.1 Estimated stimulus level at the target
Threshold radiant energy for stimulation was derived from the experiments in which CAP amplitudes were measured for increasing energy levels. Threshold was determined as the energy at which the CAP amplitude was twice the size of the noise floor. Again, the threshold energy required to produce a response increased with increasing distance between the source of fiber and the modiolus. The values were between 4.8 and 47 μJ/pulse, with an average of 16.5±15.7 μJ/pulse. After correcting for the distance between the fiber and optical source, the radiant energies were similar for all distances of the optical fiber from the modiolus, 1.4 and 16.4 μJ/pulse (7.3±5.3 μJ/pulse). The range for the corresponding radiant exposures at the modiolus was between 3.3 and 41.1 mJ/cm2 (9.5±13.7 mJ/cm2, Figure 2).
Figure 2.
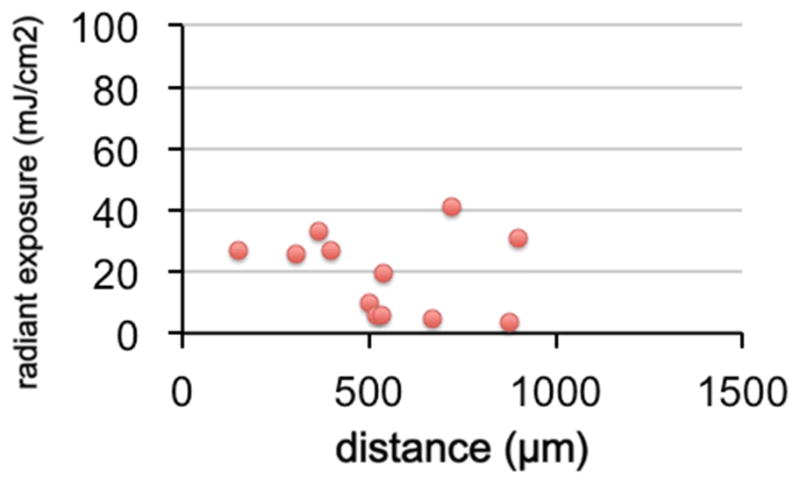
The corrected radiiant exposure for stimulation threshold is shown. Valu ues correspond well with the data previously published.
3.2 Angle polished optical fibers
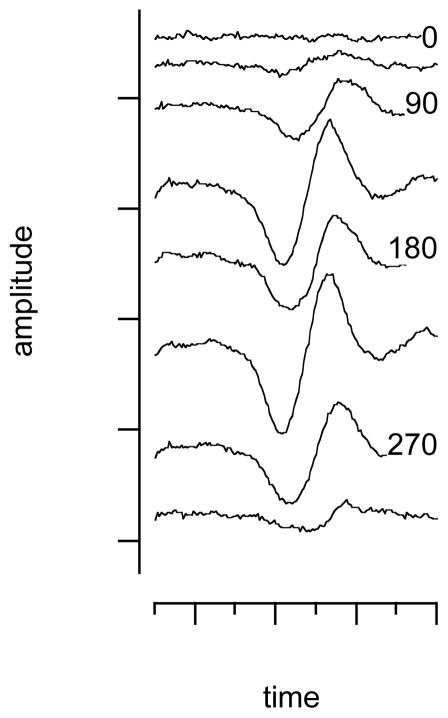
While the flat polished fibers were inserted into scala tympani, pointing with their tips towards the modiolus, the angle-polished optical fibers were inserted along a straight line into scala tympani until the fibers contacted the cochlear wall. Contact was determined visually by observing the point at which the optical fiber stopped advancing into scala tympani. After the fiber made contact with the cochlear wall it was retracted by about 100 μm and at this position the optical fiber was rotated over 360 degrees in steps of 45 degrees. At each angle, the CAP amplitude was recorded for a given fixed radiant energy (Figure 3). The angular position for which the neural responses were maximum could be identified. At approximately 180 degrees of rotation from the maximum, the INS evoked responses were minimum. The response did not always completely disappear, even when the fiber was pointed away from the spiral ganglion. This is in contrast to expected complete absence of response. Accuracy in the orientation of the fiber, potential scatter of photons from the bony cochlear wall, and the three dimensional expansion of the spiral ganglion along the cochlea may explain this discrepancy.
Figure 3.
The figure shows the CAP amplitude for a fixed energy level at different orientations of the optical fiber in a representative cochlea. It can be seen that the response is maximum for certain orientations of the fibers. The minimum occurs in the fiber is rotated 180° away from the maximum.
4. DISCUSSION
4.1 Spot size considerations
Results on INS have been published over the last decade by several groups [for review see e.g. 2, 29]. The criterion by which threshold for stimulation is defined varies largely among the studies. While some use direct electrical measurements from the nerve, others record electrical responses from the target organ, or visually determine threshold by the effect of stimulating the nerve, such muscle twitch, heart rate, or cavernous pressure. Moreover the stimulus level is reported in mJ/pulse, average power, peak power, or radiant exposure. While radiant energy/pulse, average and peak power can be directly measured at the tip of the optical fiber in air, the reporting of the radiant exposure requires the determination of the spot size.
For surgical applications, the effect of the spot size on laser tissue interaction has been discussed in the literature previously [18, 28]. The question about the spots size might be reconsidered for INS. For continuous wave (cw) lasers the irradiance [W/m2] is reported as the rate of energy delivery per second per unit area of the tissue surface, but the radiant exposure [J/cm2] is used for pulsed lasers to describe the energy delivery per pulse per unit area. Since the response to INS often includes the number of neurons stimulated synchronously it might be useful to report the radiant energy density [J/cm3], the radiant energy in an element divided by that element’s size. At a minimum, the reporting the stimulus levels required to evoke a response should be include parameters that clearly define the energy or power delivered to the tissue, and the parameter by which threshold has been defined.
Several methods have been applied to determine the spot size. It was determined with the knife edge technique as the with of the resulting energy profile at half maximum of the energy, as the area of the optical fiber, or has been calculated by using the numerical aperture and the distance of the target from the tip of the optical fiber. In two different studies either a thermal camera [30] or thermochromic ink [31] was used to determine the spot size of the laser beam at the target structure. Each of the approaches described above provides slightly different values for the spot size and makes a direct comparison in threshold radiant exposures among the studies difficult. Radiant energy or power at the tip of the optical fiber in air can be reported reliably and should be provided for each study. Furthermore, it is more than desirable that estimates of radiant energy and/or radiant exposure are reported at the target structure. For example, in this study we report the radiant energy per pulse at the tip of the optical fiber in air. The values are corrected for the absorption and scattering that might occur along the beam path, between the tip of the optical fiber and the modiolus. The spot size is calculated by using the optical properties of the different media and the optical properties of the fiber that is used to deliver the light.
4.2 INS versus electrical stimulation: power considerations
Current neural prostheses use electrical current to stimulate neurons. It has been demonstrated that stimulation threshold correlates with the charge delivered. For example, the charge per phase that is required to stimulate the auditory nerve is ~1 nC/phase [e.g. 32]. This corresponds to 20 μA/phase for a 100 μs pulse. With an electrode contact resistance of about 8 kΩ the power calculates to 3.2 μW/phase. For INS, the power that was required to reach threshold was on average 40 mW/pulse.
4.3 The cochlear implant using INS – design challenges
For the realization of an optical implant, three general approaches are directly apparent, an optical fiber bundle, light guides, and small laser sources. Optical fibers are stiff and the radius of curvature in the cochlea is small. The challenges include the optimization of the fiber size and associated coupling losses and losses that occur through bending of the fiber. Moreover, it will be challenging to predetermine the orientation of each of the side firing fiber towards the spiral ganglion. Optical light guides have not been produced and tested for the given wavelength yet. Again the challenge will be that the light guides are small enough to allow the fabrication of an array of “light sources” but big enough to delivery the energy to stimulate the neurons. Small laser sources can be placed like contacts of a contemporary electrical cochlear implant electrode. The challenge for this design will be size and temperature load of the cochlea. The efficiency of converting electrical energy into radiant energy for small light sources is about 30%. The remaining energy is lost and converted into heat, which has to be removed from the cochlea.
Design challenges clearly indicate that the placement of the optical sources, which includes control of the distance of the optical source from the target structure, and the pointing accuracy are important factors to be considered.
4.4 Pointing accuracy
The experiments with the angle polished fiber showed that the response depended on the orientation of the laser beam. When rotating the fiber, we have found an orientation that resulted in a maximum response. Further rotation of the optical fiber resulted in a decrease in response amplitude and resulted in a minimum when the beam was oriented away from the spiral ganglion neurons. We expected that the response would drop to zero when the beam was directed away from the target tissue. However, in some animals the responses did not drop to zero. While unexpected there are a few explanations that would be in agreement with the findings. It is possible that the spiral ganglion was partially located in the beam path, even though the laser is pointed in a direction that is 180° off the angle for the maximum response. Another possibility could relate to the fact that photons can be scattered from the bony wall and result in a neural response. Likewise, a laser evoked stress relaxation wave could result in an acoustic event and stimulate any remaining hair cells directly. The possibility of a photoacoustic effect cannot be ruled out completely since the optical fiber is completely immersed in the perilymph within the cochlea. Any photoacoustic effect generated by the laser would be expected to stimulate the hair cells to the same degree regardless of the orientation of the fiber. Arguments that INS in the cochlea is the direct interaction between the radiation and the neuron include the finding that neural activity evoked by INS is spatially localized and that INS is effective in chronically deaf and acutely deafened animals.
For a control, we also have compared the neural responses with the energy measured for different orientations of the optical fiber relative to an energy sensor. It can be seen, that the fibers are not ideal and the spread of the radiation occurs. From the results obtained from the CAP recordings and the measurements completed with the energy sensor, we can conclude that a pointing accuracy of ±90° is realistic.
4.5 Conclusions
In summary, optical simulation is possible with a side firing fiber. Energy values for stimulation on this little, but the placement of small optical sources in the cochlea might be a feasible approach for building a corporate employment-based optical energy. When compared with electrical stimulation, and optical implant would require similar amount of energies to simulate the neurons.
Acknowledgments
This project has been funded with federal funds from the National Institute on Deafness and Other Communication Disorders, R01 DC011855-01A1, DC011481-01A2 and by Lockheed Martin Aculight.
References
- 1.Richter CP, et al. Neural stimulation with optical radiation. Laser & Photonics Review. 2011;5(1):68–80. doi: 10.1002/lpor.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal V, et al. Acute damage threshold for infrared neural stimulation of the cochlea: functional and histological evaluation. Anatomical record. 2012;295(11):1987–99. doi: 10.1002/ar.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter CP, et al. Spread of cochlear excitation during stimulation with optical radiation: Inferior colliculus measurements. J Neural Eng. 2011;8(5):056006. doi: 10.1088/1741-2560/8/5/056006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajguru SM, et al. Optical path of infrared neural stimulation in the guinea pig and cat cochlea. Proc of SPIE. 2011;7883:788357–1. [Google Scholar]
- 5.Fried NM, et al. Laser stimulation of the cavernous nerves in the rat prostate, in vivo: optimization of wavelength, pulse energy, and pulse repetition rate. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:2777–80. doi: 10.1109/IEMBS.2008.4649778. [DOI] [PubMed] [Google Scholar]
- 6.Fried NM, et al. Noncontact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser. J Endourol. 2008;22(3):409–13. doi: 10.1089/end.2008.9996. [DOI] [PubMed] [Google Scholar]
- 7.McCaughey RG, Chlebicki C, Wong BJ. Novel wavelengths for laser nerve stimulation. Lasers Surg Med. 2010;42(1):69–75. doi: 10.1002/lsm.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells JD, et al. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg Med. 2007;39(6):513–26. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 9.Wells JD, et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys J. 2007 doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells J, et al. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10:064003. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 11.Wells J, et al. Optical stimulation of neural tissue in vivo. Optics Lett. 2005;30(5):504–506. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 12.Duke AR, et al. Spatial and temporal variability in response to hybrid electro-optical stimulation. Journal of neural engineering. 2012;9(3):036003. doi: 10.1088/1741-2560/9/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins MW, et al. Optical pacing of the embryonic heart. Nature Photonics. 2010;4:623–626. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duke AR, et al. Combined optical and electrical stimulation of neural tissue in vivo. JBO Letters. 2009;14(6):060501-1–060501-3. doi: 10.1117/1.3257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cayce JM, et al. Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. NeuroImage. 2011;57:155–166. doi: 10.1016/j.neuroimage.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cayce JM, et al. Relating Optical Signals Induced by Infrared Neural Stimulation to Electrophysiology. Biomedical Sciences and Engineering Conference (BSEC); 2010. in press. [Google Scholar]
- 17.Cayce JM, et al. Infrared neural stimulation of thalamocortical brain slices. IEEE J Sel Top Quantum Electron. 2010;16:565–572. [Google Scholar]
- 18.Welch AJ, van Gemert MJC. Optical-Thermal Response of Laser-Irradiated Tissue. 2. New York: Plenum Press; 2012. [Google Scholar]
- 19.Teudt IU, et al. Optical stimulation of the facial nerve: a new monitoring technique? Laryngoscope. 2007;117(9):1641–7. doi: 10.1097/MLG.0b013e318074ec00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells J, et al. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Methods. 2007;163(2):326–37. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells JD, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. Journal of Neuroscience Methods. 2006;163(2):326–37. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells JD, et al. Optical stimulation of neural tissue in vivo. Optics Letters. 2005;30(5):504–506. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 23.Duke AR, et al. Combined optical and electrical stimulation of neural tissue in vivo. J Biomed Opt. 2009;14(6):060501. doi: 10.1117/1.3257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajguru SM, et al. Infrared radiation of the cochlear spiral ganglion: optical spot size and spread of excitation in the inferior colliculus. Annals of Biomedical Engineering. 2013 in review. [Google Scholar]
- 25.Wells J, et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys J. 2007;93(7):2567–80. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno LE, et al. Infrared neural stimulation: beam path in the guinea pig cochlea. Hear Res. 2011 doi: 10.1016/j.heares.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stock SR. MicroComputed tomography: methodology and applications. Boca Raton, London, New York: CRC Press, Taylor & Francis Group; 2009. [Google Scholar]
- 28.Jacques SL. Laser-tissue interactions. Photochemical, photothermal, and photomechanical. Surg Clin North Am. 1992;72(3):531–58. doi: 10.1016/s0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 29.Richter CP, et al. Neural stimulation with optical radiation. Laser & photonics reviews. 2011;5(1):68–80. doi: 10.1002/lpor.200900044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells J, et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophysical journal. 2007;93(7):2567–80. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno LE, et al. Infrared neural stimulation: beam path in the guinea pig cochlea. Hearing research. 2011;282(1–2):289–302. doi: 10.1016/j.heares.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark G. Cochlear Implants: Fundamentals and Applications. Vol. 1. New York: Springer; 2003. [Google Scholar]