Abstract
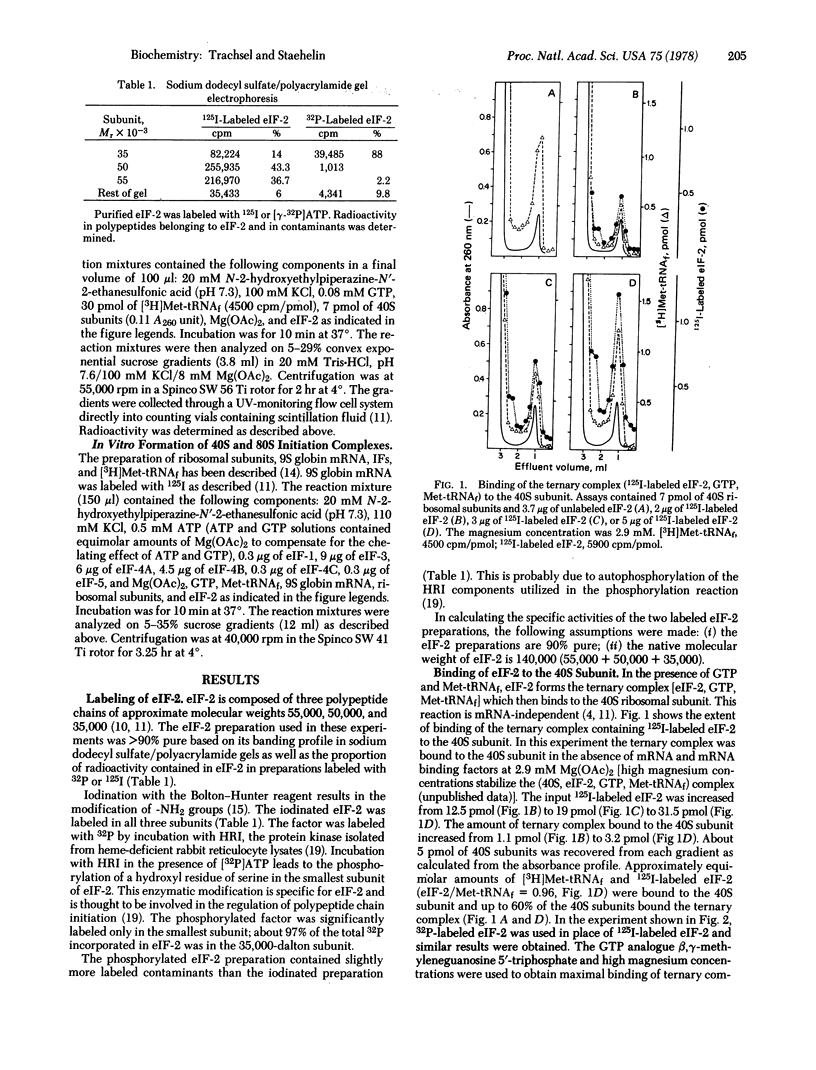
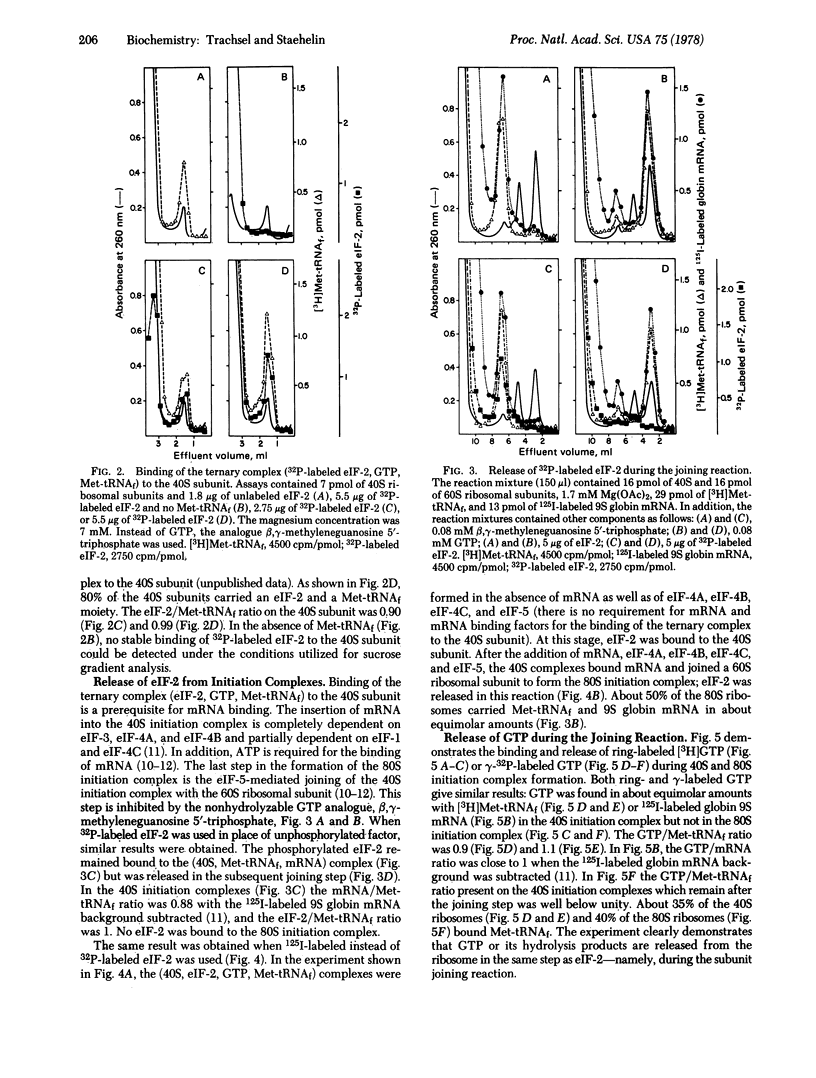
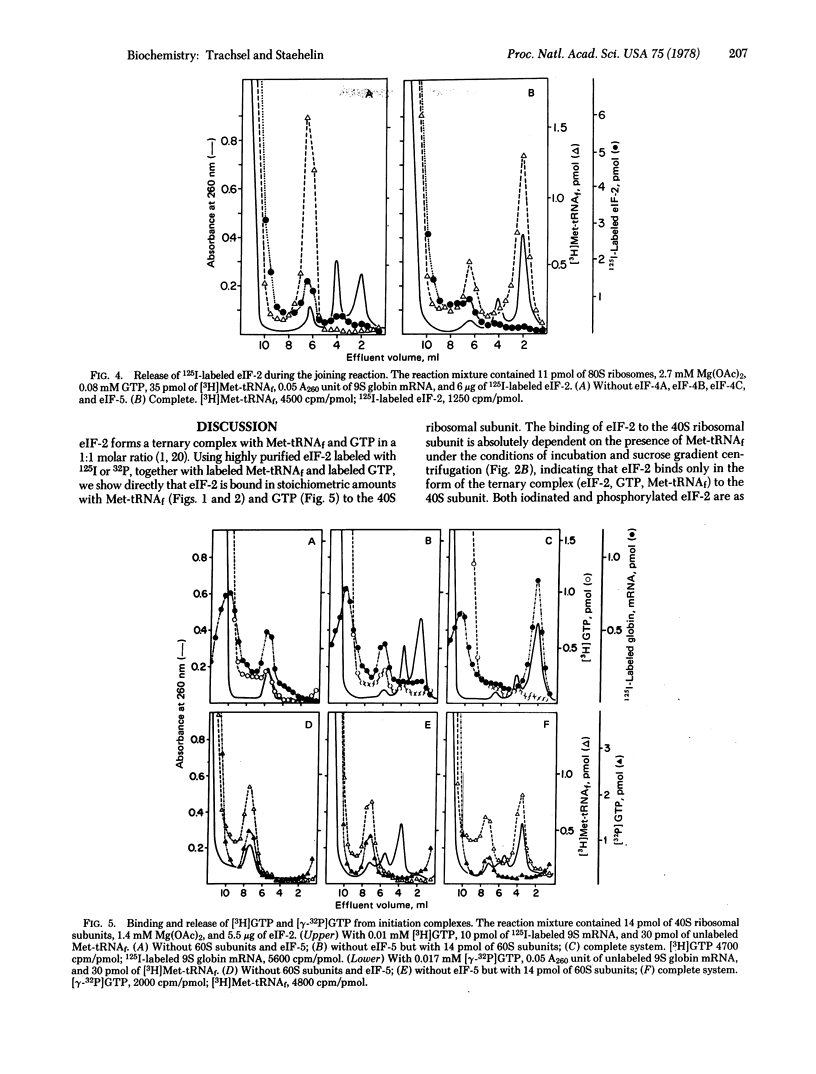
The eukaryotic initiation factor eIF-2 forms a ternary complex with Met-tRNAf and GTP. This complex binds to the 40S ribosomal subunit in the absence of mRNA and mRNA binding factors. Highly purified eIF-2 from rabbit reticulocytes was labeled with 125I by using the Bolton-Hunter reagent or with [gamma-32P]ATP by using the heme-regulated translational inhibitor protein kinase. The labeled eIF-2 was bound, together with equimolar amounts of Met-tRNAf and GTP, to the 40S subunit. In the presence of mRNA, mRNA binding factors, and 60S ribosomal subunits (complete initiation assay), eIF-2 was released from the 40S initiation complex in the subunit joining reaction. GTP also was released in this step and probably was hydrolyzed in the reaction that is dependent upon eIF-5 and the 60S subunit. The function of phosphorylated eIF-2 in initiation of protein synthesis is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Heterogeneity of the small ribosomal subunit and mechanism of chain initiation in eukaryotes. Biochim Biophys Acta. 1972 Nov 16;287(1):189–193. doi: 10.1016/0005-2787(72)90341-3. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., London I. M. On the mechanism of delayed inhibition of protein synthesis in heme-defecient rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3506–3510. doi: 10.1073/pnas.73.10.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Dettman G. L., Stanley W. M., Jr Recognition of eukaryotic initiator tRNA by an initiation factor and the transfer of the methionine moiety into peptide linkage. Biochim Biophys Acta. 1972 Nov 16;287(1):124–133. doi: 10.1016/0005-2787(72)90336-x. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gupta N. K., Woodley C. L., Chen Y. C., Bose K. K. Protein synthesis in rabbit reticulocytes. Assays, purification, and properties of different ribosomal factors and their roles in peptide chain initiation. J Biol Chem. 1973 Jun 25;248(12):4500–4511. [PubMed] [Google Scholar]
- Kramer G., Henderson A. B., Pinphanichakarn P., Wallis M. H., Hardesty B. Partial reaction of peptide initiation inhibited by phosphorylation of either initiation factor eIF-2 or 40S ribosomal proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1445–1449. doi: 10.1073/pnas.74.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein synthesis initiation in eukaryotes. Characterization of ribosomal factors from mouse fibroblasts. J Biol Chem. 1973 Sep 25;248(18):6416–6425. [PubMed] [Google Scholar]
- Pinphanichakarn P., Kramer G., Hardesty B. Partial reaction of peptide initiation inhibited by the reticulocyte hemin-controlled repressor. Biochem Biophys Res Commun. 1976 Dec 6;73(3):625–631. doi: 10.1016/0006-291x(76)90856-1. [DOI] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., Wool I. G. Preparation and characterization of eukaryotic initiation factor EIF-3. Formation of binary (EIF-3-Met-tRNAf) and ternary (EIF-3-Met-tRNAf-GTP) complexes. J Biol Chem. 1976 Apr 10;251(7):1926–1935. [PubMed] [Google Scholar]
- Safer B., Adams S. L., Anderson W. F., Merrick W. C. Binding of MET-TRNAf and GTP to homogeneous initiation factor MP. J Biol Chem. 1975 Dec 10;250(23):9076–9082. [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Laycock D. G., Crystal R. G., Anderson W. F. Requirement for GTP in the initiation process on reticulocyte ribosomes and ribosomal subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2246–2251. doi: 10.1073/pnas.68.9.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]


