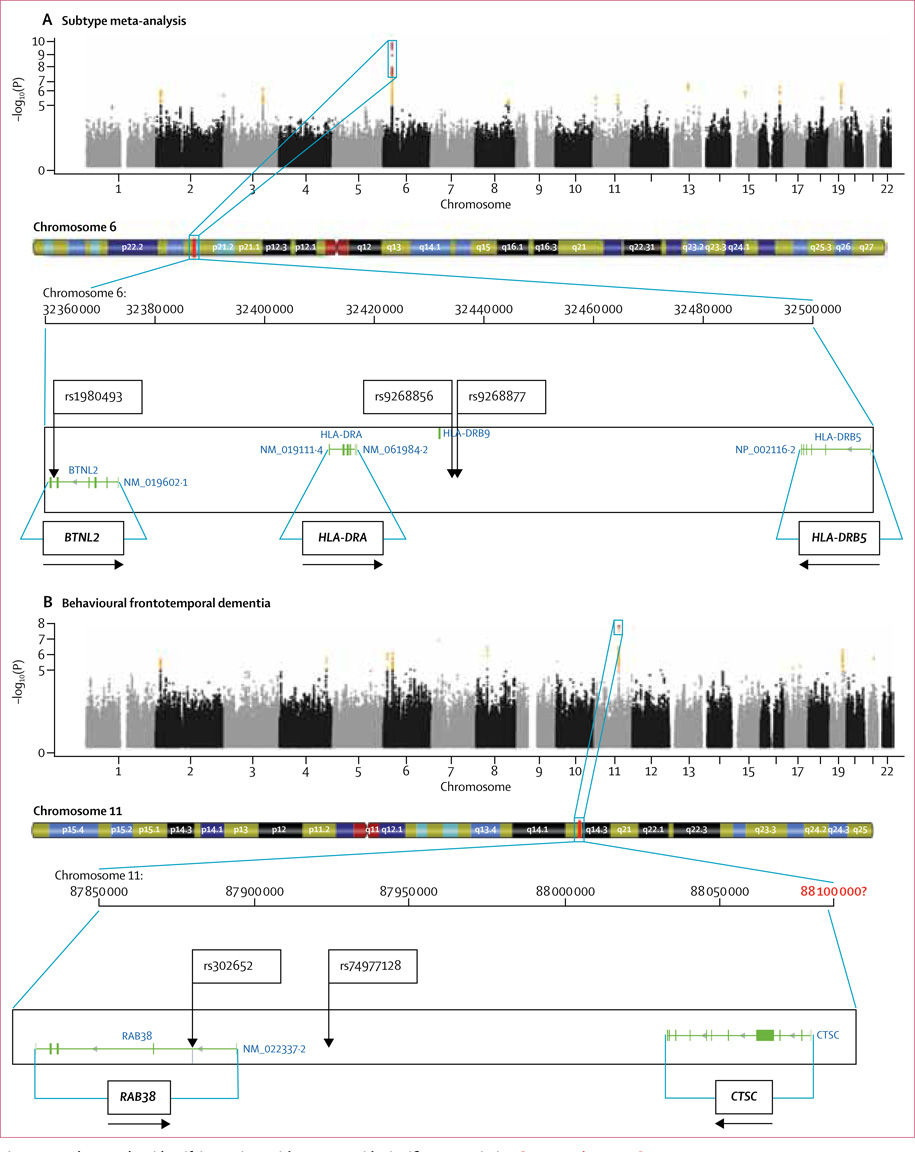
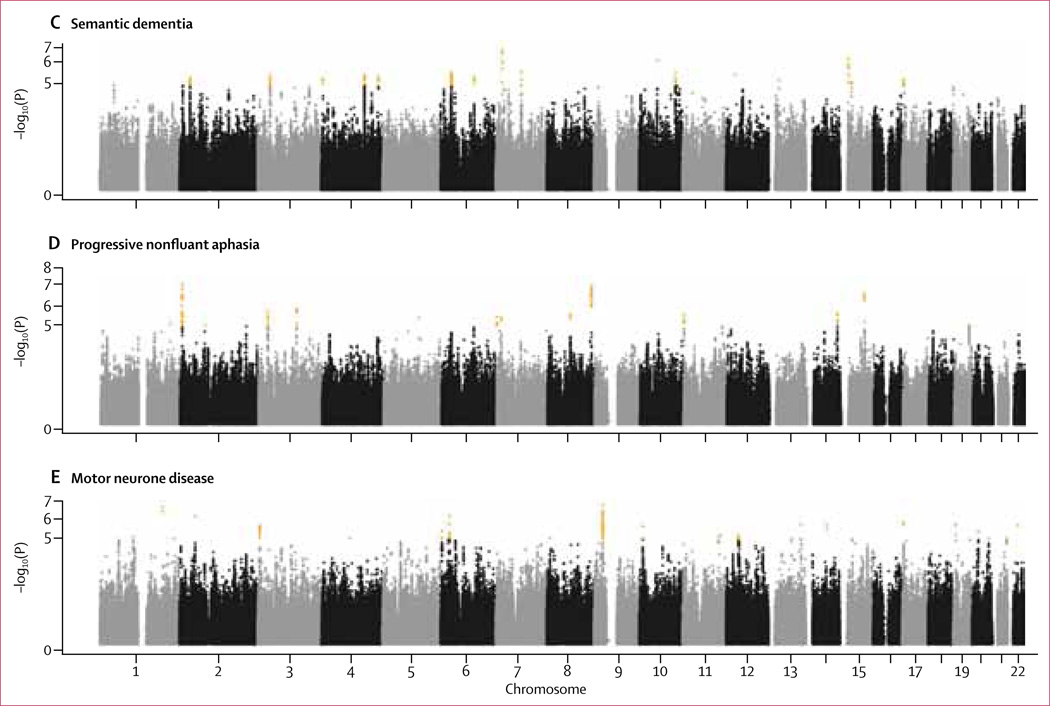
Raffaele Ferrari
Raffaele Ferrari
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,*,
Dena G Hernandez
Dena G Hernandez
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,*,
Michael A Nalls
Michael A Nalls
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,*,
Jonathan D Rohrer
Jonathan D Rohrer
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,*,
Adaikalavan Ramasamy
Adaikalavan Ramasamy
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
John B J Kwok
John B J Kwok
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Carol Dobson-Stone
Carol Dobson-Stone
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
William S Brooks
William S Brooks
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Peter R Schofield
Peter R Schofield
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Glenda M Halliday
Glenda M Halliday
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
John R Hodges
John R Hodges
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Olivier Piguet
Olivier Piguet
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Lauren Bartley
Lauren Bartley
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Elizabeth Thompson
Elizabeth Thompson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Eric Haan
Eric Haan
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Isabel Hernández
Isabel Hernández
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Agustín Ruiz
Agustín Ruiz
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Mercè Boada
Mercè Boada
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Barbara Borroni
Barbara Borroni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alessandro Padovani
Alessandro Padovani
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Carlos Cruchaga
Carlos Cruchaga
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Nigel J Cairns
Nigel J Cairns
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Luisa Benussi
Luisa Benussi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Giuliano Binetti
Giuliano Binetti
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Roberta Ghidoni
Roberta Ghidoni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Gianluigi Forloni
Gianluigi Forloni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Daniela Galimberti
Daniela Galimberti
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Chiara Fenoglio
Chiara Fenoglio
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maria Serpente
Maria Serpente
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Elio Scarpini
Elio Scarpini
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Jordi Clarimón
Jordi Clarimón
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alberto Lleó
Alberto Lleó
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Rafael Blesa
Rafael Blesa
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maria Landqvist Waldö
Maria Landqvist Waldö
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Karin Nilsson
Karin Nilsson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Christer Nilsson
Christer Nilsson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Ian R A Mackenzie
Ian R A Mackenzie
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Ging-Yuek R Hsiung
Ging-Yuek R Hsiung
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
David M A Mann
David M A Mann
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Jordan Grafman
Jordan Grafman
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Christopher M Morris
Christopher M Morris
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Johannes Attems
Johannes Attems
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Timothy D Griffiths
Timothy D Griffiths
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Ian G McKeith
Ian G McKeith
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alan J Thomas
Alan J Thomas
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
P Pietrini
P Pietrini
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Edward D Huey
Edward D Huey
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Eric M Wassermann
Eric M Wassermann
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Atik Baborie
Atik Baborie
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Evelyn Jaros
Evelyn Jaros
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Michael C Tierney
Michael C Tierney
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Pau Pastor
Pau Pastor
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Cristina Razquin
Cristina Razquin
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Sara Ortega-Cubero
Sara Ortega-Cubero
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Elena Alonso
Elena Alonso
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Robert Perneczky
Robert Perneczky
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Janine Diehl-Schmid
Janine Diehl-Schmid
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Panagiotis Alexopoulos
Panagiotis Alexopoulos
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alexander Kurz
Alexander Kurz
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Innocenzo Rainero
Innocenzo Rainero
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Elisa Rubino
Elisa Rubino
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Lorenzo Pinessi
Lorenzo Pinessi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Ekaterina Rogaeva
Ekaterina Rogaeva
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Peter St George-Hyslop
Peter St George-Hyslop
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Giacomina Rossi
Giacomina Rossi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Fabrizio Tagliavini
Fabrizio Tagliavini
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Giorgio Giaccone
Giorgio Giaccone
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
James B Rowe
James B Rowe
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
J C M Schlachetzki
J C M Schlachetzki
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
James Uphill
James Uphill
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
John Collinge
John Collinge
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
S Mead
S Mead
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Adrian Danek
Adrian Danek
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Vivianna M Van Deerlin
Vivianna M Van Deerlin
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Murray Grossman
Murray Grossman
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
John Q Trojanowsk
John Q Trojanowsk
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Julie van der Zee
Julie van der Zee
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
William Deschamps
William Deschamps
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Tim Van Langenhove
Tim Van Langenhove
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Marc Cruts
Marc Cruts
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Christine Van Broeckhoven
Christine Van Broeckhoven
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Stefano F Cappa
Stefano F Cappa
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Isabelle Le Ber
Isabelle Le Ber
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Didier Hannequin
Didier Hannequin
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Véronique Golfier
Véronique Golfier
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Martine Vercelletto
Martine Vercelletto
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alexis Brice
Alexis Brice
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Benedetta Nacmias
Benedetta Nacmias
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Sandro Sorbi
Sandro Sorbi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Silvia Bagnoli
Silvia Bagnoli
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Irene Piaceri
Irene Piaceri
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Jørgen E Nielsen
Jørgen E Nielsen
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Lena E Hjermind
Lena E Hjermind
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Matthias Riemenschneider
Matthias Riemenschneider
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Manuel Mayhaus
Manuel Mayhaus
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Bernd Ibach
Bernd Ibach
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Gilles Gasparoni
Gilles Gasparoni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Sabrina Pichler
Sabrina Pichler
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Wei Gu
Wei Gu
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Martin N Rossor
Martin N Rossor
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Nick C Fox
Nick C Fox
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Jason D Warren
Jason D Warren
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maria Grazia Spillantini
Maria Grazia Spillantini
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Huw R Morris
Huw R Morris
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Patrizia Rizzu
Patrizia Rizzu
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Peter Heutink
Peter Heutink
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Julie S Snowden
Julie S Snowden
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Sara Rollinson
Sara Rollinson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Anna Richardson
Anna Richardson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alexander Gerhard
Alexander Gerhard
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Amalia C Bruni
Amalia C Bruni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Raffaele Maletta
Raffaele Maletta
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Francesca Frangipane
Francesca Frangipane
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Chiara Cupidi
Chiara Cupidi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Livia Bernardi
Livia Bernardi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maria Anfossi
Maria Anfossi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maura Gallo
Maura Gallo
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Maria Elena Conidi
Maria Elena Conidi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Nicoletta Smirne
Nicoletta Smirne
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Rosa Rademakers
Rosa Rademakers
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Matt Baker
Matt Baker
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Dennis W Dickson
Dennis W Dickson
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Neill R Graff-Radford
Neill R Graff-Radford
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Ronald C Petersen
Ronald C Petersen
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
David Knopman
David Knopman
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Keith A Josephs
Keith A Josephs
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Bradley F Boeve
Bradley F Boeve
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Joseph E Parisi
Joseph E Parisi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
William W Seeley
William W Seeley
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Bruce L Miller
Bruce L Miller
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Anna M Karydas
Anna M Karydas
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Howard Rosen
Howard Rosen
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
John C van Swieten
John C van Swieten
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Elise G P Dopper
Elise G P Dopper
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Harro Seelaar
Harro Seelaar
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Yolande AL Pijnenburg
Yolande AL Pijnenburg
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Philip Scheltens
Philip Scheltens
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Giancarlo Logroscino
Giancarlo Logroscino
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Rosa Capozzo
Rosa Capozzo
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Valeria Novelli
Valeria Novelli
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Annibale A Puca
Annibale A Puca
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
M Franceschi
M Franceschi
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Alfredo Postiglione
Alfredo Postiglione
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Graziella Milan
Graziella Milan
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Paolo Sorrentino
Paolo Sorrentino
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Mark Kristiansen
Mark Kristiansen
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Huei-Hsin Chiang
Huei-Hsin Chiang
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Caroline Graff
Caroline Graff
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Florence Pasquier
Florence Pasquier
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Adeline Rollin
Adeline Rollin
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Vincent Deramecourt
Vincent Deramecourt
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Florence Lebert
Florence Lebert
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Dimitrios Kapogiannis
Dimitrios Kapogiannis
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Luigi Ferrucci
Luigi Ferrucci
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Stuart Pickering-Brown
Stuart Pickering-Brown
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,
Andrew B Singleton
Andrew B Singleton
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,†,
John Hardy
John Hardy
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,†,
Parastoo Momeni
Parastoo Momeni
1Laboratory of Neurogenetics, Department of Internal Medicine, Texas Tech University Health Science Center, Lubbock, Texas, USA (R Ferrari MSc, P Momeni PhD); Reta Lila Weston Research Laboratories, Department of Molecular Neuroscience, UCL Institute of Neurology, London, UK (R Ferrari, D G Hernandez MSc, J D Rohrer PhD, A Ramasamy PhD, J Hardy PhD); Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA (D G Hernandez, M A Nalls PhD, A B Singleton PhD); Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA (L Ferrucci MD); Institute of Brain, Behaviour and Mental Health, Faculty of Medical and Human Sciences, University of Manchester, Manchester, UK (J S Snowden PhD, S Rollinson PhD, S Pickering-Brown PhD); Neuroscience Research Australia, Sydney, NSW, Australia (J B J Kwok PhD, C Dobson-Stone PhD, W S Brooks MBBS, P R Schofield DSc, G M Halliday PhD, J R Hodges MD, O Piguet PhD, L Bartley MSc); University of New South Wales, Sydney, NSW, Australia (J B J Kwok, C Dobson-Stone, W S Brooks, P R Schofield, G M Halliday, J R Hodges, O Piguet); South Australian Clinical Genetics Service, SA Pathology at Women’s and Children’s Hospital, North Adelaide, SA, Australia (E Thompson MD, E Haan MBBS); Department of Paediatrics, University of Adelaide, Adelaide, SA, Australia (E Thompson, E Haan); Memory Clinic of Fundació ACE, Institut Català de Neurociències Aplicades, Barcelona, Spain (I Hernández MD, A Ruiz MD, M Boada MD); Hospital Universitari Vall d’Hebron–Institut de Recerca, Universitat Autonoma de Barcelona (VHIR-UAB), Barcelona, Spain (M Boada); Neurology Clinic, University of Brescia, Brescia, Italy (B Borroni MD, A Padovani MD); Department of Psychiatry (C Cruchaga PhD), Hope Center (C Cruchaga, N J Cairns PhD), Washington University School of Medicine, St Louis, Missouri, USA; Department of Pathology and Immunology, Washington University, St Louis, Missouri, USA (N J Cairns); NeuroBioGen Lab-Memory Clinic, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy (L Benussi PhD, G Binetti MD); Proteomics Unit, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia Italy (L Benussi, G Binetti); Biology of Neurodegenerative Disorders, IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milano, Italy (G Forloni PhD); University of Milan, Milan, Italy (D Galimberti PhD, C Fenoglio PhD, M Serpente PhD, E Scarpini MD); Fondazione Cà Granda, IRCCS Ospedale Maggiore Policlinico, Milan, Italy (D Galimberti, C Fenoglio, M Serpente, E Scarpini); Memory Unit, Neurology Department and Sant Pau Biomedical Research Institute, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain (J Clarimón PhD, A Lleó MD, R Blesa MD); Center for Networker Biomedical Research in Neurodegenerative Diseases (CIBERNED), Madrid, Spain (J Clarimón, A Lleó, R Blesa, P Pastor MD, S Ortega-Cubero MD); Unit of Geriatric Psychiatry (M L Waldö MD, K Nilsson PhD), Clinical Memory Research Unit (C Nilsson PhD), Department of Clinical Sciences, Lund University, Sweden; Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada (I R A Mackenzie MD); Division of Neurology, University of British Columbia, Vancouver, Canada (G-Y R Hsiung MD); Institute of Brain, Behaviour and Mental Health, University of Manchester, Salford Royal Hospital, Stott Lane, Salford, UK (D M A Mann PhD); Rehabilitation Institute of Chicago, Departments of Physical Medicine and Rehabilitation, Psychiatry, and Cognitive Neurology and Alzheimer’s Disease Center; Feinberg School of Medicine, Northwestern University, USA (J Grafman PhD, C M Morris PhD, J Attems MD, T D Griffiths FMedSci); Department of Psychology, Weinberg College of Arts and Sciences, Northwestern University, USA (J Grafman); Newcastle Brain Tissue Resource, Institute for Ageing and Health, Newcastle University, Newcastle upon Tyne, UK (C M Morris, J Attems MD, T D Griffiths FMedSci); Newcastle University, Institute for Ageing and Health, Campus for Ageing and Vitality, Newcastle upon Tyne, UK (C M Morris, J Attems MD, A J Thomas PhD, E Jaros PhD); Institute of Neuroscience, Newcastle University Medical School, Newcastle upon Tyne, UK (C M Morris, T D Griffiths FMedSci); Biomedical Research Building, Campus for Ageing and Vitality, Newcastle University, Newcastle upon Tyne, UK (I G McKeith MD); Clinical Psychology Branch, Pisa University Hospital, Pisa, Italy, Laboratory of Clinical Biochemistry and Molecular Biology, University of Pisa, Pisa, Italy (P Pietrini MD); Taub Institute, Departments of Psychiatry and Neurology, Columbia University, New York, NY, USA 10032 (E D Huey MD); Behavioral Neurology Unit, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA (E M Wassermann MD, M C Tierney MSc); Neuropathology Department, Walton Centre FT, Liverpool, UK (A Baborie MD); Neuropathology/Cellular Pathology, Royal Victoria Infirmary, Newcastle upon Tyne, UK (E Jaros); Neurogenetics Laboratory, Division of Neurosciences, Center for Applied Medical Research, Universidad de Navarra, Pamplona, Spain (P Pastor, C Razquin PhD, S Ortega-Cubero, E Alonso BSc); Department of Neurology, Clínica Universidad de Navarra, University of Navarra School of Medicine, Pamplona, Spain (P Pastor); Neuroepidemiology and Ageing Research Unit, School of Public Health, Faculty of Medicine, The Imperial College of Science, Technology and Medicine, London, UK (R Perneczky MD); West London Cognitive Disorders Treatment and Research Unit, West London Mental Health Trust, London TW8 8 DS, UK (R Perneczky); Department of Psychiatry and Psychotherapy, Technische Universität München, Munich, Germany (R Perneczky, J Diehl-Schmid MD, P Alexopoulos MD, A Kurz MD); Neurology I, Department of Neuroscience, University of Torino, Italy, AO Città della Salute e della Scienza di Torino, Italy (I Rainero MD, E Rubino MD, L Pinessi MD); Tanz Centre for Research in Neurodegenerative Diseases and Department of Medicine, University of Toronto, Toronto, Ontario, Canada (E Rogaeva PhD, P St George-Hyslop MD); Cambridge Institute for Medical Research and the Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK (P St George-Hyslop); Division of Neurology V and Neuropathology, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano Italy (G Rossi PhD, F Tagliavini MD, G Giaccone MD); Cambridge University Department of Clinical Neurosciences, Cambridge, CB2 0SZ, UK (J B Rowe PhD); MRC Cognition and Brain Sciences Unit, Cambridge, UK (J B Rowe); Behavioural and Clinical Neuroscience Institute, Cambridge, UK (J B Rowe); Department of Psychiatry and Psychotherapy, University of Freiburg Medical School, Germany (J C M Schlachetzki MD); Department of Molecular Neurology, University Hospital Erlangen, Erlangen, Germany (J C M Schlachetzki); MRC Prion Unit, Department of Neurodegenerative Disease, UCL Institute of Neurology, London, UK (J Uphill BSc, J Collinge MD, S Mead PhD); Neurologische Klinik und Poliklinik, Ludwig-Maximilians-Universität, Munich, Germany (A Danek MD); German Center for Neurodegenerative Diseases (DZNE), Munich, Germany (A Danek); University of Pennsylvania Perelman School of Medicine, Department of Neurology and Penn Frontotemporal Degeneration Center, Philadelphia, PA, USA (V M Van Deerlin PhD, M Grossman MD, J Q Trojanowski PhD); Neurodegenerative Brain Diseases group, Department of Molecular Genetics, VIB, Antwerp, Belgium (J van der Zee PhD, W Deschamps MSc, T Van Langenhove MD, M Cruts PhD, C Van Broeckhoven PhD); Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium (J van der Zee, W Deschamps, T Van Langenhove, M Cruts, C Van Broeckhoven); Neurorehabilitation Unit, Deptartment Of Clinical Neuroscience, Vita-Salute University and San Raffaele Scientific Institute, Milan, Italy (S F Cappa MD); Inserm, UMR_S975, CRICM, F-75013; UPMC Univ Paris 06, UMR_S975, F-75013; and CNRS UMR 7225, F-75013, Paris, France (I Le Ber MD, A Brice MD); AP-HP, Hôpital de la Salpêtrière, Département de neurologie-centre de références des démences rares, F-75013, Paris, France (I Le Ber, A Brice); Service de Neurologie, Inserm U1079, CNR-MAJ, Rouen University Hospital, France (D Hannequin MD); Service de neurologie, CH Saint Brieuc, France (V Golfier MD); Service de Neurologie, CHU Nantes, France (M Vercelletto MD); Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA) University of Florence, Florence, Italy (B Nacmias PhD, S Sorbi PhD, S Bagnoli PhD, I Piaceri PhD); Danish Dementia Research Centre, Neurogenetics Clinic, Department of Neurology, Rigshospitalet, Copenhagen University Hospital, Denmark (J E Nielsen MD, L E Hjermind MD); Department of Cellular and Molecular Medicine, Section of Neurogenetics, The Panum Institute, University of Copenhagen, Denmark (J E Nielsen MD, L E Hjermind MD); Saarland University Hospital, Department for Psychiatry and Psychotherapy, Homburg/Saar, Germany (M Riemenschneider MD); Saarland University, Laboratory for Neurogenetics, Kirrberger, Homburg/Saar, Germany (M Riemenschneider, M Mayhaus PhD, G Gasparoni PhD, S Pichler MSc, W Gu PhD); University Regensburg, Department of Psychiatry, Psychotherapy and Psychosomatics, Universitätsstr 84, Regensburg, Germany (B Ibach PhD); Luxembourg Centre For Systems Biomedicine (LCSB), University of Luxembourg, Luxembourg (W Gu); Dementia Research Centre, Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK (J D Rohrer, M N Rossor MD, N C Fox MD, J D Warren PhD); University of Cambridge, Department of Clinical Neurosciences, John Van Geest Brain Repair Centre, Cambridge, UK (M G Spillantini PhD); MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, School of Medicine, Cardiff, UK (H R Morris PhD); German Center of Neurodegenerative Diseases-Tübingen, Tübingen, Germany (P Rizzu PhD, P Heutink PhD); Salford Royal Foundation Trust, Faculty of Medical and Human Sciences, University of Manchester, UK (A Richardson MB); Institute of Brain, Behaviour and Mental Health, The University of Manchester, Withington, Manchester, UK (A Gerhard MD); Regional Neurogenetic Centre, ASPCZ, Lamezia Terme, Italy (A C Bruni MD, R Maletta MD, F Frangipane MD, C Cupidi MD, L Bernardi PhD, M Anfossi PhD, M Gallo PhD, M Elena Conidi PhD, N Smirne BSc); Department of Neuroscience (R Rademakers PhD, M Baker BSc, D W Dickson MD), Department of Neurology (N R Graff-Radford MD), Mayo Clinic Jacksonville, Jacksonville, FL, USA; Department of Neurology(R C Petersen MD, D Knopman MD, K A Josephs MD, B F Boeve MD), Department of Pathology (J E Parisi MD), Mayo Clinic Rochester, Rochester, MN USA; Department of Neurology (W W Seeley MD, B L Miller MD, A M Karydas BA, H Rosen MD), University of California, San Francisco, CA, USA; Department of Neurology, Erasmus Medical Centre, Rotterdam, The Netherlands (J C van Swieten MD, E G P Dopper MD, H Seelaar PhD); Department of Medical Genetics, VU university Medical Centre, Amsterdam, The Netherlands (J C van Swieten); Alzheimer Centre and Department of Neurology, VU University medical centre, Amsterdam, The Netherlands (Y A L Pijnenburg MD, P Scheltens MD); Department of Basic Medical Sciences, Neurosciences and Sense Organs of the Aldo Moro University of Bari, Italy (G Logroscino MD, R Capozzo MD); Department of Medical and Molecular Genetics, King1s College London, Guy’s Hospital, London, UK (A Ramasamy); Department of Molecular Cardiology, IRCCS Fondazione S Maugeri, Pavia, Italy (V Novelli PhD); Cardiovascular Research Unit, IRCCS Multimedica, Milan, Italy (An A Puca MD); Department of Medicine and Surgery, University of Salerno, Baronissi (SA), Italy (An A Puca); Neurology Department, IRCCS Multimedica, Milan, Italy (M Franceschi MD); Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy (A Postiglione MD); Geriatric Center Frullone- ASL Napoli 1 Centro, Naples, Italy (G Milan MD, P Sorrentino MD); UCL Genomics, Institute of Child Health (ICH), UCL, London, UK (M Kristiansen PhD); Karolinska Institutet, Department NVS, KI-Alzheimer Disease Research Center, Stockholm, Sweden (H-H Chiang PhD, C Graff MD); Department of Geriatric Medicine, Genetics Unit, Karolinska Universtiy Hospital, Stockholm (H-H Chiang, C Graff); Université Lille Nord de France, Lille, France (F Pasquier MD, A Rollin MD, V Deramecourt MD, F Lebert MD); and National Institute on Aging (NIA/NIH),, Baltimore, MD, USA (D Kapogiannis MD)
1,†