Abstract
This review summarizes the historical and more recent developments of multiphoton microscopy, as applied to dermatology. Multiphoton microscopy offers several advantages over competing microscopy techniques: there is an inherent axial sectioning, penetration depths that compete well with confocal microscopy on account of the use of near-infrared light, and many two-photon contrast mechanisms, such as second-harmonic generation, have no analogue in one-photon microscopy. While the penetration depths of photons into tissue are typically limited on the order of hundreds of microns, this is of less concern in dermatology, as the skin is thin and readily accessible. As a result, multiphoton microscopy in dermatology has generated a great deal of interest, much of which is summarized here. The review covers the interaction of light and tissue, as well as the various considerations that must be made when designing an instrument. The state of multiphoton microscopy in imaging skin cancer and various other diseases is also discussed, along with the investigation of aging and regeneration phenomena, and finally, the use of multiphoton microscopy to analyze the transdermal transport of drugs, cosmetics and other agents is summarized. The review concludes with a look at potential future research directions, especially those that are necessary to push these techniques into widespread clinical acceptance.
Keywords: Multiphoton microscopy, skin, cancer, aging and regeneration
1. Introduction
The skin is the largest and most easily accessible organ in the body, and consequently, many optical methods are first developed for dermatological studies. The skin is also comparatively thin, having thickness compatible with ballistic penetration depth of light in tissues: on the order of hundreds of microns.1 These factors contributed to the early adoption of multiphoton microscopy to study skin physiology2 and the subsequent application of multiphoton microscopy to the diagnosis of skin cancer and other diseases, the monitoring of skin aging and regeneration processes, and transport studies of chemicals, drugs and nanoparticles through the skin, with implications in drug delivery, cosmetics and sun protection.
There are other excellent reviews of this field and readers looking for another perspective are invited to pursue these further. Previous reviews include an extensive review by Schenke-Layland et al. that summarized the field up to 20063 and a shorter review by Lin et al. with a more clinical perspective.1 More recently, Wang et al. published a review focused primarily on ophthalmology but with a substantial section on skin.4 In another work, König et al. discussed the use of multiphoton imaging in drug delivery research, with emphasis on the treatment of photoaging.5 The use of multi-photon fluorescence lifetime imaging microscopy (FLIM) for skin imaging has attracted recent interest, with reviews by Cicchi and Pavone,6 Seidenari et al.,7 and König.8 Campagnola and Dong have summarized the use of second-harmonic generation in disease diagnosis, including its use in dermatology.9 Finally, Perry et al. have published a review on the use of multiphoton imaging in cancer research which contains a lengthy discussion on skin cancers.10
While many researchers have contributed to this field, it is easy to recognize the works of König and his co-workers as being singularly substantial. König and co-workers have contributed to almost every aspect of dermatological research based on multiphoton microscopy. More importantly, this group was the first to successfully commercialize multiphoton dermatological instrumentation that is compatible with clinical use. Their work has established the safety and efficacy of multiphoton imaging in the skin.11 They have further obtained regulatory approval for the clinical use of these instruments in the European Union and several other countries. The availability of commercial instruments has significantly increased the routine use of multiphoton microscopic imaging by clinicians; progress in this area has been summarized in several articles.12–16
This review will first focus on skin physiology and its optical properties. The historical development of multiphoton techniques for skin imaging will be briefly described. The focus of this review will then shift to cover the different dermatological diseases and phenomena that can be studied using multiphoton microscopy, and will close with a discussion on the future directions within this field.
2. Optical Considerations in Multiphoton Microscope Skin Imaging
The physiology and optical properties of the skin have been studied extensively. Knowledge of the optical properties of the skin allows the rational design of multiphoton microscopy technologies for dermal imaging. In this section, we will provide a brief overview of skin physiology with an emphasis on its optical properties. A brief history of the development of multiphoton microscope technologies for skin imaging will also be provided.
2.1. Skin physiology and optical properties
Physiologically, skin may appear to be simple but is actually a complex organ consisting of two structurally distinct regions.17 The outer region, known as the epidermis, consists of stratified cells and is relatively thin, on the order of 100-150 μm. The exterior of the epidermis is the stratum corneum, consisting of five or six layers of cornified dead cells that are in the process of being sloughed off. The next two strata, the stratum granulosum and the stratum spinosum, each consist of several layers of living keratinocytes. The keratinocytes in the stratum spinosum tend to be polyhedral in shape. The bottom stratum of the epidermal region is the basal cell layer, where the cells are in the process of proliferating and differentiating into new cells that migrate toward the surface. Four types of cells are located within the living epidermis. The majority are keratinocytes, with the few remaining percent being dendritic cells: Langerhans cells, melanocytes and Merkel cells. Melanocytes produce the pigment melanin which is formed into vesicles called melanosomes. Melanin is not a homogenous chemical species but can be roughly classified as either eumelanin or pheomelanin, each with distinct spectroscopic properties. Eventually the melanosomes are transferred from the melanocytes into the basal epithelial cells. The epidermis also contains other physiological structures, including free nerve endings, hair, sweat and apocrine glands.
The dermal region is located under the epidermis. It is important to note that the epidermal–dermal junction is not a flat surface, but has a characteristic, undulating reteridge structure. The dermis primarily consists of extracellular matrix tissue, including collagen and elastin fibers, and a sparse population of cells including fibroblasts, macrophages and adipocytes. Within the dermis, there are also many important functional organs, including hair follicles, sweat, sebaceous and apocrine glands, nerve fibers and their receptors. The hair follicle structure is particularly important as recent studies have shown that the skin stem cells are located in niches close to the base of the hair follicle; these stem cells are required for the repair of the skin after injury. The vasculature and the lymphatic vessels can also be found within the dermis. The capillary loops of the vasculature extending into the tips of the reteridge supply oxygen to the living cells both in the dermis and the epidermis. The thickness of the dermis ranges from hundreds of micrometers to millimeters, with the actual thickness depending on the location on the body and the species of the organism.
While skin structure and biochemistry can be studied with multiphoton microscopy using extrinsic contrast agents such as organic fluorophores or genetically expressible fluorescent proteins, it is important to note that many skin components can be imaged based on endogenous contrast. Keratinocytes can be visualized based on fluorescence from reduced pyridine nucleotides and oxidized flavin proteins. Both flavin proteins and NAD(P)H are localized in the cellular mitochondria, but NAD(P) H is also present in the rest of the cytosol. Importantly, cellular metabolism can be noninvasively monitored by redox fluorometry.18,19 Endogenous fluorescence imaging of the structural protein keratin that is abundant in the stratum corneum has also been reported.20 In the dermis, collagen and elastin are also observable based on their fluorescence. However, several isoforms of collagen, including type I, often produce a higher second harmonic generation (SHG) signal due to their noncentrosymmetric molecular structure and crystalline organization. The combination of the SHG signal with polarization-resolved imaging can further provide information on the elements of the χ2 susceptibility matrix, which in turn can provide more detailed information regarding the molecular level organization of the collagen fibrils, such as their chirality. Because of the importance of melanoma as a disease, melanin has also been an important target for multiphoton imaging. A recent study21 based on pump-probe transient absorption imaging has demonstrated the distinction between pheomelanin and eumelanin; their relative abundances has been postulated to be an oncogenic factor.22 Other reports suggest that melanin can be imaged based on a stepwise-multiphoton excitation process.23
Multiphoton imaging of the skin is complicated by its stratified structure, since the different structural layers have very distinct refractive indices, as studied by Tearney and co-workers.24 As a result of refractive index variation, the actual change in the focal depth of focused light differs from that expected by the physical translation of the specimen, and this difference can be measured quantitatively using optical coherence tomography. They found that the refractive indices in stratum corneum, epidermis and dermis have values of 1.51, 1.34 and 1.41, respectively. Effectively, the stratum corneum has reflective index close to oil, while the epidermis has index close to water and the dermis lies in between. For multiphoton imaging, this multi-layered structure with different indices of refraction causes spherical aberration and distorts the excitation focus, resulting in signal loss and a reduction in image resolution. In other more homogenous tissues, spherical aberration can be mostly eliminated by choosing a microscope objective that matches the refractive index of the tissue. However, in heterogenous structures such as the skin, effective matching is very difficult and significant aberration remains, even when using objectives equipped with correction collars.25
Another limitation imposed by tissue physiology on multiphoton skin imaging lies in possible photodamage. While multiphoton microscopy generates less photodamage in thick tissue as compared with most one-photon modalities such as confocal fluorescence microscopy, at the focal volume where photochemical interactions occur, multiphoton processes can still cause considerable photodamage. Today, three multiphoton photodamage mechanisms are well recognized. (a) Oxidative photo-damage can be caused by two or higher photon excitation of endogenous and exogenous fluorophores, with a photodamage pathway similar to that of ultraviolet irradiation. These fluorophores act as photosensitizers in photo-oxidative processes.26,27 Photo-activation of these fluorophores results in the formation of reactive oxygen species (ROS) which trigger the subsequent biochemical damage cascade in cells. Current studies found that the degree of photodamage follows a quadratic dependence on excitation power, indicating that two-photon processes are the primary damage mechanism.28–32 Flavin-containing oxidases have been identified as one of the primary endogenous targets for photodamage.28 (b) Photodamage may also be caused by mechanisms resulting from the high peak power of the fem to second laser pulses. There are indications that dielectric breakdown occasionally occurs.30 (c) Most importantly, one-and two-photon absorption of high-power infrared radiation may also produce thermal damage. The temperature change resulting from two-photon absorption has been estimated to be on the order of 1 mK for typical excitation power, and has been shown to be insignificant.33,34 However, in the presence of a strong infrared absorber such as melanin,35,36 there can be appreciable heating due to one-photon absorption. Thermal damage has been observed in the basal layer of human skin when irradiated by lasers with high average excitation powers.37 Masters et al. subsequently performed an in-depth study, firmly establishing one-photon absorption by melanin as the primary photodamage mechanism that limits the maximum power that can be used for skin imaging.38
2.2. The development of multiphoton technology for skin imaging
The demonstration of noninvasive imaging of living embryos by Denk and co-workers firmly established multiphoton microscopy as the method of choice for the high-resolution study of optically thick specimens.39 Piston and co-workers performed the first multiphoton ex vivo tissue study by imaging cornea structures of rabbit cornea.40 Given the optical accessibility of the skin, it is not surprising that the second application of multiphoton microscopy in tissue focused on the skin.2 In this study, Masters and co-workers demonstrated that multiphoton microscopy can image the skin down to a penetration depth of 150 μm, resolving all the stratified cellular layers in the epidermis and partly into the dermis. In the stratum spinosum, granulosum, and the basal layer, living keratinocytes were observed; the cytosol and the mitochondrial structures were imaged using NAD(P)H fluorescence. Cellular nuclei can be seen as circular voids where fluorescent species are mostly absent. Morphological changes of keratinocytes from a cuboidal geometry (in the basal layer) to a flattened geometry (in the stratum spinosum) were observed, consistent with previous histological studies and confocal microscopy. A bright fluorescent signal was also observed in the stratum corneum that may be assigned to keratin today but was not identified in the work of Masters and co-workers. The reteridge morphology of the epidermal-dermal junction was also reconfirmed in this study. In the dermis, a signal from extracellular matrix was also clearly observed, showing the characteristic fibrous structure. Finally, it is important to note that this study was also the first in vivo human application of multiphoton microscopy.
Multiphoton microscopy not only allows the imaging of skin morphology in 3D with sub-micron resolution, but the incorporation of spectroscopic measurement further allows quantification of the tissue biochemical state. Excitation/emission spectroscopy and lifetime resolved spectroscopy are two of the most widely used spectroscopic modalities for skin characterization. The utility of both spectroscopic techniques for skin studies were both first demonstrated by Masters and co-workers in the initial multiphoton study of skin.2 In this study, emission spectra and lifetime decay kinetics were measured at selected points in the skin, but no spectrally resolved imaging was performed. In the epidermis, the emission spectral measurements and the fluorescence-lifetime resolved measurements both established that NAD(P)H is primarily responsible for the fluorescent signals from living keratinocytes, when excited at approximately 800 nm. In the dermis, the emission spectra show characteristic sharp emission peaks of SHG superimposed on a broad background fluorescence, that today are well recognized as the SHG from collagen and the endogenous fluorescence from both collagen and elastin, but Masters and co-workers did not make the correct spectral assignments in this early study. Subsequently, Laiho and co-workers extended excitation/emission spectroscopy studies in the skin from point measurements to 3D-resolved imaging at several excitation and emission wave-lengths.41 They further applied chemometric analysis in order to identify the principal components responsible for the skin endogenous fluorescent signal, resolving contributions from NAD(P)H, collagen and elastin that are well accepted today. They also assigned components corresponding to tryptophan and melanin that are less well supported today. With further instrument improvement, Radosevich and co-workers extended excitation/emission spectroscopic measurement to demonstrate that up to eight different luminescence components can be resolved in the skin.42 The eight components correspond to fluorescence signals from NAD(P)H, collagen, elastin, keratin, sebum and flavin protein, combined with second harmonic signals originating from collagen and keratin. Subsequently, excitation/ emission spectrally resolved multiphoton imaging has been applied in a broad range of skin physiology studies.43–47 After Masters et al. demonstrated life-time-resolved spectroscopy at selected points in the skin, multiphoton microscopes with the capability for lifetime-resolved measurements also saw rapid development. Konig and Riemann demonstrated the first lifetime-resolved imaging of the skin, based on a time-correlated single photon counting (TCSPC) approach.48 In conjunction with the development of low-cost, easy-to-use TCSPC modules that can be readily integrated into multiphoton microscopes,49 the work of Konig et al. established TCSPC as the method of choice for lifetime-resolved skin imaging. Today, the acquisition of 3D multiphoton image stacks of the skin with lifetime-resolved spectroscopic information at every voxel has become feasible, allowing very sensitive discrimination of tissue physiological and pathological states.11,50–52
Other nonlinear optical modalities have also found unique applications in dermal studies. Coherent anti-Stokes Raman scattering (CARS) has been shown to be a powerful method to monitor the dermal transport of small drug molecules,53 while imaging based on time-resolved transient pump-probe absorption microscopy allows the detection and discrimination of different melanin species.21,54 The combination of multiphoton microscopy with other imaging modalities, such as confocal microscopy,55 optical coherence tomography,56,57 and ultrasound imaging,52 have also been shown to be very useful in the study of skin.
3. Applications of Multiphoton Microscopy in Different Areas of Dermatological Studies
3.1. Skin cancers
A major motivation for the development of multi-photon imaging in skin is the diagnosis and monitoring of skin cancer. The major types of skin cancer by decreasing levels of incidence are basal cell carcinoma (originating from cells that make up the stratum basale of the epidermis), squamous cell carcinoma (originating from squamous cells, which make up the major part of the epidermis) and malignant melanoma (originating from melanocytes).58 Despite the comparatively low proportion of malignant melanoma cases, it is by far the most deadly form of skin cancer, with a case mortality rate of approximately 20% in the United States.59 Multiphoton imaging is particularly applicable for diagnosing this type of cancer, since the identification of abnormally located melanocytes is facilitated by the dark melanin pigment that they produce, unlike basal or squamous cell carcinomas which are much less readily located.
Early examples of multiphoton imaging for skin cancer include a brief mention by Teuchner et al., who built a multiphoton microscope and used it to measure the two-photon fluorescence of melanin in excised skin tissue.60,61 This was followed later by animal model studies such as in Skala et al.,62 where a hamster cheek model was used. Tumors were biopsied, imaged in 3D using a titanium-sapphire laser at 780 nm, and five features were determined that could distinguish normal, precancerous and cancerous (squamous cell carcinoma) tissue; this work was later extended by including fluorescence lifetime measurements to quantify the concentration of NADH,63 noting that the concentration of protein-bound NADH and FAD was different in tumors when compared to normal tissue.64 The use of mouse models of carcinoma is still relevant today; a more convenient xenograft mouse model has recently been developed in order to reduce the need for clinical samples of melanoma tissue.65 For skin cancer animal models, dedicated imaging stations have been created in order to perform long-term imaging on mouse carcinoma models.66 Time-lapse imaging of tumor-specific CD8 T-cells tagged with green fluorescent protein was performed in vivo using two-photon fluorescence. In order to ensure that the same location was imaged over a period of several days, microtattoos were used to facilitate image registration.
While the use of animal models is helpful, the study of human tissues is an important step toward eventual clinical applications. Conventional instruments use two-photon fluorescence of endogenous fluorophores in order to perform imaging. This technique was performed on a tumor biopsy taken from a patient with basal cell carcinoma by Cicchi et al.67; an increase in fluorescence intensity was observed in cancerous tissue, although it should be stressed that there was only one patient in the study. Another early study imaging malignant melanoma in nevi, used fluorescence with excitation at 760 nm for NAD(P)H, elastin and pigmented cells, followed by excitation at 840nm to highlight pigmented cells and collagen. Melanoma cells were observed to fluoresce much more brightly than surrounding cells.68 A much larger trial, with 250 patients, was performed, also imaging melanoma.69 The increased fluorescence from cancerous melanocytes was confirmed, and morphological differences could also be seen. A study by Zhang et al. further confirmed that the morphology of cancerous melanocytes was different; the cells were more elongated than normal, and the melanocytes appeared to be migrating together. While only one patient was in the study, this marked the first time that this had been observed in vivo.70 En face geometry was also used to image nonmelanoma skin cancer by Paoli et al.71 and Ericson et al.,72 who noted that morphological features reported in histopathology could also be observed using multiphoton imaging. In another large study, 115 patients were recruited to study the sensitivity and specificity of two-photon fluorescence for imaging melanoma73; values of up to 95% sensitivity and up to 97% specificity were reported. The overarching message of these reports seems to be that while multiphoton fluorescence intensity imaging is suitable for cancer diagnosis, like in histopathology, the morphology is still very important; fluorescence intensity does not serve to highlight cancer cells with enough specificity to be useful.
A potential solution to the problem of not being able to distinguish tumor cells from normal cells is to provide additional contrast mechanisms. Despite the comparatively large number of papers employing fluorescence intensity, it is not the only contrast mechanism that can be used in tissue; fluorescence lifetime imaging54,74–76 and spectrally resolved en face two-photon fluorescence.77 have also been performed, both of which promise better classification of tissue samples. Multiphoton tomography and lifetime imaging were also used to image nevi in an attempt to diagnose malignant melanoma,78 and a 37-patient study on the use of spectrally resolved fluorescence lifetime imaging to diagnose melanoma concluded that while different melanized cell types (such as keratinocytes or melanocytes) could be readily distinguished using a single measurement point, morphological data was still necessary to distinguish benign from malignant melanocytic skin lesions.79 Morphological differences in basal cell carcinoma and squamous cell carcinoma cells were also observed by Xiong et al. using SHG and two-photon fluorescence.80 Because SHG is sensitive to type-I collagen, on account of the fact that type-I collagen is one of the few skin components to exhibit noncentrosymmetry, it can be used to distinguish ordered collagen (observed in healthy skin) from disordered collagen which is more common in tumor regions. These two techniques were also used by Chernyavskiy et al.81 who combined them with confocal reflectance and fluorescence imaging to image the effects of microwave-induced hyperthermia in mice as a treatment for melanoma.
Several other new contrast mechanisms for skin cancer diagnosis are currently in the early stages of development, and their clinical utility remains uncertain. Excited state absorption has been shown to effectively image melanin, and this was developed into a portable imaging system for the diagnosis of melanoma by Teuchner et al.82 Pump-probe optical coherence microscopy was used to image melanoma by Wan and Applegate,83,85 and melanoma was also studied using two-photon photoacoustic microscopy by Oh et al.,84 who exploited the fact that melanin has a high two-photon absorption cross section. CARS has been combined with SGH and two-photon fluorescence by Vogler et al. in order to image basal cell carcinoma.85 Opposing the trend of increasing the number of imaging modes, Chen et al. limited themselves to just second- and third-harmonic generation using a 1230-nm Cr:Forsterite laser in order to image many different skin features and diseases; they claim that their approach permits greater tissue penetration depth than lasers with shorter wavelengths.86
One new trend in melanoma imaging stresses the importance of quantifying the proportion of pheomelanin and eumelanin, since it has been argued that higher proportions of pheomelanin in skin are positively correlated with skin cancer.87 This has the potential to be the first case where it is possible to distinguish cancer cells based on spectroscopy alone, rather than having to interpret the morphological data. Fu et al. initially built an instrument,88 and used transient (or excited state) absorption measurements to distinguish between the two types of melanin in hair, skin and in phantoms consisting of capillary tubes filled with red hair, black hair or Rhodamine 6G.89 It was discovered that the excited-state lifetime for eumelanin was much longer, permitting the two species to be imaged separately. This was exploited by Matthews et al. to image pigmented lesions that were excised during biopsy.21 Contrary to expectation, it was found that there was significantly increased eumelanin in the melanoma samples, and that this fact (combined with other morphological observations) could be used to identify all the melanomas while excluding 75% of the dysplastic nevi, albeit with a sample size of only 21 patients. Further studies have been performed using mouse models,90 as well as a study in humans that attempted to image melanogenesis on excised tissue samples.91 Fluorescence lifetime has also been used to separate melanoma from nevi,92 and attempts have also been made to use ordinary two-photon fluorescence93,94 along with a demonstration of the use of two-spectral-channel lifetime imaging to classify nevi and basal cell carcinoma.51 Today, while the identification of melanoma based on the spectral differences between pheomelanin and eumelanin is far from proven, this approach does show enough promise to justify further investigation.
While it is fair to say that the majority of multiphoton microscopy cancer research is focused on melanoma, other types of cancer are investigated as well. Cicchi et al. used multiphoton imaging to image a number of conditions; besides melanoma, they studied basal cell carcinoma, scarring and keloid formation.95,96 More recently, a number of studies on the diagnosis of basal cell carcinoma in excised tissue were performed by groups affliated with König, employing multiphoton tomography,97 a combination of multiphoton tomography and fluorescence lifetime,98 just fluorescence lifetime,50 and a combination of multiphoton and optical coherence tomography,99 as well as comparing multiphoton tomography and confocal reflectance for the diagnosis of basal cell carcinoma.100,101 Aside from more conventional skin cancers, Hoeller et al. offered a unique investigation into T-cell lymphoma, a form of cancer characterized by the accumulation of malignant CD4+ T-cells in the skin.102 The investigation was performed by imaging fluorescently labeled malignant T-cells in a mouse model, and several conclusions were drawn as to the biological methods by which malignant T-cells adhere to E-selectin in the skin.
All the papers cited above use some form of endogenous contrast agent in order to perform imaging. The use of endogenous contrast agents for the diagnosis of skin cancer is highly desirable from a complexity, safety, cost, ubiquity and applicability point of view, but exogenous contrast agents may provide spectral contrast sufficient to highlight disease features that cannot be observed using endogenous signals alone. 5-Aminolevulinic acid (ALA) was used by Cicchi et al. during a larger study on the imaging of basal cell carcinoma,103,104 as well as by Riemann et al.105; ALA is a precursor to protoporphyrin-IX which is highly fluorescent and accumulates in tumor cells. Gold nanorods are also being pursued on account of their strong luminescence and biocompatibility; Durr et al. have spearheaded this research,106–108 which has also been pursued by the group of Tunnell.109,110 The use of exogenous contrast agents may push the sensitivity and specificity of multiphoton imaging to a point where it can be relied upon in the clinic; however, the regulatory hurdles to the use of any exogenous image contrast agent are considerable.
While conventional histopathology remains the gold standard of any clinical diagnosis including skin cancer, the accuracy of histopathology depends greatly on the skill of the pathologist. A number of research groups are working toward making pathological analysis more quantitative. In the diagnosis of skin lesions based on multiphoton imaging, several research groups advocate the creation of simple, sensitive and robust diagnosis measures. The multifluorescence to SHG index (MFSI) has been proposed as a means of distinguishing basal cell carcinoma from normal tissue,111,112 and was used to locate precancerous melanocytes in mice.113 The autofluorescence to SHG index (ASI) has also been proposed, and was tested on dorsal skin-fold chambers in nude mice.114 Levitt et al. developed a much more complex series of image processing metrics for two-photon fluorescence that were used on a tissue model.115 Unfortunately, as with many of these studies, only very few of them perform well enough to warrant a large clinical trial in order to evaluate whether they can provide a sensitivity and specificity comparable to, or above that of a trained pathologist.
Two studies published recently highlight possible limitations to multiphoton microscopy or treatment protocols. Kantere et al. have noted that multiphoton protoporphyrin-IX fluorescence does not increase tumor contrast; they recommend one-photon anti-Stokes fluorescence instead.116 Nadiarnykh et al. have an even more striking conclusion; that with a diffraction-limited focal spot and a peak power of around 1 kW, significant DNA damage can occur by multiphoton absorption, as measured by the presence of cyclobutane–pyrimidine dimers.117 The effect was strongly dependent on wavelength, with 695-nm excitation being particularly damaging, and wavelengths longer than 780nm being less so (see Fig. 1). This is broadly consistent with the results of Le Harzic et al., who noted that 1064-nm femtosecond laser pulses were much less damaging than wavelengths of 532nm and shorter,118 and has clear implications for the clinical use of multiphoton imaging in cancer diagnosis.
Fig. 1.

Spectral dependence of CPD damage production. Pixel dwell time: 30 μs, pulse width at the focal plane: 164 fs. Reproduced with permission from Ref. 117.
Overall, the use of multiphoton imaging methods for cancer diagnosis and monitoring shows considerable promise. Many studies have demonstrated that tumors can be identified through a variety of different contrast mechanisms, and while the small field of view precludes the use of multiphoton imaging for wide area screening, it may find use in the investigation of suspicious nevi or other neoplasms, especially in locations such as the head and neck, where the consequences of prophylactic surgical excision are more pronounced. In addition, multiphoton imaging may also find use in margin determination, where surgeons need effective imaging tools to determine whether they have completely resected the whole lesion. The availability of clinically compatible instruments from Jenlab goes a long way in proving that these techniques can be applied not just in a laboratory environment, but work acceptably well in the clinic, and the increasing number of clinical trials that involve a substantial number of patients should provide a statistically significant evaluation of the utility of multiphoton imaging as a clinically useful tool for skin cancer diagnosis.
3.2. Other dermatological diseases
In addition to cancer, multiphoton imaging has been used in the diagnosis and monitoring of many different skin diseases and conditions. In the clinic, it has been used to image diseases like Jadassohn–Pellizzari anetoderma,119 scleroderma,120 lymphedema,121 atopic dermatitis in a mouse model,122 and actinic keratosis.123 In some of these diseases, it is often the fact that both collagen and elastin can be easily resolved by SHG and two-photon autofluorescence, respectively, that makes multiphoton imaging particularly suitable. Jadassohn–Pellizzari anetoderma is characterized by a loss of dermal elastin, whereas scleroderma is characterized by the abnormal accumulation of collagen. Similarly, one of the most important clinical parameters for establishing the extent of lymphedema can be the extent of collagen restructuring. Atopic dermatitis can result in hyperkeratosis (a thickening of the stratum corneum) and fibrosis of the upper dermis, both of which can be successfully imaged. Huck et al. have pursued atopic dermatitis further, incorporating fluorescence lifetime imaging to measure the proportion of free versus proteinbound NADH as a measure of cellular activity in 20 patients and 20 control subjects.5,124 Actinic keratosis has been imaged in vivo, with the increased average nuclear diameter being observable both in histopathology and two-photon fluorescence.123 Larger studies have been performed by König and coworkers, who imaged a variety of different diseases such as seborrheic keratoses, angioma, actinic keratoses, psoriasis, pemphigus vulgaris, scarring, and autoimmune bullous skin diseases.125,126 Skin disease due to infectious agents has also been monitored by multiphoton imaging. Lin et al. noted that fungal infections can be monitored by two-photon fluorescence; Microsporum canis, for example, is highly autofluorescent and can be readily distinguished from the stratum corneum in a mouse model.127
Systematic disorders can sometimes also be studied by how they alter dermal structures. Dong and coworkers have studied diabetes. They observed protein glycation, thought to be a major cause of complications caused by diabetes mellitus and aging, by the increased autofluorescence due to the advanced glycation endproducts (AGEs) and slight reduction in SHG in the skin, as well as in the cornea and aorta.128,129 Gunawardana et al. report on the potential for treating typeI diabetes using tissue engineering: In a mouse model, embryonic pancreatic tissue was implanted subcutaneously,130 and two-photon fluorescence imaging used to monitor proper endocrine differentiation.
3.3. Skin aging studies
Aside from disease diagnosis and treatment, the cosmetic and plastic surgery industries have considerable interest in multiphoton imaging for the investigation of chronological aging and extrinsic (often photo induced) aging of skin. Lin et al. first attempted to create a measure that correlated with the chronological age of a subject; the SHG to autofluorescence aging index of dermis (SAAID) offered a simple means to estimate the age of skin by taking the ratio of SHG to autofluorescence.131,132 This measure has been supported by a study which noted that there was a difference in SAAID scores between men and women of the same age,133 and another study which confirmed the correlation with age by measuring sites on the face.134 The discrepancy between men and women was overcome by a more subjective score, the MLT-based dermis morphology score (MDMS, where MLT stands for multiphoton laser-scanning tomography) which also had a better correlation with age than SAAID.135 Depth-resolved measurements of SAAID were taken by Kaatz et al., in order to quantify to what extent the measure varied with imaging depth.136 The difficulty in using SAAID to distinguish between photoaged and nonphotoaged skin was noted by Sanchez et al. and an improvement, incorporating lifetime imaging of NAD(P)H, was made.137 Life-time imaging was also used by Koehler et al. to diagnose dermal elastosis, which is often a sign of extrinsic aging.138
Other researchers have proposed similar measures; Puschmann et al. proposed the elastin to collagen ratio (ELCOR) which explicitly includes autofluorescence contributions from just elastin by manual masking of the image, and also takes the ratio of the area of each skin component as opposed to the fluorescent intensity.139 Wu et al. proposed a measure based on the fast Fourier transform of the SHG image140 or the gray-level cooccurrence matrix.141 Cicchi et al. have also proposed a similar method based on the fast Fourier transform.142
Other studies were more qualitative, seeking to investigate the changes that occur during chronological aging or photoaging. Koehler et al. have investigated the acceleration of aging induced by sunbeds; while the sample was too small to quantify the damage from the sunbed, differences were observed between the dorsal and volar forearm, demonstrating the effect that sun exposure has on skin.143 Similar observations were made by Benati et al.144 and Baldeweck et al. who performed 3D-resolved measurements to further investigate the differences between exposed and unexposed skin.145 Decenciere et al. extended this 3D resolution further, developing a segmentation algorithm which could be used to quantify the size and shape of various skin components, and in the process, estimate the effect of aging.146 yasui et al. used polarization-resolved SHG to show that wrinkles in skin were aligned with the underlying collagen fibers,147,148 and Lutz et al. argued that collagen cross-linking could be quantified in vitro by noting the increase in SHG and a decrease in the fluorescence lifetime.149,150
The therapeutic effects of certain skin treatments have also been investigated using multiphoton imaging. Pena et al. investigated a potential treatment for wrinkles, by noting that fibroblasts can cause contraction in the skin. The effect of Y-27632, a RhoA-kinase inhibitor, was investigated, and found to have potential as a means to inhibit this contraction151,152 as seen in Fig. 2. Bazin et al. also investigated a potential anti-wrinkle treatment consisting of soy and jasmine extracts, and showed a statistically significant increase in dermal collagen content.
Fig. 2.

Multiphoton imaging of collagen matrix remodeling induced by fibroblast contraction. Combined 2PEF (red) and SHG (green) images of fibroblasts within control and Y-27632 treated collagen gels. The images were acquired at T0-before samples contraction; T+24 h and T+48 h — 24 h and 48 h after free contraction of the samples. At T+24 h, the fibroblast inhibitor was removed from the culture medium and replaced with a control culture medium in order to assess the reversibility of the inhibitor effect. Scale bar: 30 m. Excitation: 60 mW at 730 nm. Objective: 20×, 0.9 NA. Acquisition time: 6.9s/image of 681681 pixels. Reproduced with permission from Ref. 152.
Aside from biochemical agents, the after effects of laser treatment for wrinkles have been investigated using two-photon fluorescence and SHG; laser fractional micro-ablative rejuvenation involves using a laser to induce a thermal shock to fibroblasts in the skin, which then produce more collagen. This increased collagen production can be imaged using multiphoton microscopy.153–155 A very similar study was previously performed by Tsai et al., investigating the effect of Er:YAG laser irradiation on skin for the treatment of skin hyperplasia and tumors.156
3.4. Skin regeneration studies
Wound healing and dermal regeneration processes have also been studied with multiphoton imaging, since type I collagen can be imaged particularly well using SHG imaging. As the structure and morphology of the collagen that forms around the wound plays a large part in determining whether a scar forms, or whether the scar is normal, atrophic, hypertrophic or keloid, this application is particularly suited to SHG imaging. Initial studies focused on observing wound healing process in animal models. Navarro et al. imaged the different stages of skin wound closure in guinea pig models using two-photon fluorescence at several time points after full-thickness wounds were induced surgically. Growth of blood vessels and collagen fibers was observed.157 This was pursued further, incorporating SHG imaging, and imaging the wound with greater time resolution and over a longer period, in order to yield more insight into the wound-healing process.93 Later, Luo et al. used SHG and image analysis to investigate wound healing in KunMing mice over a period of 14 days.158
Human scarring has been studied with ex vivo specimens. Brewer et al. imaged samples excised from normal and keloid scars. While the study was very limited (with a total of two patients), a difference in collagen density was observed, although the trend was contrary to expectations, with a greater collagen density observed in the normal scar as opposed to the keloid one.159 Meshkinpour et al. used SHG to image biopsies taken from keloid and hypertrophic scars undergoing treatment with the ThermaCool (TC) device from Thermage Inc. Significant variation was found in the collagen structure of the four biopsied patients.160 This finding was echoed by Da Costa et al. who found swirling collagen structure in keloids, as opposed to more wavy structure in normal skin.161
In vivo study of human scarring was first studied by Riemann et al., imaging a biopsy scar of a single patient over a period of 60 days, with images taken every one to three days after surgery. Two-photon fluorescence and SHG were both employed, and the organization of the new collagen fibers was noted.162 Zhu et al. imaged much older scars by sampling from women who had previously undergone caesarian section. Their data showed a slight decrease in elastin fluorescence and SHG from collagen as the wound aged.163
The skin is known to possess adult stem cells, specifically within the hair follicles. These stem cells may be found in the bulge area as well as in the dermal papilla. Using two-photon microscopy, Rompolas et al. studied the growth regulation of these stem cells in vivo in mice.164 Through the observation of hair follicle regeneration, they found that there exists a spatial organization to these stem cell progeny divisions. Likewise, cell-to-cell signaling allows for coordinated and rapid movement of the follicle. Through targeted laser ablation, Rompolas et al. also showed that the mesenchyme plays an important role in hair regeneration. Similarly, Liu et al. studied the pluripotency of the nestin expressing stem cells found within the bulge area and the dermal papilla.165 These cells migrate from the bulge area to the dermal papilla, suggesting that the bulge area is the source of skin stem cells166 as seen in Fig. 3. By seeding these cells on Gelfoam and subsequently transplanting them into mice with spinal cord injury it was observed that the transplanted cells migrated toward adjacent spinal cord segments. Mice that were transplanted with these stem cells experienced plantar placing of the affected paw within three days of transplantation whereas the negative control group transplanted with only Gelfoam took seven days. Full recovery took at least 28 days for the mice transplanted with stem cells, while only a few mice in the untransplanted group achieved locomotor recovery.
Fig. 3.
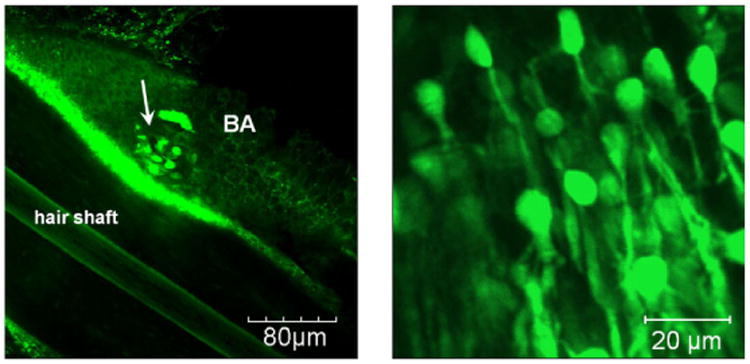
Typical nestin-GFP expressing stem cells within the hair follicle bulge (white arrow). The cells have an oval-shaped body with a typical size of 7mm and dendritic-like arms. Images were obtained from fresh isolated hair follicles by confocal 3D optical slicing. Reproduced with permission from Ref. 166.
Several researchers have noted that the ratio of two-photon fluorescence to SHG can be used to classify scars167,168; however, as collagen structure is deemed to be important in wound healing, several researchers have attempted to quantify the degree of order within a collagen structure. Chen et al. contrasted three different approaches; an edge-detecting filter to determine the gradient at each pixel, a simple threshold to measure the collagen density for the image and a complex semi-arbitrary geometric morphology approach. These were then weighted to form a final measure.169
Some collagen classification metrics that are discussed in the skin aging section, also find applications in wound healing. Cicchi et al. also assessed three different approaches, including employing a gray-level co-occurrence matrix (which was studied further by Ferro et al.170), a fast Fourier transform and a measure known as the SAAID, or Second-harmonic Autofluorescence Aging Index of Dermis.142 Rather than determining one superior technique, it was found that each was effective at a particular length scale. Jiang et al. used a fast Fourier transform as well, in order to define a Collagen Orientation Index and a Bundle Distance, which was then used to characterize collagen in the deep, middle and superficial dermis of keloid tissue excised from patients undergoing reconstructive surgery.171 Taking the above approaches further, it is possible that image processing combined with multiphoton imaging can help determine the border of a scar, to aid the surgeon in determining where to excise or intervene. Chen et al. used two-photon fluorescence and SHG to image skin samples taken from six patients, five of whom had hypertrophic scars. After processing the images, a number of features were proposed in order to distinguish scar tissue from normal tissue.172 Shortly afterward, another set of researchers discovered that the volume density of elastin could be used to distinguish keloid, hypertrophic and normal scars,173 and that the two-photon fluorescence and SHG from collagen could be used to distinguish atrophic and keloid scars.174
In terms of instrument development, Su et al. published two papers demonstrating polarization-resolved SHG, and showed that the second-order susceptibility ratio d33=d31 could be used to distinguish normal tissue from keloid, morphea and dermal elastolysis.175,176 The most significant developments in applying multiphoton imaging to patients in vivo have been made primarily by König and co-workers; in particular, the use of a GRIN lens to allow imaging from within atrophic scars and other recessed skin features, and to image ulcers in vivo.11,177–179 Later, a GRIN lens with a higher numerical aperture of 0.8 was introduced, providing increased spatial resolution.180
3.5. Transdermal transport of drugs, cosmetics, sunscreens and nano-particles
The skin forms a natural barrier that protects the body by keeping potentially toxic substances out. This barrier comprises of a physical layer (the stratum corneum), as well as immunological and enzymatic defenses.181 Many cosmetic and pharmacological products are designed and sold for topical application, and their efficacy sometimes depends on their penetration into the interior of the skin. The dermal distribution of these products has been studied, and much work has gone into designing formulations that can effectively overcome the skin barrier.182
Grewal and co-workers were the first to demonstrate that penetrant distribution in skin can be noninvasively visualized using multiphoton microscopy.183 They further demonstrated that the fluorescently labeled dextran distribution can be modulated by topical application of different enhancers. Subsequently, Yu et al. determined the distribution of fluorescent hydrophobic and hydrophilic penetrants in the skin both before and after treatment with oleic acid, a common enhancer. Coupled with biochemical diffusion rate measurement data, they first quantitatively extracted transport parameters such as concentration gradient enhancement factor and the probe vehicle to skin partition-coefficient enhancement factor . They further proved that hydrophobic and hydrophilic agents penetrate through the skin stratum corneum and the epidermis through different routes.184 Yu and co-workers further showed that, due to skin heterogeneity, high throughput large area multiphoton imaging is critical to minimize errors in determining penetrant transport properties.185 Finally, they have also quantitatively evaluated the effect of ultrasound in skin transport enhancement.186
Besides transport, the safety of topically applied cosmetics, lotions and creams is of key importance for the cosmetic and drug industry. Furthermore, with the development of nanoparticles for a variety of applications, the biosafety of these particles due to inadvertent absorption through skin is also an important concern. For example, nanoparticles such as zinc oxide or titanium oxide are between 20 and 30 nm in size and are often used in sunscreens.18 Using two-photon microscopy on excised human skin from volunteers, it was found that these nanoparticles remained in the stratum corneum. Higher concentrations were found in the skin folds or hair follicle roots; on the order of 800 particles/ μm3 .188,189 Pigment particles remaining in the skin after tattooing represent another class of common nanoparticles in human skin. Konig investigated how nanoparticles from tattoo pigments could be imaged, showing that they could be distinguished from other autofluorescent species in the skin by their fluorescence lifetime and emission spectrum.190 Efforts have also been made to devise a multidimensional quantitative approach toward skin penetration by pharmacological formulations. Recently, a multimodal approach utilizing multi-photon imaging has seen early clinical trials as a way of performing optical biopsies, as well as testing the efficacy of cosmetics.192,193 In addition, Saar and co-workers used stimulated Raman scattering (SRS) to investigate, noninvasively and label-free, the penetration of drugs into the skin. Using ibuprofen and ketoprofen in propylene glycol applied to an excised mouse ear, they were able to image a three-dimensional volume (250 × 250 × 100 μm3 ) of SRS data, which extended from the applied drug on the surface to the subcutaneous fat. In their study, they found that both drugs penetrated through the intercellular lipids of the stratum corneum and the hair shafts. Saar and co-workers were able to use SRS microscopy to track chemical uptake and transport kinetics. They found proof that transport through the stratum corneum was slower, taking over 2 h, compared to penetration through the hair shaft, which reached steady-state in 26min. With the development of video-rate SRS microscopy194,195 and the label-free capability of SRS, real-time tracking of the efficacy of cosmetic and sunscreen compounds can be imaged with both high spatial and temporal resolution.
The importance of being able to study the efficacy of such compounds noninvasively as well as with high resolution is demonstrated by the work by Hanson and Clegg.196 In their study, they observed and quantified the generation of ROS in ex vivo skin irradiated with UV light. In this study, ex vivo human skin samples were incubated with dihy-drorhodamine-123 (DHR), which only converts to a fluorescent form (rhodamine-123) after reacting with ROS. The samples were then irradiated with varying doses of UVB and the amount of rhodamine-123 generated is imaged with a two-photon microscope and quantified. It was found that for an average adult-sized face exposed to 2h to UVB generated 14:7 × 10−3 moles of ROS (as measured by their reaction with DHR-123 to form fluorescent rhodamine-123) in the stratum corneum. A further 10−4 moles were generated in the lower epidermal strata. A subsequent study further revealed that some UV blockers actually increased the amount of ROS generated (see Fig. 4), and therefore increased the chances of skin cancer.197 Since pH gradient may affect the transport of polar chemical species, a high resolution study of the stratum corneum pH gradient was also performed, and it was found that the acidity decreases with increasing depth.198
Fig. 4.

Concentration of R123 as a function of UVB irradiation time (upper x-axes) or UVB dose (Jm−2, lower x-axes) in the stratum corneum (a) and the viable epidermal strata (b-d) for lightly pigmented skin. Variability in the signal most likely results from histological, pigmentation and age difference between samples. Reproduced with permission from Ref. 196.
The effect on the skin of common chemical warfare agents are clearly of importance from the standpoint of protection and treatment. Werrlein et al. have investigated the effects of sulfur mustard, a potent vesicant, on human epidermal keratinocyte cultures.199 They noted a disruption to actin filaments, large punctuate inclusions and a lack of stress fibers in exposed cells, as compared to controls.
4. Conclusion
While skin is one of the most accessible organs in the body, the physiology of the skin is complex and far from being fully understood. The development of powerful imaging tools based on multiphoton techniques enables in vivo minimal invasive imaging of skin physiology throughout the epidermis and into a substantial fraction of the dermis. The in vivo imaging of stem cells and their physiological functions in the native skin environment by the König group will likely provide significant insights for stem cell technology and regenerative medicine.171 Similarly, skin pathologies are medically important. While chronic diseases, such as dermatites, are not life threatening, they can significantly compromise the quality of life for patients. Melanoma, the most dangerous form of skin cancer, can be very effectively cured if the lesion is discovered sufficiently early, but the 10-year mortality rate can be in excess of 70% after metastasis has occurred. Interestingly, a recent report has shown in a mouse model that melanoma may develop from “invisible” nevi that contain difficult-to-visualize lightly colored eumelanin instead of the darker pheomelanin.22 The possibility of the presence of melanoma-causing invisible nevi in light skin color population is an important medical hypothesis that should be investigated. Recent multiphoton imaging technologies21 that can effectively distinguish eumelanin from pheomelanin can play an important role in these studies, and may play an important future diagnostic role if this hypothesis is validated. Lastly, skin products, used for cosmetic, sun-protection, anti-aging or regeneration purposes, are commercial products that we use daily. Despite their financial significance, the efficacy of many of these products is mostly judged subjectively. Importantly, the toxicology evaluation of many of these products is often phenomenological and sometimes lacking the vigor of modern physiological investigation. The advent of multiphoton microscopy, which enables the in vivo study of many of these products on animal models and human volunteers is promising to change the research of this large and commercially important field.
Shortly after the advent of biological multiphoton microscopy with the publication of Denk and co-workers in 1990, the imaging of skin was recognized as an important biomedical area, and one in which this exciting imaging modality will find unique application. Significant technological progress has occurred over the past decade. Multiphoton skin imaging has progressed from using bulky, slow laboratory-based microscopes2 to ergonomic, fast, regulatory agency approved clinical devices.11 The significance of gaining regulatory approval of this technology and the establishment of the safe operation limit cannot be over-emphasized. This important step has now pushed open the doors of clinics around the world for the multiphoton microscopic investigation of skin physiology and pathology, which will, in turn, probably ease the way for clinical trials using multiphoton microscopy to study the other organs.
While multiphoton skin imaging technology has progressed significantly along many fronts, the format of a review provides a forum for the authors to speculate on the most important future directions in multiphoton skin imaging instrumentation. Though multiphoton imaging has been demonstrated in the brain up to a depth of almost 2 mm, the typical imaging depth in skin is substantially smaller, in the region of 150 to 200 μm. A major direction in technique development must therefore be to image deeper into the skin. In a way, the fact that tissue penetration is superior in the brain is not surprising, given the higher scattering coefficient of the skin compared with the brain.200 However, just the difference in scattering coefficients does not appear to fully account for this difference. This difference may be partly explained by the fact that skin imaging is based on endogenous fluorophores such as NAD(P)H, which emit at approximately 450 nm, a wavelength that has short mean free path in tissues. On the other hand, the deep imaging work in the brain is often based on bright exogenous fluorophores emitting more at red wavelengths. The two-photon excitation wavelength of NAD(P)H is 730–800 nm, which is substantially shorter than the deep brain imaging work at 1200 or 1800 nm. This difference may also be partly explained by the fact that deep brain imaging is targeting relatively larger vascular structures, while skin imaging focuses more on detailed features like the morphology of individual cells or that of elastin fibers. Finally, we believe that the difference in penetration depth between the brain and the skin is also partly due to the layered structures of the skin, each with a different refractive index, which result in significant aberration as one images deeper into the tissue. This is a clear opportunity for suitable correction using adaptive optics. While adaptive optics systems have been developed for multiphoton microscopy,201 its actual use in in vivo clinical imaging is very limited. This is partly because, in the imaging of many biological systems, the advantage of using adaptive optics is relatively modest in most cases.202,203 However, it is possible that adaptive optics is perfectly suitable for skin imaging and may substantially improve imaging depth. It would be interesting to label dermal capillaries with red emitting dye and study the depth of penetration in the skin using excitation wavelength in either 1200- or 1800-nm range. The drawback is that, while this approach may work with exogenous organic probes or deep red fluorescent proteins, endogenous contrast agents like NAD(P)H cannot be readily excited. Of course, this provokes the question: Can we identify other endogenous proteins that can provide multiphoton contrast, either based on fluorescence or absorption? The answer is that potentially, the imaging of flavins, cytochromes and porphyrins with longer excitation and absorption wavelengths may result in some improvement in penetration depth, but it remains to be proven.
Another major development that is revolutionizing the whole multiphoton imaging field is the use of newer contrast mechanisms that complement older nonlinear optical contrast mechanisms such as multiphoton fluorescence and SHG. As discussed in this review, techniques based on Raman and absorption contrast hold tremendous promise. Absorption contrast between eumelanin and phomelanin is one of the highlights of recent research, and potentially has significant clinical relevance. Hypothetically, the combination of absorption and fluorescence may allow the study of tissue metabolism with an accuracy and precision that was not previously available.
Metabolic imaging is partly limited by the fact that NAD(P)H is fluorescent while NAD is not; similarly, only oxidized flavins are fluorescent while the reduced forms are not. The paradigm established by Chance and co-worker almost half-a-century ago of ratioing oxidized flavin with NAD(P)H to obtain metabolic imaging was a breakthrough.19 However, if one can obtain the direct ratios of NAD versus NAD(P)H and oxidized versus reduced flavin, one may potentially be able to dissect cellular and tissue redox pathways with much greater precision.
The importance of imaging using chemical signature based on Raman imaging has been demonstrated in many fields, and only partly in skin. While there are some successes in the imaging of fluorescent penetrant distributions, the presence of a bulky fluorophore linked to the small molecule of interest greatly compromises the relevance of many of these past studies. Although some drugs and chemicals are intrinsically fluorescent, they are in a minority, so the work by Xie and co-workers in imaging the penetration through skin by small, nonfluorescent molecules, such as retinol and propylene glycol, is a breakthrough.201,204 It is likely that Raman-based skin imaging will become the method of choice for imaging drug and chemical transport processes in the skin.
Finally, while it is a significant advance to port a laboratory-scale multiphoton microscope to a robust articulated arm system that is compatible with clinical applications, the ability to drastically miniaturize this system to a hand-held probe or an endomicroscope format will even further enhance the clinical acceptance of multiphoton imaging of skin. It is also important to note that while the system has been significantly improved, the current acquisition speed of the clinical system is not significantly faster than that of the laboratory model, being limited to a frame rate of several Hz in order to achieve a good signal to noise ratio. While there are many high-speed multiphoton imaging approaches being developed that preserve the image signal-to-noise ratio while greatly improving image speed,205 most of these techniques, such as selective imaging based on acousto-optical deflectors, and parallelized imaging based on either multi-foci or temporal focused wide field excitation, have not been adapted for clinical imaging. Clearly, the adaptation of these more-advanced multiphoton imaging techniques in a miniaturized endomicroscope format will present a significant challenge.
In this short review, we have discussed the physiology and optical properties of the skin and the historical trajectory of multiphoton technology developments in the imaging of this complex organ. Most importantly, we have provided a fairly thorough review of the major applications areas that use multiphoton microscope for skin imaging, including the diagnosis of skin cancer and other diseases, the assessment of skin aging and the regeneration process, and the monitoring of transdermal transport of different drugs, chemicals and nanoparticles. There is no doubt that, with further technological advances, multiphoton microscopic imaging will become the method of choice for the scientific study of skin physiology and pathology, but if a number of additional challenging clinical, regulatory, economical hurdles can be overcome, it is possible that multiphoton imaging may one day become an important tool in routine patient care as well.
Acknowledgments
PTCSO acknowledges support from: NIH 9P41 EB015871-26A1, R01-EX017656, 5 R01 NS051320, 4R44EB012415-02, NSF CBET-0939511, the Singapore-MIT Alliance 2 and the MIT SkolTech initiative. EYYS is supported by the National Research Foundation Singapore through the Singapore MIT Alliance for Research and Technology's BioSym research programme. CJR is funded by a Wellcome Trust MIT Postdoctoral Research Fellowship 093831/Z/10/Z.
References
- 1.Lin SJ, Jee SH, Dong CY. Multiphoton microscopy: A new paradigm in dermatological imaging. Eur J Dermatol. 2007;17:361–366. doi: 10.1684/ejd.2007.0232. [DOI] [PubMed] [Google Scholar]
- 2.Masters BR, So PT, Gratton E. Multiphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin. Biophys J. 1997;72:2405–2412. doi: 10.1016/S0006-3495(97)78886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenke-Layland K, Riemann I, Damour O, Stock UA, Koenig K. Two-photon microscopes and in vivo multiphoton tomographs — Powerful dicagnostic tools for tissue engineering and drug delivery. Adv Drug Deliv Rev. 2006;58:878–896. doi: 10.1016/j.addr.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Wang BG, Koenig K, Halbhuber KJ. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J Microscopy-Oxford. 2010;238:1–20. doi: 10.1111/j.1365-2818.2009.03330.x. [DOI] [PubMed] [Google Scholar]
- 5.Koenig K, Weinigel M, Breunig HG, Gregory A, Fischer P, Kellner-Hoefer M, Bueckle R, Schwarz M, Riemann I, Stracke F, Huck V, Gorzelanny C, Schneider SW. 5D-intravital tomography as a novel tool for non-invasive in-vivo analysis of human skin. Proc SPIE. 2010;7555:75551I75551–75551I75556. [Google Scholar]
- 6.Cicchi R, Pavone FS. Non-linear fluorescence lifetime imaging of biological tissues. Analy Bioanal Chem. 2011;400:2687–2697. doi: 10.1007/s00216-011-4896-4. [DOI] [PubMed] [Google Scholar]
- 7.Seidenari S, Arginelli F, Bassoli S, Cautela J, French PMW, Guanti M, Guardoli D, Konig K, Talbot C, Dunsby C. Multiphoton laser microscopy and fluorescence lifetime imaging for the evaluation of the skin. Dermatol Res Prac. 2012;2012:1–8. doi: 10.1155/2012/810749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig K. Clinical multiphoton FLIM tomography. Proc SPIE. 2012;8226:822601–822612. [Google Scholar]
- 9.Campagnola PJ, Dong CY. Second harmonic generation microscopy: Principles and applications to disease diagnosis. Laser Photon Rev. 2011;5:13–26. [Google Scholar]
- 10.Perry SW, Burke RM, Brown EB. Two-photon and second harmonic microscopy in clinical and translational cancer research. Ann Biomed Eng. 2012;40:277–291. doi: 10.1007/s10439-012-0512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konig K, Ehlers A, Riemann I, Schenkl S, Buckle R, Kaatz M. Clinical two-photon micro-endoscopy. Microsc Res Tech. 2007;70:398–402. doi: 10.1002/jemt.20445. [DOI] [PubMed] [Google Scholar]
- 12.Koenig K. Multiphoton tomography for tissue engineering. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2008;6858:685801–685807. [Google Scholar]
- 13.Koenig K, Mueller J, Hoefer M, Mueller C, Weinigel M, Bueckle R, Elsner P, Kaatz M, Messerschmidt B. Invited review: Two-photon scanning systems for clinical high-resolution in vivo tissue imaging. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2008;6860:6860141–6860146. [Google Scholar]
- 14.Koenig K, Bueckle R, Weinigel M, Elsner P, Kaatz M. Clinical multiphoton tomography and clinical two-photon microendoscopy. Proc SPIE. 2009;7183:7183191–7183199. [Google Scholar]
- 15.Koenig K, Bueckle R, Weinigel M, Koehler J, Elsner P, Kaatz M. In vivo multiphoton tomography in skin aging studies. Proc SPIE-The International Society for Optical Engineering. 2009;7161:716101–716109. [Google Scholar]
- 16.Koenig K. New developments in multimodal clinical multiphoton tomography. Proc SPIE. 2011;7903:7903051–7903058. [Google Scholar]
- 17.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77:13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 18.Masters B, Chance B. Redox confocal imaging: Intrinsic fluorescent probes of cellular metabolism. Fluorescent and Luminescent Probes for Biological Activity. 1993:44–56. [Google Scholar]
- 19.Chance B. Pyridine nucleotide as an indicator of the oxygen requirements for energy-linked functions of mitochondria. Circ Res. 1976;38:I31–38. [PubMed] [Google Scholar]
- 20.Pena A, Strupler M, Boulesteix T, Schanne-Klein M. Spectroscopic analysis of keratin endogenous signal for skin multiphoton microscopy. Opt Express. 2005;13:6268–6274. doi: 10.1364/opex.13.006268. [DOI] [PubMed] [Google Scholar]
- 21.Matthews TE, Piletic IR, Selim MA, Simpson MJ, Warren WS. Pump-probe imaging differentiates melanoma from melanocytic nevi. Sci Transl Med. 2011;3:1–19. doi: 10.1126/scitranslmed.3001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, Robinson KC, Devi SP, Vanover JC, D'Orazio JA, McMahon M, Bosenberg MW, Haigis KM, Haber DA, Wang Y, Fisher DE. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in thered hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai Z, Kerimo J, Mega Y, Dimarzio CA. Stepwise multiphoton activation fluorescence reveals a new method of melanin detection. J Biomed Opt. 2013;18:0612251–0612257. doi: 10.1117/1.JBO.18.6.061225. [DOI] [PubMed] [Google Scholar]
- 24.Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Hee MR, Fujimoto JG. Determination of the refractive index of highly scattering human tissue by optical coherence tomography. Opt Lett. 1995;20:2258–2260. doi: 10.1364/ol.20.002258. [DOI] [PubMed] [Google Scholar]
- 25.Lo W, Sun Y, Lin SJ, Jee SH, Dong CY. Spherical aberration correction in multiphoton fluorescence imaging using objective correction collar. J Biomed Opt. 2005;10:0340061–0340065. doi: 10.1117/1.1924614. [DOI] [PubMed] [Google Scholar]
- 26.Tyrrell RM, Keyse SM. New trends in photobiology the interaction of UVA radiation with cultured cells. J Photochem Photobiol B: Biol. 1990;4:349–361. doi: 10.1016/1011-1344(90)85014-n. [DOI] [PubMed] [Google Scholar]
- 27.Keyse SM, Tyrrell RM. Induction of the heme oxygenase gene in human skin fibroblasts by hydrogen peroxide and UVA (365 nm) radiation: Evidence for the involvement of the hydroxyl radical. Carcinogenesis. 1990;11:787–791. doi: 10.1093/carcin/11.5.787. [DOI] [PubMed] [Google Scholar]
- 28.Hockberger PE, Skimina TA, Centonze VE, Lavin C, Chu S, Dadras S, Reddy JK, White JG. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc Natl Acad Sci USA. 1999;96:6255–6260. doi: 10.1073/pnas.96.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koester HJ, Baur D, Uhl R, Hell SW. Ca2+ fluorescence imaging with pico and femtosecond two-photon excitation: Signal and photodamage. Biophys J. 1999;77:2226–2236. doi: 10.1016/S0006-3495(99)77063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konig K, So PT, Mantulin WW, Tromberg BJ, Gratton E. Two-photon excited life-time imaging of autofluorescence in cells during UVA and NIR photostress. J Microsc. 1996;183:197–204. [PubMed] [Google Scholar]
- 31.Konig K, Becker TW, Fischer P, Riemann I, Halbhuber KJ. Pulse-length dependence of cellular response to intense near-infrared laser pulses in multiphoton microscopes. Opt Lett. 1999;24:113–115. doi: 10.1364/ol.24.000113. [DOI] [PubMed] [Google Scholar]
- 32.Sako Y, Sekihata A, Yanagisawa Y, Yamamoto M, Shimada Y, Ozaki K, Kusumi A. Comparison of two-photon excitation laser scanning microscopy with UV-confocal laser scanning microscopy in three-dimensional calcium imaging using the fluorescence indicator Indo-1. J Microsc. 1997;185:9–20. doi: 10.1046/j.1365-2818.1997.1480707.x. [DOI] [PubMed] [Google Scholar]
- 33.Schonle A, Hell SW. Heating by absorption in the focus of an objective lens. Opt Lett. 1998;23:325–327. doi: 10.1364/ol.23.000325. [DOI] [PubMed] [Google Scholar]
- 34.Denk W, Sugimori M, Llinas R. Two types of calcium response limited to single spines in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1995;92:8279–8282. doi: 10.1073/pnas.92.18.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacques SL, McAuliffe DJ, Blank IH, Parrish JA. Controlled removal of human stratum corneum by pulsed laser. J Invest Dermatol. 1987;88:88–93. doi: 10.1111/1523-1747.ep12465112. [DOI] [PubMed] [Google Scholar]
- 36.Pustovalov V. Initiation of explosive boiling and optical breakdown as a result of the action of laser pulses on melanosome in pigmented biotissues. Quantum Electron. 1995;25:1055–1059. [Google Scholar]
- 37.Buehler C, Kim KH, Dong CY, Masters BR, So PT. Innovations in two-photon deep tissue microscopy. IEEE Eng Med Biol Mag. 1999;18:23–30. doi: 10.1109/51.790988. [DOI] [PubMed] [Google Scholar]
- 38.Masters BR, So PT, Buehler C, Barry N, Sutin JD, Mantulin WW, Gratton E. Mitigating thermal mechanical damage potential during two-photon dermal imaging. J Biomed Opt. 2004;9:1265–1270. doi: 10.1117/1.1806135. [DOI] [PubMed] [Google Scholar]
- 39.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 40.Piston DW, Masters BR, Webb WW. Three-dimensionally resolved NAD(P)H cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J Microsc. 1995;178:20–27. doi: 10.1111/j.1365-2818.1995.tb03576.x. [DOI] [PubMed] [Google Scholar]
- 41.Laiho LH, Pelet S, Hancewicz TM, Kaplan PD, So PT. Two-photon 3-D mapping of ex vivo human skin endogenous fluorescence species based on fluorescence emission spectra. J Biomed Opt. 2005;10:0240161–024016110. doi: 10.1117/1.1891370. [DOI] [PubMed] [Google Scholar]
- 42.Radosevich AJ, Bouchard MB, Burgess SA, Chen BR, Hillman EM. Hyperspectral in vivo two-photon microscopy of intrinsic contrast. Opt Lett. 2008;33:2164–2166. doi: 10.1364/ol.33.002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Lee A, Zhao J, Wang H, Lui H, McLean DI, Zeng H. Spectroscopic characterization and microscopic imaging of extracted and in situ cutaneous collagen and elastic tissue components under two-photon excitation. Skin Res Technol. 2009;15:418–426. doi: 10.1111/j.1600-0846.2009.00381.x. [DOI] [PubMed] [Google Scholar]
- 44.Breunig HG, Studier H, Koenig K. Excitation-wavelength dependence of multiphoton excitation of fluorophores of human skin in vivo. Proc SPIE. 2010;7548:7548061–7548065. doi: 10.1364/OE.18.007857. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Lee AMD, Wang H, Tang S, Zhao J, Lui H, Zeng H. Imaging-guided two-photon excitation-emission-matrix measurements of human skin tissues. J Biomed Opt. 2012;17:0770041–0770048. doi: 10.1117/1.JBO.17.7.077004. [DOI] [PubMed] [Google Scholar]
- 46.Palero JA, de Bruijn HS, van der Ploeg van den Heuvel A, Sterenborg HJCM, Gerritsen HC. Three-dimensional multiphoton autofluorescence spectral imaging of live tissues. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2006;6191:619101–619109. [Google Scholar]
- 47.Palero JA, de Bruijn HS, van den Heuvel AvdP, Sterenborg HJCM, Gerritsen HC. Spectrally-resolved multiphoton imaging of post-mortem biopsy and in-vivo mouse skin tissues. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6442:644211–6442110. [Google Scholar]
- 48.Konig K, Riemann I. High-resolution multi-photon tomography of human skin with subcellular spatial resolution and picosecond time resolution. J Biomed Opt. 2003;8:432–439. doi: 10.1117/1.1577349. [DOI] [PubMed] [Google Scholar]
- 49.Becker W, Bergmann A, Hink MA, Konig K, Benndorf K, Biskup C. Fluorescence life-time imaging by time-correlated single-photon counting. Microsc Res Tech. 2004;63:58–66. doi: 10.1002/jemt.10421. [DOI] [PubMed] [Google Scholar]
- 50.Patalay R, Talbot C, Alexandrov Y, Lenz MO, Kumar S, Warren S, Munro I, Neil MA, Konig K, French PM, Chu A, Stamp GW, Dunsby C. Multiphoton multispectral fluorescence lifetime tomography for the evaluation of basal cell carcinomas. PLoS One. 2012;7:434601–434609. doi: 10.1371/journal.pone.0043460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patalay R, Talbot C, Alexandrov Y, Munro I, Neil MA, Konig K, French PM, Chu A, Stamp GW, Dunsby C. Quantification of cellular autofluorescence of human skin using multiphoton tomography and fluorescence lifetime imaging in two spectral detection channels. Biomed Opt Express. 2011;2:3295–3308. doi: 10.1364/BOE.2.003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konig K, Speicher M, Kohler MJ, Scharenberg R, Kaatz M. Clinical application of multi-photon tomography in combination with high-frequency ultrasound for evaluation of skin diseases. J Biophotonics. 2010;3:759–773. doi: 10.1002/jbio.201000074. [DOI] [PubMed] [Google Scholar]
- 53.Breunig G, Weinigel M, Darvin ME, Lademann J, Koenig K. Clinical multiphoton and CARS microscopy. Proc SPIE. 2012;8226:8226231–8226237. [Google Scholar]
- 54.Fu D, Ye T, Matthews TE, Yurtsever G, Warren SW. Two-color, two-photon, and excited-state absorption microscopy. J Biomed Opt. 2007;12:0540041–0540048. doi: 10.1117/1.2780173. [DOI] [PubMed] [Google Scholar]
- 55.Matthews TE, Piletic IR, Selim MA, Simpson MJ, Warren WS. Pump-probe imaging differentiates melanoma from melanocytic nevi. Sci Trans Med. 2011;3:1–19. doi: 10.1126/scitranslmed.3001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen WL, Chou CK, Lin MG, Chen YF, Jee SH, Tan HY, Tsai TH, Kim KH, Kim D, So PTC, Lin SJ, Dong CY. Single-wavelength reflected confocal and multiphoton microscopy for tissue imaging. J Biomed Opt. 2009;14:0540261–0540268. doi: 10.1117/1.3247157. [DOI] [PubMed] [Google Scholar]
- 57.Yazdanfar S, Chen YY, So PTC, Laiho LH. Multifunctional imaging of endogenous contrast by simultaneous nonlinear and optical coherence microscopy of thick tissues. Micros Res Tech. 2007;70:628–633. doi: 10.1002/jemt.20447. [DOI] [PubMed] [Google Scholar]
- 58.Koenig K, Weinigel M, Breunig HG, Gregory A, Fischer P, Kellner-Hoefer M, Bueckle R. Current developments in clinical multiphoton tomography. Proc SPIE. 2010;7569:7569151–7569157. [Google Scholar]
- 59.Koenig K, Speicher M, Koehler MJ, Scharenberg R, Kaatz M. Clinical application of multi-photon tomography in combination with high-frequency ultrasound for evaluation of skin diseases. J Biophotonics. 2010;3:759–773. doi: 10.1002/jbio.201000074. [DOI] [PubMed] [Google Scholar]
- 60.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer — The role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 61.Marks R. An overview of skin cancers — incidence and causation. Cancer. 1995;75:607–612. doi: 10.1002/1097-0142(19950115)75:2+<607::aid-cncr2820751402>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Teuchner K, Freyer W, Leupold D, Volkmer A, Birch DJS, Altmeyer P, Stucker M, Hoffmann K. Femtosecond two-photon excited fluorescence of melanin. Photochem Photobiol. 1999;70:146–151. [PubMed] [Google Scholar]
- 63.Teuchner K, Ehlert J, Freyer W, Leupold D, Altmeyer P, Stucker M, Hoffmann K. Fluorescence studies of melanin by stepwise two-photon femto-second laser excitation. J Fluoresc. 2000;10:275–281. [Google Scholar]
- 64.Skala MC, Squirrell JM, Vrotsos KM, Eickhoff VC, Gendron-Fitzpatrick A, Eliceiri KW, Ramanujam N. Multiphoton microscopy of endogenous fluorescence differentiates normal, pre-cancerous, and cancerous squamous epithelial tissues. Cancer Res. 2005;65:1180–1186. doi: 10.1158/0008-5472.CAN-04-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skala MC, Riching KM, Bird DK, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, Keely PJ, Ramanujam N. In vivo multiphoton flurorescence lifetime imaging of protein-bound and free nicotinamide adenine dinucleotide in normal and precancerous epithelia. J Biomed Opt. 2007;12:1–19. doi: 10.1117/1.2717503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, Ramanujam N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence life-times, and cellular morphology in precancerous epithelia. Proc Nat Acad Sci USA. 2007;104:19494–19499. doi: 10.1073/pnas.0708425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson JW, Degan S, Mitropoulos T, Selim MA, Zhang JY, Warren WS. In vivo pump-probe microscopy of melanoma and pigmented lesions. Proc SPIE. 2012;8226:8226021–8226028. [Google Scholar]
- 68.Entenberg D, Aranda L, Li Y, Toledo-Crow R, Schaer D, Li Y. Multimodal microscopy of immune cells and melanoma for longitudinal studies. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2006;6081:608101–6081012. [Google Scholar]
- 69.Cicchi R, Massi D, Sestini S, Carli P, De Giorgi V, Lotti T, Pavone FS. Multidimensional non-linear laser imaging of basal cell carcinoma. Opt Express. 2007;15:10135–10148. doi: 10.1364/oe.15.010135. [DOI] [PubMed] [Google Scholar]
- 70.Riemann I, Dimitrow E, Kaatz MT, Fluhr J, Elsner P, Kobow J, Konig K. In vivo multiphoton tomography of inflammatory tissue and melanoma. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2005;5686:97–104. [Google Scholar]
- 71.Koenig K, Riemann I, Ehlers A, Bueckle R, Dimitrow E, Kaatz M, Fluhr J, Elsner P. In vivo multiphoton tomography of skin cancer. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2006;6089:118–124. [Google Scholar]
- 72.Zhang K, Zhang W, Yang CY, Yang H. Bipolar cellular morphology of malignant melanoma in unstained human melanoma skin tissue. J Biomed Opt. 2009;14:0240421–0240429. doi: 10.1117/1.3120491. [DOI] [PubMed] [Google Scholar]
- 73.Paoli J, Smedh M, Wennberg AM, Ericson MB. Multiphoton laser scanning microscopy on non-melanoma skin cancer: Morphologic features for future non-invasive diagnostics. J Invest Dermatol. 2008;128:1248–1255. doi: 10.1038/sj.jid.5701139. [DOI] [PubMed] [Google Scholar]
- 74.Ericson MB, Paoli J, Ljungblad C, Odu A, Smedh M, Wennberg AM. Two-photon microscopy of non-melanoma skin cancer: Initial experience and diagnostic criteria ex vivo. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6630:663001–663008. [Google Scholar]
- 75.Dimitrow E, Ziemer M, Koehler MJ, Norgauer J, Konig K, Elsner P, Kaatz M. Sensitivity and specificity of multiphoton laser tomography for in vivo and ex vivo diagnosis of malignant melanoma. J Invest Dermatol. 2009;129:1752–1758. doi: 10.1038/jid.2008.439. [DOI] [PubMed] [Google Scholar]
- 76.Patalay R, Talbot C, Munro I, Breunig HG, Koenig K, Alexandrov Y, Warren S, Neil MAA, French PWM, Chu A, Stamp GW, Dunsby C. Fluorescence lifetime imaging of skin cancer. Proc SPIE. 2011;7883:788301–788307. [Google Scholar]
- 77.Patalay R, Talbot C, Alexandrov Y, Munro I, Breunig HG, Koenig K, Warren S, Neil MAA, French PMW, Chu A, Stamp GW, Dunsby C. Non-invasive imaging of skin cancer with fluorescence lifetime imaging using two photon tomography. Proc SPIE. 2011;8087:808718–808718. [Google Scholar]
- 78.Riemann I, Ehlers A, Le Harzic R, Dimitrow E, Kaatz M, Elsner P, Bueckle R, Koenig K. Non-invasive analysis/diagnosis of human normal and melanoma skin tissues with two-photon FLIM in vivo. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2008;6842:6842051–6842055. [Google Scholar]
- 79.De Giorgi V, Massi D, Sestini S, Cicchi R, Pavone FS, Lotti T. Combined non-linear laser imaging (two-photon excitation fluorescence microscopy, fluorescence lifetime imaging microscopy, multispectral multiphoton microscopy) in cutaneous tumours: First experiences. J Eur Acad Dermatol Venereology. 2009;23:314–316. doi: 10.1111/j.1468-3083.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 80.Arginelli F, Manfredini M, Bassoli S, Dunsby C, French P, Koenig K, Magnoni C, Ponti G, Talbot C, Seidenari S. High resolution diagnosis of common nevi by multiphoton laser tomography and fluorescence lifetime imaging. Skin Res Technol. 2013;19:194–204. doi: 10.1111/srt.12035. [DOI] [PubMed] [Google Scholar]
- 81.Dimitrow E, Riemann I, Ehlers A, Koehler MJ, Norgauer J, Elsner P, Koenig K, Kaatz M. Spectral fluorescence lifetime detection and selective melanin imaging by multiphoton laser tomography for melanoma diagnosis. Exp Dermatol. 2009;18:509–515. doi: 10.1111/j.1600-0625.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- 82.Xiong SY, Yang JG, Zhuang J. Nonlinear spectral imaging of human normal skin, basal cell carcinoma and squamous cell carcinoma based on two-photon excited fluorescence and second-harmonic generation. Laser Phys. 2011;21 [Google Scholar]
- 83.Chernyavskiy O, Vannucci L, Bianchini P, Difato F, Saieh M, Kubinova L. Imaging of mouse experimental melanoma in vivo and ex vivo by combination of confocal and nonlinear microscopy. Microsc Res Tech. 2009;72:411–423. doi: 10.1002/jemt.20687. [DOI] [PubMed] [Google Scholar]
- 84.Teuchner K, Mory S, Leupold D. A mobile, intensified femtosecond fiber laser based TPF spectrometer for early diagnosis of malignant melanoma. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2004;5516:63–71. [Google Scholar]
- 85.Wan Q, Applegate BE. Multiphoton coherence domain molecular imaging with pump-probe optical coherence microscopy. Opt Lett. 2010;35:532–534. doi: 10.1364/OL.35.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oh JT, Li ML, Zhang HF, Maslov K, Stoica G, Wang LV. Three-dimensional imaging of skin melanoma in vivo by dual-wavelength photoacoustic microscopy. J Biomed Opt. 2006;11:0340321–0340324. doi: 10.1117/1.2210907. [DOI] [PubMed] [Google Scholar]
- 87.Vogler N, Heuke S, Akimov D, Latka I, Kluschke F, Roewert-Huber HJ, Lademann J, Dietzek B, Popp J. Discrimination of skin diseases using the multimodal imaging approach. Proc SPIE. 2012;8427:8427101–8427108. [Google Scholar]
- 88.Chen SY, Chen SU, Wu HY, Lee WJ, Liao YH, Sun CK. In vivo virtual biopsy of human skin by using noninvasive higher harmonic generation microscopy. IEEE J Sel Top Quant. 2010;16:8231–8242. [Google Scholar]
- 89.Takeuchi S, Zhang WG, Wakamatsu K, Ito S, Hearing VJ, Kraemer KH, Brash DE. Melanin acts as a cause an atypical potent UVB photosensitizer to mode of cell death in murine skin. Proc Nat Acad Sci USA. 2004;101:15076–15081. doi: 10.1073/pnas.0403994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fu D, Ye T, Matthews TE, Yurtsever G, Hong L, Simon JD, Warren WS. Two-color excited-state absorption imaging of melanins — art. no. 642402. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6424:424021–424027. [Google Scholar]
- 91.Fu D, Ye T, Matthews TE, Grichnik J, Hong L, Simon JD, Warren WS. Probing skin pigmentation changes with transient absorption imaging of eumelanin and pheomelanin. J Biomed Opt. 2008;13:0540361–0540367. doi: 10.1117/1.2976424. [DOI] [PubMed] [Google Scholar]
- 92.Matthews TE, Wilson JW, Degan S, Simpson MJ, Jin JY, Zhang JY, Warren WS. In vivo and ex vivo epimode pump-probe imaging of melanin and microvasculature. Biomed Opt Express. 2011;2:1576–1583. doi: 10.1364/BOE.2.001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leupold D, Scholz M, Stankovic G, Reda J, Buder S, Eichhorn R, Wessler G, Stuecker M, Hoffmann K, Bauer J, Garbe C. The stepwise two-photon excited melanin fluorescence is a unique diagnostic tool for the detection of malignant transformation in melanocytes. Pigment Cell & Melanoma Res. 2011;24:438–445. doi: 10.1111/j.1755-148X.2011.00853.x. [DOI] [PubMed] [Google Scholar]
- 94.Cicchi R, Sacconi L, Jasaitis A, O'Connor RP, Massi D, Sestini S, De Giorgi V, Lotti T, Pavone FS. Multidimensional custom-made non-linear microscope: From ex-vivo to in-vivo imaging. Appl Phys B-Lasers and Opt. 2008;92:359–365. [Google Scholar]
- 95.Eichhorn R, Wessler G, Scholz M, Leupold D, Stankovic G, Buder S, Stuecker M, Hoffmann K. Early diagnosis of melanotic melanoma based on laser-induced melanin fluorescence. J Biomed Opt. 2009;14:0340331–0340337. doi: 10.1117/1.3155511. [DOI] [PubMed] [Google Scholar]
- 96.Krasieva TB, Liu F, Sun CH, Kong Y, Balu M, Meyskens FL, Tromber BJ. Two-photon excited fluorescence spectroscopy and imaging of melanin in-vitro and in-vivo. Proc SPIE. 2012;8226:822621–822627. doi: 10.1117/1.JBO.18.3.031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patalay R, Talbot C, Alexandrov Y, Munro I, Neil MAA, Koenig K, French PMW, Chu A, Stamp GW, Dunsby C. Quantification of cellular autofluorescence of human skin using multiphoton tomography and fluorescence lifetime imaging in two spectral detection channels. Biomed Opt Express. 2011;2:3295–3308. doi: 10.1364/BOE.2.003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cicchi R, Sestini S, De Giorgi V, Massi D, Lotti T, Pavone FS. Multidimensional two-photon imaging of diseased skin. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2008;6859:6859031–68590311. [Google Scholar]
- 99.Cicchi R, Sestini S, De Giorgi V, Stambouli D, Carli P, Massi D, Lotti T, Pavone FS. Non-linear laser imaging of skin lesions. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6633:663301–6633012. [Google Scholar]
- 100.Seidenari S, Arginelli F, Bassoli S, Cautela J, Cesinaro AM, Guanti M, Guardoli D, Magnoni C, Manfredini M, Ponti G, Koenig K. Diagnosis of BCC by multiphoton laser tomography. Skin Res Technol. 2013;19:E297–E304. doi: 10.1111/j.1600-0846.2012.00643.x. [DOI] [PubMed] [Google Scholar]
- 101.Seidenari S, Arginelli F, Dunsby C, French P, Koenig K, Magnoni C, Manfredini M, Talbot C, Ponti G. Multiphoton laser tomography and fluorescence lifetime imaging of basal cell carcinoma: Morphologic features for non-invasive diagnostics. Exp Dermatol. 2012;21:831–836. doi: 10.1111/j.1600-0625.2012.01554.x. [DOI] [PubMed] [Google Scholar]
- 102.Alex A, Weingast J, Weinigel M, Kellner-Hoefer M, Nemecek R, Binder M, Pehamberger H, Koenig K, Drexler W. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352–362. doi: 10.1002/jbio.201200085. [DOI] [PubMed] [Google Scholar]
- 103.Ulrich M, Klemp M, Darvin ME, Konig K, Lademann J, Meinke MC. In vivo detection of basal cell carcinoma: Comparison of a reflectance confocal microscope and a multiphoton tomograph. J Biomed Opt. 2013;18:612291–612297. doi: 10.1117/1.JBO.18.6.061229. [DOI] [PubMed] [Google Scholar]
- 104.Manfredini M, Arginelli F, Dunsby C, French P, Talbot C, Koenig K, Pellacani G, Ponti G, Seidenari S. High-resolution imaging of basal cell carcinoma: A comparison between multiphoton microscopy with fluorescence lifetime imaging and reflectance confocal microscopy. Skin Res Technol. 2013;19:E433–E443. doi: 10.1111/j.1600-0846.2012.00661.x. [DOI] [PubMed] [Google Scholar]
- 105.Hoeller C, Richardson SK, Ng LG, Valero T, Wysocka M, Rook AH, Weninger W. In vivo imaging of cutaneous T-cell lymphoma migration to the skin. Cancer Res. 2009;69:2704–2708. doi: 10.1158/0008-5472.CAN-08-2891. [DOI] [PubMed] [Google Scholar]
- 106.Cicchi R, Sestini S, De Giorgi V, Stambouli D, Carli P, Massi D, Pavone FS. Time-resolved multiphoton imaging of basal cell carcinoma. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6442:644211–644219. [Google Scholar]
- 107.Riemann I, Ehlers A, Dill-Mueller D, Martin S, Koenig K. Multiphoton tomography of skin tumors after ALA application. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6424:6424051–6424056. [Google Scholar]
- 108.Durr NJ, Larson T, Smith DK, Korgel BA, Sokolov K, Ben-Yakar A. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007;7:941–945. doi: 10.1021/nl062962v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park J, Estrada A, Schwartz JA, Diagaradjane P, Krishnan S, Dunn AK, Tunnell JW. Intra-organ biodistribution of gold nanoparticles using intrinsic two-photon-induced photoluminescence. Lasers Surg Med. 2010;42:630–639. doi: 10.1002/lsm.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Puvanakrishnan P, Diagaradjane P, Kazmi SMS, Dunn AK, Krishnan S, Tunnell JW. Narrow band imaging of squamous cell carcinoma tumors using topically delivered anti-EGFR anti-body conjugated gold nanorods. Lasers Surg Med. 2012;44:310–317. doi: 10.1002/lsm.22019. [DOI] [PubMed] [Google Scholar]
- 111.Lin SJ, Jee SH, Kuo CJ, Wu R, Jr, Lin WC, Chen JS, Liao YH, Hsu CJ, Tsai TF, Chen YF, Dong CY. Discrimination of basal cell carcinoma from normal dermal stroma by quantitative multiphoton imaging. Opt Lett. 2006;31:2756–2758. doi: 10.1364/ol.31.002756. [DOI] [PubMed] [Google Scholar]
- 112.Lin SJ, Hsu CJ, Wu R, Jr, Kuo CJ, Chen JS, Chan JY, Lin WC, Jee SH, Dong CY. Quantitative multiphoton imaging for guiding basal-cell carcinoma removal. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6424:6424041–64240411. [Google Scholar]
- 113.Wang CC, Li FC, Lin WC, Chen YF, Chen JS, Lin SJ, Dong CY. Early development of cutaneous cancer revealed by intravital non-linear optical microscopy. Appl Phys Lett. 2010;97:1137021–1137023. [Google Scholar]
- 114.Wang CC, Li FC, Lin SJ, Lo W, Dong CY. Utilizing nonlinear optical microscopy to investigate the development of early cancer in nude mice in vivo — art. no. 66300Y. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6630:663001–663008. [Google Scholar]
- 115.Levitt JM, McLaughlin-Drubin ME, Muenger K, Georgakoudi I. Automated biochemical, morphological, and organizational assessment of precancerous changes from endogenous two-photon fluorescence images. PLoS One. 2011;6:1–11. doi: 10.1371/journal.pone.0024765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kantere D, Guldbrand S, Paoli J, Goksor M, Hanstorp D, Wennberg AM, Smedh M, Ericson MB. Anti-stokes fluorescence from endogenously formed protoporphyrin IX implications for clinical multiphoton diagnostics. J Biophotonics. 2013;6:409–415. doi: 10.1002/jbio.201200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nadiarnykh O, Thomas G, Van Voskuilen J, Sterenborg HJCM, Gerritsen HC. Carcinogenic damage to deoxyribonucleic acid is induced by near-infrared laser pulses in multiphoton microscopy via combination of two- and three-photon absorption. J Biomed Opt. 2012;17:1160241–1160247. doi: 10.1117/1.jbo.17.11.116024. [DOI] [PubMed] [Google Scholar]
- 118.Le Harzic R, Weinigel M, Riemann I, Konig K, Messerschmidt B. Nonlinear optical endoscope based on a compact two axes piezo scanner and a miniature objective lens. Opt Express. 2008;16:20588–20596. doi: 10.1364/oe.16.020588. [DOI] [PubMed] [Google Scholar]
- 119.Zhao J, Chen J, Yang Y, Zhuo S, Jiang X, Tian W, Ye X, Lin L, Xie S. Jadassohn-Pellizzari anetoderma: Study of multiphoton microscopy based on two-photon excited fluorescence and second harmonic generation. Eur J Dermatol. 2009;19:570–575. doi: 10.1684/ejd.2009.0797. [DOI] [PubMed] [Google Scholar]
- 120.Lu K, Chen J, Zhuo S, Zheng L, Jiang X, Zhu X, Zhao J. Multiphoton laser scanning microscopy of localized scleroderma. Skin Res Technol. 2009;15:489–495. doi: 10.1111/j.1600-0846.2009.00395.x. [DOI] [PubMed] [Google Scholar]
- 121.Wu X, Zhuo S, Chen J, Liu N. Real-time in vivo imaging collagen in lymphedematous skin using multiphoton microscopy. Scanning. 2011;33:463–467. doi: 10.1002/sca.20266. [DOI] [PubMed] [Google Scholar]
- 122.Lee JH, Chen SY, Yu CH, Chu SW, Wang LF, Sun CK, Chiang BL. Noninvasive in vitro and in vivo assessment of epidermal hyperkeratosis and dermal fibrosis in atopic dermatitis. J Biomed Opt. 2009;14:0140081–01400815. doi: 10.1117/1.3077182. [DOI] [PubMed] [Google Scholar]
- 123.Koehler MJ, Zimmermann S, Springer S, Elsner P, Koenig K, Kaatz M. Keratinocyte morphology of human skin evaluated by in vivo multiphoton laser tomography. Skin Res Technol. 2011;17:479–486. doi: 10.1111/j.1600-0846.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 124.Huck V, Gorzelanny C, Thomas K, Mess C, Dimitrova V, Schwarz M, Riemann I, Niemeyer V, Luger TA, Koenig K, Schneider SW. Intravital multiphoton tomography as an appropriate tool for non-invasive in vivo analysis of human skin affected with Atopic Dermatitis. Proc SPIE. 2011;7883:788301–788306. [Google Scholar]
- 125.Koehler MJ, Speicher M, Lange-Asschenfeldt S, Stockfleth E, Metz S, Elsner P, Kaatz M, Koenig K. Clinical application of multiphoton tomography in combination with confocal laser scanning microscopy for in vivo evaluation of skin diseases. Exp Dermatol. 2011;20:589–594. doi: 10.1111/j.1600-0625.2011.01279.x. [DOI] [PubMed] [Google Scholar]
- 126.Koenig K, Speicher M, Bueckle R, Reckfort J, McKenzie G, Welzel J, Koehler MJ, Elsner P, Kaatz M. Clinical optical coherence tomography combined with multiphoton tomography for evaluation of several skin disorders. Proc SPIE. 2010;7554:755421–755426. [Google Scholar]
- 127.Lin SJ, Lo W, Sun Y, Jee SH, Dong CY. Multiphoton fluorescence and second harmonic generation microscopy of different skin states. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2005;5686:67–72. [Google Scholar]
- 128.Ghazaryan AA, Tseng JY, Lo W, Chen YF, Hovhanissyan V, Chen SJ, Tan HY, Dong CY. Multiphoton imaging and quantification of tissue glycation. Proc SPIE. 2011;7895:7895091–7895095. [Google Scholar]
- 129.Tseng JY, Ghazaryan AA, Lo W, Chen YF, Hovhannisyan V, Chen SJ, Tan HY, Dong CY. Multiphoton spectral microscopy for imaging and quantification of tissue glycation. Biomed Opt Express. 2011;2:218–230. doi: 10.1364/BOE.2.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gunawardana SC, Benninger RKP, Piston DW. Subcutaneous transplantation of embryonic pancreas for correction of type 1 diabetes. Am J Physiol Endocrinol Metab. 2009;296:323–332. doi: 10.1152/ajpendo.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin SJ, Wu R, Jr, Tan HY, Lo W, Lin WC, Young TH, Hsu CJ, Chen JS, Jee SH, Dong CY. Evaluating cutaneous photoaging by use of multiphoton fluorescence and second-harmonic generation microscopy. Opt Lett. 2005;30:2275–2277. doi: 10.1364/ol.30.002275. [DOI] [PubMed] [Google Scholar]
- 132.Lin SJ, Jee SH, Chan JY, Wu R, Jr, Lo W, Tan HY, Lin WC, Chen JS, Young TH, Hsu CJ, Dong CY. Monitoring photoaging by use of multiphoton fluorescence and second harmonic generation microscopy. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2006;6078:6078031–6078037. [Google Scholar]
- 133.Koehler MJ, Koenig K, Elsner P, Bueckle R, Kaatz M. In vivo assessment of human skin aging by multiphoton laser scanning tomography. Opt Lett. 2006;31:2879–2881. doi: 10.1364/ol.31.002879. [DOI] [PubMed] [Google Scholar]
- 134.Sugata K, Osanai O, Sano T, Takema Y. Evaluation of photoaging in facial skin by multi-photon laser scanning microscopy. Skin Res Technol. 2011;17:1–3. doi: 10.1111/j.1600-0846.2010.00475.x. [DOI] [PubMed] [Google Scholar]
- 135.Koehler MJ, Hahn S, Preller A, Elsner P, Ziemer M, Bauer A, Koenig K, Bueckle R, Fluhr JW, Kaatz M. Morphological skin ageing criteria by multiphoton laser scanning tomography: Non-invasive in vivo scoring of the dermal fibre network. Exp Dermatol. 2008;17:519–523. doi: 10.1111/j.1600-0625.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 136.Kaatz M, Sturm A, Elsner P, Koenig K, Bueckle R, Koehler MJ. Depth-resolved measurement of the dermal matrix composition by multi-photon laser tomography. Skin Res Technol. 2010;16:131–136. doi: 10.1111/j.1600-0846.2009.00423.x. [DOI] [PubMed] [Google Scholar]
- 137.Sanchez WY, Obispo C, Ryan E, Grice JE, Roberts MS. Changes in the redox state and endogenous fluorescence of in vivo human skin due to intrinsic and photoaging, measured by multi-photon tomography with fluorescence lifetime imaging. J Biomed Opt. 2013;18:0612171–06121712. doi: 10.1117/1.JBO.18.6.061217. [DOI] [PubMed] [Google Scholar]
- 138.Koehler MJ, Preller A, Elsner P, Koenig K, Hipler UC, Kaatz M. Non-invasive evaluation of dermal elastosis by in vivo multiphoton tomography with autofluorescence lifetime measurements. Exp Dermatol. 2012;21:48–51. doi: 10.1111/j.1600-0625.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 139.Puschmann S, Rahn CD, Wenck H, Gallinat S, Fischer F. Approach to quantify human dermal skin aging using multiphoton laser scanning microscopy. J Biomed Opt. 2012;17:0360051–0360056. doi: 10.1117/1.JBO.17.3.036005. [DOI] [PubMed] [Google Scholar]
- 140.Wu S, Li H, Yang H, Zhang X, Li Z, Xu S. Quantitative analysis on collagen morphology in aging skin based on multiphoton microscopy. J Biomed Opt. 2011;16:0405021–0405023. doi: 10.1117/1.3565439. [DOI] [PubMed] [Google Scholar]
- 141.Wu S, Li H, Zhang X, Li Z, Xu S. The analysis of aging skin based on multiphoton microscopy. Proc SPIE-The International Society for Optical Engineering. 2010;7845:784501–784508. [Google Scholar]
- 142.Cicchi R, Kapsokalyvas D, De Giorgi V, Maio V, Van Wiechen A, Massi D, Lotti T, Pavone FS. Scoring of collagen organization in healthy and diseased human dermis by multiphoton microscopy. J Biophotonics. 2010;3:34–43. doi: 10.1002/jbio.200910062. [DOI] [PubMed] [Google Scholar]
- 143.Koehler MJ, Preller A, Kindler N, Elsner P, Koenig K, Bueckle R, Kaatz M. Intrinsic, solar and sunbed-induced skin aging measured in vivo by multiphoton laser tomography and biophysical methods. Skin Res Technol. 2009;15:357–363. doi: 10.1111/j.1600-0846.2009.00372.x. [DOI] [PubMed] [Google Scholar]
- 144.Benati E, Bellini V, Borsari S, Dunsby C, Ferrari C, French P, Guanti M, Guardoli D, Koenig K, Pellacani G, Ponti G, Schianchi S, Talbot C, Seidenari S. Quantitative evaluation of healthy epidermis by means of multiphoton microscopy and fluorescence lifetime imaging microscopy. Skin Res Technol. 2011;17:295–303. doi: 10.1111/j.1600-0846.2011.00496.x. [DOI] [PubMed] [Google Scholar]
- 145.Baldeweck T, Tancrede E, Dokladal P, Koudoro S, Morard V, Meyer F, Decenciere E, Pena AM. In vivo multiphoton microscopy associated to 3D image processing for human skin characterization. Proc SPIE. 2012;8226:822631–822638. [Google Scholar]
- 146.Decenciere E, Tancrede-Bohin E, Dokladal P, Koudoro S, Pena AM, Baldeweck T. Automatic 3D segmentation of multiphoton images: A key step for the quantification of human skin. Skin Res Technol. 2013;19:115–124. doi: 10.1111/srt.12019. [DOI] [PubMed] [Google Scholar]
- 147.Yasui T, Takahashi Y, Araki T. Polarization-resolved second-harmonic-generation imaging of photoaged dermal collagen fiber. Proc SPIE. 2009;7183:718311–718319. [Google Scholar]
- 148.Yasui T, Takahashi Y, Fukushima S, Ogura Y, Yamashita T, Kuwahara T, Hirao T, Araki T. Observation of dermal collagen fiber in wrinkled skin using polarization-resolved second-harmonic-generation microscopy. Opt Express. 2009;17:912–923. doi: 10.1364/oe.17.000912. [DOI] [PubMed] [Google Scholar]
- 149.Bazin R, Flament F, Colonna A, Le Harzic R, Bueckle R, Piot B, Laize F, Kaatz M, Koenig K, Fluhr JW. Clinical study on the effects of a cosmetic product on dermal extracellular matrix components using a high-resolution multiphoton tomograph. Skin Res Technol. 2010;16:305–310. doi: 10.1111/j.1600-0846.2010.00432.x. [DOI] [PubMed] [Google Scholar]
- 150.Lutz V, Sattler M, Gallinat S, Wenck H, Poertner R, Fischer F. Impact of collagen cross-linking on the second harmonic generation signal and the fluorescence lifetime of collagen autofluorescence. Skin Res Technol. 2012;18:168–179. doi: 10.1111/j.1600-0846.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 151.Lutz V, Puschmann S, Gallinat S, Wenck H, Wittern KP, Fischer F. Collagen crosslink status analysed in vitro using second harmonic generation (SHG) and fluorescence lifetime imaging (FLIM) Proc SPIE. 2012;8207:8207031–82070315. [Google Scholar]
- 152.Pena AM, Olive C, Michelet JF, Galey JB, Fagot D, Leroy F, Martin JL, Colonna A, Schanne-Klein MC. Multiphoton microscopy of engineered dermal substitutes: Assessment of 3D collagen matrix remodeling induced by fibroblasts contraction. Proc SPIE. 2010;7548:7548021–7548025. doi: 10.1117/1.3503411. [DOI] [PubMed] [Google Scholar]
- 153.Pena AM, Fagot D, Olive C, Michelet JF, Galey JB, Leroy F, Beaurepaire E, Martin JL, Colonna A, Schanne-Klein MC. Multiphoton microscopy of engineered dermal substitutes: Assessment of 3-D collagen matrix remodeling induced by fibroblast contraction. J Biomed Opt. 2010;15:0560181–0560187. doi: 10.1117/1.3503411. [DOI] [PubMed] [Google Scholar]
- 154.Cicchi R, Kapsokalyvas D, Troiano M, Campolmi P, Morini C, Cosci A, Massi D, Lotti T, Pavone FS. In-vivo multiphoton imaging of collagen remodeling after microablative fractional rejuvenation. Proc SPIE. 2011;7883:788301–788308. [Google Scholar]
- 155.Gong W, Xie S, Huang Y. Evaluating thermal damage induced by pulsed light with multiphoton microscopy. Proc SPIE. 2009;7161:716101–716105. [Google Scholar]
- 156.Cicchi R, Kapsokalyvas D, Troiano M, Campolmi P, Morini C, Lotti T, Pavone FS. In vivo TPEF-SHG microscopy for detecting collagen remodeling after laser micro-ablative fractional resurfacing treatment. Proc SPIE. 2011;8087:808711–808716. [Google Scholar]
- 157.Tsai TH, Lee JN, Chan JY, Hsu JT, Tan HY, Dong CY, Lee WR, Lin SJ. Monitoring laser-tissue interaction by non-linear optics. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2008;6842:6842021–6842029. [Google Scholar]
- 158.Navarro FA, So PTC, Driessen A, Kropf N, Park CS, Huertas JC, Lee HB, Orgill DP. Two photon confocal microscopy in wound healing. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2001;2:27–40. [Google Scholar]
- 159.Luo T, Chen JX, Zhuo SM, Lu KC, Jiang XS, Liu QG. Visualization of collagen regeneration in mouse dorsal skin using second harmonic generation microscopy. Laser Phys. 2009;19:478–482. [Google Scholar]
- 160.Brewer MB, Yeh A, Torkian B, Sun CH, Tromberg BJ, Wong BJ. Multiphoton imaging of excised normal skin and keloid scar: Preliminary investigations. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2004;5312:204–208. [Google Scholar]
- 161.Meshkinpour A, Ghasri P, Pope K, Lyubovitsky JG, Risteli J, Krasieva TB, Kelly KM. Treatment of hypertrophic scars and keloids with a radiofrequency device: A study of collagen effects. Lasers Surg Med. 2005;37:343–349. doi: 10.1002/lsm.20268. [DOI] [PubMed] [Google Scholar]
- 162.Da Costa V, Wei R, Lim R, Sun CH, Brown JJ, Wong BJF. Nondestructive imaging of live human keloid and facial tissue using multi-photon microscopy. Arch Facial Plast Surg. 2008;10:38–43. doi: 10.1001/archfacial.2007.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Riemann I, Ehlers A, LeHarzic R, Martin S, Reif A, Koenig K. In vivo multiphoton tomography of skin during wound healing and scar formation. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6442:6442261–6442268. [Google Scholar]
- 164.Zhu X, Zhuo S, Zheng L, Jiang X, Chen J, Lin B. Characteristics of scar margin dynamic with time based on multiphoton microscopy. Lasers Med Sci. 2011;26:239–245. doi: 10.1007/s10103-010-0851-4. [DOI] [PubMed] [Google Scholar]
- 165.Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, Mii S, Amoh Y, Katsuoka K, Hoffman RM. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830–839. doi: 10.4161/cc.10.5.14969. [DOI] [PubMed] [Google Scholar]
- 167.Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- 168.Chen J, Zhuo S, Jiang X, Zhu X, Zheng L, Xie S, Lin B, Zeng H. Multiphoton microscopy study of the morphological and quantity changes of collagen and elastic fiber components in keloid disease. J Biomed Opt. 2011;16:0513051–0513056. doi: 10.1117/1.3569617. [DOI] [PubMed] [Google Scholar]
- 169.Zhu X, Zhuo S, Zheng L, Jiang X, Chen J, Lin B. Marginal characteristics of skin scarred dermis quantitatively extracted from multiphoton microscopic imaging. Proc SPIE-The International Society for Optical Engineering. 2010;7845:7845281–7845285. [Google Scholar]
- 170.Chen G, Xie Z, Chen R, Lin J, Yang K. Texture analysis on two-photon excited microscopic images of human skin hypertrophic scar tissue. Br J Dermatol. 2008;161:48–55. [Google Scholar]
- 171.Ferro DP, Vieira-Damiani G, Adam RL, Cesar CL, Metze K. Non-linear optics for the study of human scar tissue. Proc SPIE. 2012;8226:822631–822637. [Google Scholar]
- 172.Jiang XS, Chen S, Chen JX, Zhu XQ, Zheng LQ, Zhuo SM, Wang DJ. Monitoring process of human keloid formation based on second harmonic generation imaging. Laser Phys. 2011;21:1661–1664. [Google Scholar]
- 173.Chen G, Chen J, Zhuo S, Xiong S, Zeng H, Jiang X, Chen R, Xie S. Nonlinear spectral imaging of human hypertrophic scar based on two-photon excited fluorescence and second-harmonic generation. Br J Dermatol. 2009;161:48–55. doi: 10.1111/j.1365-2133.2009.09094.x. [DOI] [PubMed] [Google Scholar]
- 174.Chen S, Jiang XS, Chen JX, Zhu XQ, Zheng LQ, Zhuo SM, Yang HQ, Wang DJ. Differentiating keloids from normal and hypertrophic scar based on multiphoton microscopy. Laser Phys. 2010;20:900–903. [Google Scholar]
- 175.Zhu X, Zhuo S, Zheng L, Jiang X, Chen J, Lin B. Quantification of scar margin in keloid different from atrophic scar by multiphoton microscopic imaging. Scanning. 2011;33:195–200. doi: 10.1002/sca.20230. [DOI] [PubMed] [Google Scholar]
- 176.Su PJ, Chen WL, Hong JB, Li TH, Wu R, Jr, Chou CK, Chen SJ, Hu C, Lin SJ, Dong CY. Discrimination of collagen in normal and pathological skin dermis through second-order susceptibility microscopy. Opt Express. 2009;17:11161–11171. doi: 10.1364/oe.17.011161. [DOI] [PubMed] [Google Scholar]
- 177.Su PJ, Chen WL, Hong JB, Li TH, Wu R, Jr, Chou CK, Lin SJ, Dong CY. Discrimination of collagen in normal and pathological dermis through polarization second harmonic generation. Proc SPIE. 2010;7569:756921–756926. [Google Scholar]
- 178.Schenkl S, Ehlers A, Le Harzic R, Stark M, Riemann I, Messerschmidt B, Kaatz M, Koenig K. Rigid and high NA multiphoton fluorescence GRIN-endoscopes. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6631:1–7. [Google Scholar]
- 179.Ehlers A, Schenkl S, Riemann I, Messerschmidt B, Kaatz M, Bueckle R, Koenig K. In vivo multiphoton endoscopy of endogenous skin fluorophores. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6442:644211–644217. [Google Scholar]
- 180.Koenig K, Ehlers A, Riemann I, Schenkl S, Bueckle R, Kaatz M. Clinical two-photon micro endoscopy. Micro Res Tech. 2007;70:398–402. doi: 10.1002/jemt.20445. [DOI] [PubMed] [Google Scholar]
- 181.Koenig K, Ehlers A, Riemann I, Schenkl S, Messerschmidt B, Bueckle R, LeHarzic R, Elsner P, Kaatz M. Clinical in vivo two-photon micro-endoscopy for intradermal high-resolution imaging with GRIN optics. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2007;6442:6442151–6442156. [Google Scholar]
- 182.Weinigel M, Breunig HG, Fischer P, Kellner-Hoefer M, Bueckle R, Koenig K. Compact clinical high-NA multiphoton endoscopy. Proc SPIE. 2012;8217:8217061–8217068. [Google Scholar]
- 183.Baroli B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J Pharm Sci. 2010;99:21–50. doi: 10.1002/jps.21817. [DOI] [PubMed] [Google Scholar]
- 184.Barry BW. Breaching the skin's barrier to drugs. Nat Biotechnol. 2004;22:165–167. doi: 10.1038/nbt0204-165. [DOI] [PubMed] [Google Scholar]
- 185.Grewal BS, Naik A, Irwin WJ, Gooris G, de Grauw CJ, Gerritsen HG, Bouwstra JA. Transdermal macromolecular delivery: Real-time visualization of iontophoretic and chemically enhanced transport using two-photon excitation microscopy. Pharm Res. 2000;17:788–795. doi: 10.1023/a:1007595822786. [DOI] [PubMed] [Google Scholar]
- 186.Yu B, Kim KH, So PT, Blankschtein D, Langer R. Visualization of oleic acid-induced transdermal diffusion pathways using two-photon fluorescence microscopy. J Invest Dermatol. 2003;120:448–455. doi: 10.1046/j.1523-1747.2003.12061.x. [DOI] [PubMed] [Google Scholar]
- 187.Yu B, Hean Kim K, So PT, Blankschtein D, Langer R. Topographic heterogeneity in transdermal transport revealed by high-speed two-photon microscopy: Determination of representative skin sample sizes. J Invest Dermatol. 2002;118:1085–1088. doi: 10.1046/j.1523-1747.2002.01796.x. [DOI] [PubMed] [Google Scholar]
- 188.Kushner JT, Kim D, So PT, Blankschtein D, Langer RS. Dual-channel two-photon microscopy study of transdermal transport in skin treated with low-frequency ultrasound and a chemical enhancer. J Invest Dermatol. 2007;127:2832–2846. doi: 10.1038/sj.jid.5700908. [DOI] [PubMed] [Google Scholar]
- 189.Nohynek GJ, Dufour EK, Roberts MS. Nanotechnology, cosmetics and the skin: Is there a health risk? Skin Pharm Physiol. 2008;21:136–149. doi: 10.1159/000131078. [DOI] [PubMed] [Google Scholar]
- 190.Song Z, Kelf TA, Sanchez WH, Roberts MS, Ricka J, Frenz M, Zvyagin AV. Characterization of optical properties of ZnO nanoparticles for quantitative imaging of transdermal transport. Biomed Opt Express. 2011;2:3321–3333. doi: 10.1364/BOE.2.003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zvyagin AV, Zhao X, Gierden A, Sanchez W, Ross JA, Roberts MS. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J Biomed Opt. 2008;13:0640311–0640319. doi: 10.1117/1.3041492. [DOI] [PubMed] [Google Scholar]
- 192.Koenig K. Multiphoton tomography of intra-tissue tattoo nanoparticles. Proc SPIE. 2012;8207:820701–820706. [Google Scholar]
- 193.Labouta HI, Kraus T, El-Khordagui LK, Schneider M. Combined multiphoton imaging-pixel analysis for semiquantitation of skin penetration of gold nanoparticles. Int J Pharm. 2011;413:279–282. doi: 10.1016/j.ijpharm.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 194.Roberts MS, Dancik Y, Prow TW, Thorling CA, Lin LL, Grice JE, Robertson TA, König K, Becker W. Non-invasive imaging of skin physiology and percutaneous penetration using fluorescence spectral and lifetime imaging with multiphoton and confocal microscopy. Eur J Pharm Biopharm. 2011;77:469–488. doi: 10.1016/j.ejpb.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 195.Stracke F, Weiss B, Lehr CM, Konig K, Schaefer UF, Schneider M. Multiphoton microscopy for the investigation of dermal penetration of nanoparticle-borne drugs. J Invest Dermatol. 2006;126:2224–2233. doi: 10.1038/sj.jid.5700374. [DOI] [PubMed] [Google Scholar]
- 196.Ozeki Y, Umemura W, Otsuka Y, Satoh S, Hashimoto H, Sumimura K, Nishizawa N, Fukui K, Itoh K. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat Photon. 2012;6:845–851. [Google Scholar]
- 197.Saar BG, Freudiger CW, Reichman J, Stanley CM, Holtom GR, Xie XS. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science. 2010;330:1368–1370. doi: 10.1126/science.1197236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hanson KM, Clegg RM. Observation and quantification of ultraviolet-induced reactive oxygen species in ex vivo human skin. Photochem Photobiol. 2002;76:57–63. doi: 10.1562/0031-8655(2002)076<0057:oaqoui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 199.Hanson KM, Gratton E, Bardeen CJ. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic Biol Med. 2006;41:1205–1212. doi: 10.1016/j.freeradbiomed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 200.Hanson KM, Behne MJ, Barry NP, Mauro TM, Gratton E, Clegg RM. Two-photon fluorescence lifetime imaging of the skin stratum corneum pH gradient. Biophys J. 2002;83:1682–1690. doi: 10.1016/S0006-3495(02)73936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Werrlein RJ, Braue CR, Dillman JF. Multiphoton imaging the disruptive nature of sulfur mustard lesions. Proc Society of Photo-Optical Instrumentation Engineers (SPIE) 2005;5700:240–248. [Google Scholar]
- 202.Uchugonova A, Duong J, Zhang N, Konig K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–2050. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- 203.Cheong WF, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. Quantum Electron IEEE J. 1990;26:2166–2185. [Google Scholar]
- 204.Girkin JM, Poland S, Wright AJ. Adaptive optics for deeper imaging of biological samples. Curr Opin Biotechnol. 2009;20:106–110. doi: 10.1016/j.copbio.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 205.Rueckel M, Mack-Bucher JA, Denk W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. Proc Natl Acad Sci USA. 2006;103:17137–17142. doi: 10.1073/pnas.0604791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cha JW, Ballesta J, So PT. Shack-Hartmann wavefront-sensor-based adaptive optics system for multiphoton microscopy. J Biomed Opt. 2010;15:0460221–046022110. doi: 10.1117/1.3475954. [DOI] [PMC free article] [PubMed] [Google Scholar]


