Abstract
The nematode Caenorhabditis (C.) elegans, a long time work horse for behavioral genetic studies of locomotion, has recently been studied for quiescent behavior. Methods previously established for the study of C. elegans locomotion are not well-suited for the study of quiescent behavior. We describe in detail two computer vision approaches to distinguish quiescent from movement bouts focusing on the behavioral quiescence that occurs during fourth larval stage lethargus, a transition stage between the larva and the adult. The first is the frame subtraction method, which consists of subtraction of temporally adjacent images as a sensitive way to detect motion. The second, which is more computationally intensive, is the posture analysis method, which consists of analysis of the rate of local angle change of the animal’s body. Quiescence measurements should be done continuously while minimizing sensory perturbation of the animal.
Keywords: Quiescence, elegans, sleep, molting, lethargus, machine vision
1. Introduction
It has been our experience that a common response to observing C. elegans for the first time under the microscope is “wow, they move!”. Indeed, the undulating movements of this 1-mm nematode have been the subject of intense study by researchers starting with the days of Sidney Brenner [1]. But to a seasoned C. elegans researcher used to gazing daily at crawling animals in a Petri dish, the behavior that stands out is, in fact, not movement but, rather, a lack of movement. We refer to such absence of movement as “quiescence”. Quiescence is rare, occurring during typical laboratory conditions almost exclusively during larval development as the animal transitions from one larval stage to another or from the fourth larval stage to the adult stage. This transition stage is known as lethargus [2, 3].
Quiescent behavior has correlates across phylogeny including in mammals. Many mammals are quiescent during seasonal hibernation or daily torpor and all mammals have been shown to sleep. Unlike torpor, which is unique to homeothermic animals, sleep behavior has been observed widely outside mammals. The observation of sleep in insects has led to the suggestion that this behavior is universal to all animals [4]. Despite its apparent universality, the function of sleep remains a deep mystery and the regulation of sleep is poorly understood. The similarity of C. elegans quiescence to sleep in more complex animals has motivated a number of labs to use this model system as a means to understand mechanisms and functions of sleep. Even in these early days of this field, a few conclusions can be drawn. Lethargus quiescence behavior is, like mammalian sleep, controlled by the nervous system [5–7]. Second, several genes that regulate sleep in other animals have comparable effects in C. elegans [5, 8–11]. This suggests that at least some of the underlying biochemistry of lethargus quiescence is shared with sleep in other animals, and has motivated continued research in this system.
C. elegans methods to study quiescence diverge from well-established methods to study movement in a number of respects. First, the behavior is slow, occurring on the time scale of hours rather than the seconds-to-minutes time scale of most previously-studied behavior. Individual animals must therefore be tracked continuously for several hours. Confining the animals to the field of view of the camera can be accomplished by various approaches. In early work, the animal’s food was limited to a small (<1 cm2) area in the camera view, thereby encouraging it to dwell in the monitored arena. But this method has a limited throughput, with just one animal monitored per camera. In addition, mutant animals that cannot detect food, fail to slow their movement in the presence of food, or are generally hyperactive, will escape from the field of view. For example, adult males, which are motivated to find a mate, cannot be imaged by this method. A promising new method, which makes use of small concave lenses filled with agar to limit the animal’s arena to the field of view of the camera has recently been reported [12], though this method has not yet been used for the quantitative study of quiescence. Placing the animals in miniature (~100 nanoLiter) droplets confines them to the field of view and, in theory, allows for massive up scaling of the throughput. However, Belfer et al observed severely reduced survival in the droplets [10], suggesting that conditions are not optimal. Two other confinement methods, both involving the use of microfluidics chambers to confine and monitor several individual animals simultaneously, have received more traction. One is the use of agarose hydrogels to confine animals and the second is the use of microfluidics chambers fashioned to mimic dirt [13]. We use this latter method in this paper. The artificial dirt chamber is designed to optimally accommodate a worm the size of an L4 to early adult. To monitor behavior in older animals, for example those undergoing quiescence following a fasting/refeeding cycle [14], one would need to design a properly-sized chamber. Regardless of the method used to house animals during the experiments, a potential influence of the particular environment on the animals’ behavior should be considered when interpreting the data.
The second aspect of quiescence methods that differs from prior behavioral methods relates to the machine vision used for measurement of the behavior. To track movements, most researchers have used centroid tracking. The centroid is the geometric center of the smallest rectangle that captures the animal. However, the centroid assumes a constant geometry of the tracked object, and an animal can move yet retain the same centroid location because its shape has changed. Therefore, centroid tracking to identify quiescence is insensitive to small movements, which are typical in larvae [15]. In addition, centroid tracking does not make use of the posture of the animal, which we have found to have specific features during quiescent bouts in lethargus [5, 15]. We here describe approaches to measuring quiescence that does not make use of centroid but, rather, analyzes differences between temporally adjacent images. In addition, we describe our methods for analyzing worm posture.
The third unique aspect of quiescence measurements is the sensitivity of the animal to sensory stimulation. Just as an alarm clock can wake a sleeping human and cause a disruption of his quiescent posture, so can strong sensory stimulation wake a sleeping nematode and disrupt its quiescent behavior. Even weak sensory stimuli may affect quiescence during lethargus. Therefore, recordings should be done in an environment in which mechanical, photic, and chemical stimulation can be kept to a minimum.
Finally, prior investigations of lethargus quiescence have shown that the behavioral quiescence is interrupted by movement bouts and that quiescence dynamics change during the course of the two-hour lethargus period. This emphasizes the need for continuous measurements throughout lethargus.
In this methods paper, we describe methods currently used by our labs to study quiescence in C. elegans. We explain some of the deficiencies of established centroid tracking methods for identifying quiescence. We present the frame subtraction analysis, arguably the conceptually and computationally simplest method for identifying quiescent epochs. We then present a more computationally-intense method that makes use of posture information. We use npr-1 mutant analysis as an example to demonstrate the importance of performing continuous measurements with minimal sensory perturbations throughout lethargus. In addition, we use the behavior of egl-30 mutants to demonstrate that quiescence may be disrupted differentially from other behavioral correlates of lethargus.
2. Materials and Methods
2.1 Animal husbandry and strains used in the study
Animals were cultivated as hermaphroditic cultures at 20 deg Celsius on the agar surface of nematode growth medium (NGM) containing 1.5% agar. The animals were fed with the Escherichia coli bacterial strain OP50 [1]. Strains used were obtained from the CGC and included N2, a strain widely used as a laboratory reference strain, CX4148 npr-1(ky13); DA609 npr-1(ad609), and CG21 egl-30(tg26); him-5(e1490). All experiments were performed on hermaphrodites.
2.2 Experimental recording set up
Artificial dirt chambers were fashioned as previously described [13]. Late fourth larval stage (L4) animals were identified based on the appearance of their reproductive structures under stereomicroscopy. They were individually transferred to the observation chambers and videos were recorded for 10 consecutive hours at a frame rate of 10 fps. The camera was a Prosilica GC2450 (Allied Vision Technologies, Stadtroda, Germany) containing 2448 × 2050 pixels. The camera was controlled using custom Labview scripts [5]. Images were captured at 8-bit grayscale resolution.
2.3 Frame subtraction method for identifying quiescence
Frame subtraction data was obtained from the raw images using custom Matlab script (Mathworks Inc., Natick MA) that utilized the Matlab image processing toolbox. In order to vary the interval of frame subtraction, the images were acquired at 10 frames per second and down-sampled appropriately.
2.4 Posture dynamics method for identifying quiescence
The posture was identified using a custom suite of tools, called PyCelegans, for image analysis on high performance parallel computing resources. In brief, PyCelegans identifies the midline and the edges of the body of the animal in each frame, as well as the positions of the head and the tail. Each midline was divided into 20 equal segments and the local angle at each of the inner 18 segments was calculated in order to measure locomotion, quiescence, and posture as described in Nagy et al [15].
2.5 Vibration stimulus to disrupt quiescence in wild-type and npr-1 mutant animals
Vibrations were applied by gluing two 50mm piezo buzzer elements (Digikey part no. 668-1190-ND) to a plastic clamp. The clamp enabled tight mechanical coupling of the buzzers to a standard petri dish which contained the artificial dirt chamber, soaked in NGM buffer. The piezo elements were supplied with a 15-second 8V pulse every 15 minutes. In our hands, this level of stimulation produced a robust, but not saturated, locomotion response.
2.5 Statistical analysis
The Akaike Information Criterion was used to select the best model to the data presented in Fig. 4. In Fig. 5, statistical significance was tested using a one-way ANOVA test. Post-hoc correction for multiple comparisons was performed using the Bonferroni adjustment.
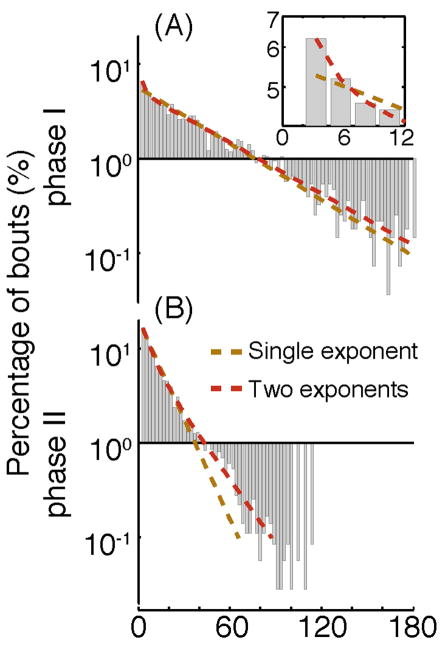
Figure 4. The distribution of durations of quiescence bouts measured using the posture-based method.
(A) The distribution of durations of quiescence bouts during the first phase of L4leth (20–90 minutes from the onset of quiescence), characterized by a high QF, decreasing body curvature, and detectable correlations between durations of consecutive bouts of motion and quiescence [5]. The Y-axis is a logarithmic depiction of the frequency and the x-axis is the duration of bouts. Dashed lines depict two possible statistical models for this distribution: a single exponential distribution (brown), and a double exponential distribution (red). Using the Akaike Information Criteria, the double exponential was a better fit to the data (p<0.01), yielding two sub-populations of bouts with mean durations of 47.9 and 2.3 sec. Inset: An enlarged view of the bins corresponding to short bouts (semilog scale). The double exponential is more successful than the single exponential in capturing the frequency distribution of short bouts in the data. (B) The same as (A), but measured during the second phase of L4leth (90 minutes from onset of quiescence until the end of quiescence), characterized by a rapidly decreasing QF, increasing body curvature, and increasing spontaneous activity in mechanosensory neurons. The double exponential distribution was still the best model, although the mean durations of the two sub-populations of bouts were more similar to each other: 19.1 and 6.2 sec.
Figure 5. The fraction of quiescence and overall motion of npr-1 mutants, as detected using the posture-based method.
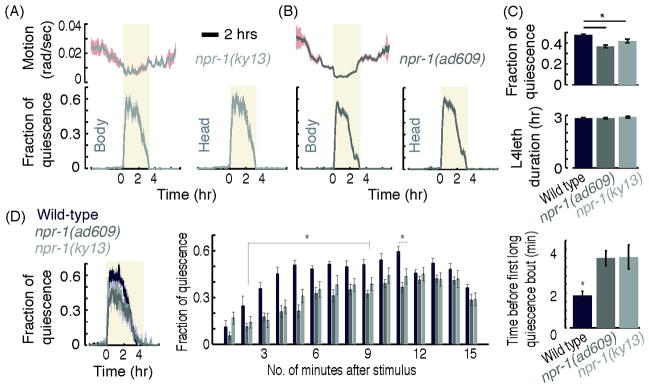
(A–B) Left: the overall motion and QF of npr-1(ky13) mutants (A) and npr-1(ad609) mutants (B), measured as described in Fig. 3D. Right: the QF of the heads of npr-1(ky13) and npr-1(ad609) mutants, measured as described in Fig. 3B. Shaded areas depict ± s.e.m. (C) The mean QF of both npr-1 mutants was slightly reduced as compared to the QF of wild-type animals, but no significant difference was found between the two npr-1 alleles. The duration of L4leth of both npr-1 mutants was similar to the L4leth duration of wild-type animals (bottom). N(wild-type) = 10, N(ad609) = 10, N(ky13) = 10, error bars depict ± s.e.m. (D) The QF of wild-type animals and npr-1 mutants that were exposed to 15 sec pulses of weak vibrations every 15 minutes. Left: the QF of wild-type animals is minimally affected by the mild stimulus. In contrast, the same stimulus causes a 20% reduction in the QF of npr-1 mutants. Middle: the QF of all three strains, measured separately during minutes 1 to 15 following the stimulus. Right: the time from the termination of the stimulus to the onset of the first quiescence bout that was longer than 10 seconds in duration. Asterisks denote that each of the mutants was significantly different from wild-type (N(wild type) = 17, N(ad609) = 13, N(ky13) = 14, p<0.05).
3. Results
3.1 Inferring quiescence from centroid tracking
Traditional machine vision algorithms for tracking C. elegans behavior under bright field microscopy make use of the centroid of the animal as a proxy for its location. The centroid is the geometric center of the smallest rectangle encompassing all pixels in the image identified to be part of the worm. The approach is first to identify worm pixels by a standard segmentation approach (appropriate functions are implemented in virtually all commercial image processing toolboxes), then calculate the centroid as above. Fig. 1 depicts the fraction of time that wild-type animals spend in quiescence during the fourth lethargus stage (L4leth) as inferred from centroid tracking. To calculate the fraction of quiescence (QF), a time interval, Δt, and a threshold number of pixels for motion, θ, are defined. If the position of the centroid shifted by a sub-threshold amount, the corresponding time interval is defined as “quiescent”. The QF is calculated as the fraction of quiescent intervals in a moving time window, typically 5–10 min wide. The data shown in Fig. 1 was obtained of an animal in a 10-hour time period spanning L4 lethargus. The time interval was kept constant at Δt=500 ms, and the threshold for detecting motion was varied from, θ=2 to θ=330 pixels. In this experiment, a single pixel corresponds to approximately 4×4 μm2. At the highest threshold of 330 pixels (~5,000 μm2), the QF was estimated to be large and was likely a gross overestimate of the actual QF, since a movement of this magnitude corresponds to movement of 1/8 fraction of the total area of the worm’s body. At the lowest threshold of 2 pixels (16 μm2), the QF was small and likely underestimated the actual QF. Using a threshold setting of θ=70 pixels (~1,000 μm2), the QF was comparable to that obtained by other methods. These results demonstrate a limitation of centroid tracking method, namely that Δt and θ set an arbitrary velocity scale. This arbitrary scale may vary between different genetic backgrounds and may be sensitive to a variety of known and unknown experimental conditions.
Figure 1. The fraction of quiescence detected using the centroid method.
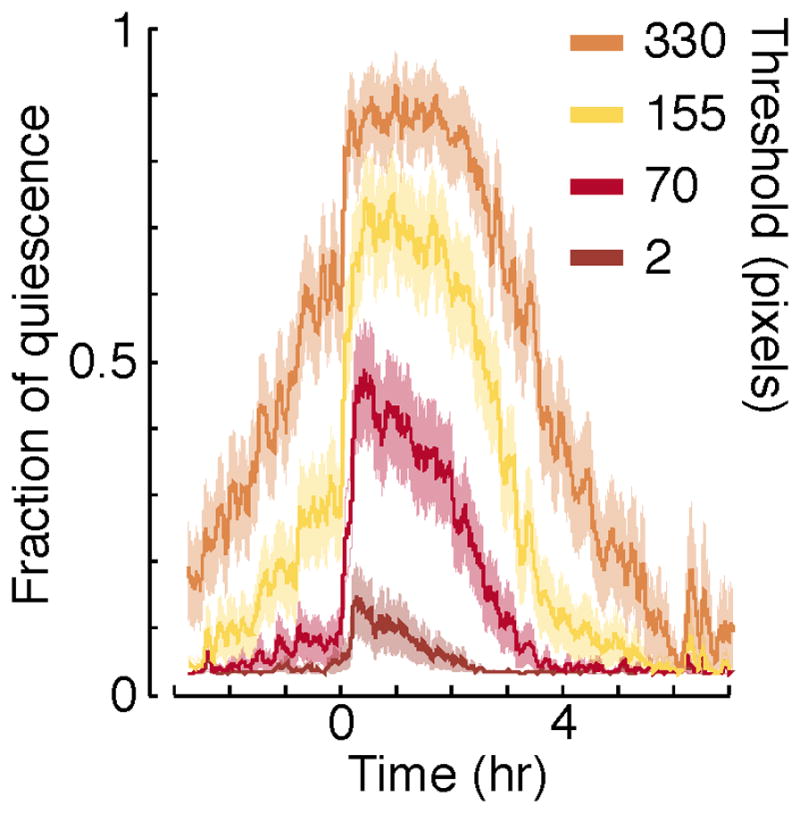
The motion of the centroid was assessed over a time interval of 500 ms, and the threshold for separating motion form quiescence was varied from 2 to 330 pixels. Each plot depicts the average fraction of quiescence (QF) over a moving time window of five minutes. Traces derived from analysis of 30 wild-type animals imaged starting four hours before L4 lethargus were aligned at the onset of lethargus quiescence and averaged. The onset of lethargus quiescence was identified manually as the time when the QF increased by at least 0.3 in a 30-minute time period. Shaded areas depict ± s.e.m..
While useful for measuring speed of the animal under conditions of rapid locomotion, centroid tracking has limitations for the study of quiescence. A major drawback of this method is that the centroid position of a deformable body can be a complex, non-linear, function of deformation – the relation between a cutoff on this function and complete absence of movement may not be straightforward. In addition, the required segmentation step typically introduces small frame-to-frame variations that reduce the sensitivity of the measurement, e.g. noise due to including dark pixels caused by shadows in the bacterial tracks or by excluding pixels of the less optically-dense head of the animal. Deformations of the shape of the animal may thus result in no detected change of centroid location within the accuracy of the measurement, while measurement noise may be interpreted as animal motion. Two commonly observed examples of motion that may be difficult to detect are head oscillations, which can persist without directed progression of the body, or minor fluctuations (5–15% of the body length) in positions of body-bends, often observed in a state of dwelling. The dynamics of head motion and quiescence is of particular interest in the context of C. elegans lethargus, since it was found to approximate those of the entire animal [15, 16]. Therefore, a method such as centroid tracking, which is not sensitive to head motion, is expected to incur measurement errors and over-estimate quiescence.
In the Drosophila melanogaster sleep research field, researchers use the crossing of an infrared beam located at the center of a small (6 cm) tube containing the fly. When the fly crosses the path of the beam, the animal is considered awake. This is similar to hard encoding the threshold, θ, in the dimensions of the experimental apparatus. When the fly does not cross the beam for five continuous minutes, it is considered asleep [17]. In the C. elegans field too, Golombeck and colleagues used beam crossings to monitor slow (circadian) behavioral rhythms [18]. While beam-crossing method has the advantage of providing convincing evidence of animal movement when it crosses the path of the infrared beam, the animal can clearly be moving yet not cross the path of the beam. Hence, it can greatly over-estimate quiescence, as demonstrated directly in Drosophila [19]
3.2 Detecting quiescence using frame subtraction
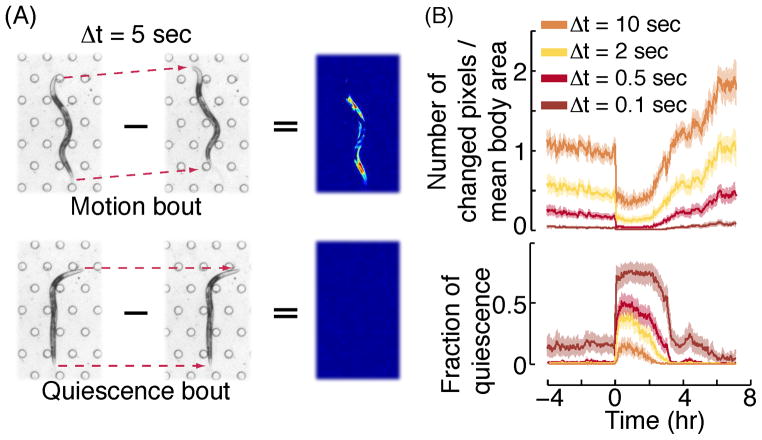
We developed a video machine vision method, which we call “frame subtraction”, based on the beam crossing principle, only taken to the extreme of a single pixel [9]. Pairs of temporally adjacent video images are subtracted, as shown in Fig. 2A. If there is any movement between the two frames, pixel gray scale values will change (Figure 2A, top). The frame rate of the imaging setup thus sets the timescale, Δt, and it typically varies between 0.5 – 10 sec, depending on the type of camera and magnification used. The threshold for motion is, by definition, θ=0, although in some cases a small threshold (1–2 pixels) may be used to compensate for experimental noise. Therefore, one can consider the frame subtraction method as a beam-crossing method with the spatial resolution for detecting movement of one pixel, which corresponds to 3 – 20 μm in a typical video acquisition experiment. Since the brightness of the pixels of a digital camera can fluctuate slightly, a threshold change in the greyscale value is needed in order to distinguish animal motion from electronic noise. Optimizing the optical set up to achieve a strong contrast between the worm and its surrounding eliminates the need to finely tune this threshold, and is thus important. The data shown in Fig. 2B was obtained with θ=0, a threshold change of 30 in greyscale values, and a pixel size corresponding to 4 μm. The data were collected at a rate of 10 frames per second. Because animals of different genotypes may have different sizes, we normalized the number of pixels moved to the average total number of pixels that defined the worms’ bodies (Fig 2B, top).
Figure 2. The fraction of quiescence during L4leth detected using the frame subtraction method.
(A) The subtraction of one frame from the frame preceding it by 5 seconds during the fourth intermolt larval stage, L4int, (top) and during an L4leth bout of quiescence (bottom). Animals were confined to the field of view using a microfluidic “artificial dirt” microfluidics chamber [13] which contains a hexagonal array of posts visible in the picture. In the resulting difference image, the magnitude of the absolute value of the grey scale difference of individual pixels is shown in pseudocolor where red is large and blue is small. Pixels with grey scale values less than the threshold value of 30 are shown as not having changed. (B) Top: the number of pixels changed in the difference image was normalized by the total number of pixels in the animal’s body (identified by standard thresholding methods) and used as a proxy for overall motion. The mean body area was calculated as the average number of pixels constituting the worm’s body during L4leth, averaged over all of the animals tested. Bottom: fraction of quiescence was determined by calculating the fraction of instances, in a moving window of 5 minutes, in which either one or zero pixels changed their value between consecutive frames. The four curves in each plot correspond to the results of analysis using a variable subtraction interval Δt=0.1 – 10 seconds. Each plot depicts the average QF over a moving window of 5 minutes. Traces derived from analysis of 30 wild-type animals were aligned at the onset of lethargus quiescence and averaged. The onset of lethargus quiescence was identified manually based on the frame subtraction data (using Δt=0.5) as the time when the QF increased by at least 0.3 in a 30-minute time period. Shaded areas depict ± s.e.m.
We tested the effect of varying the interval of subtraction Δt. At very short Δt=0.1 seconds, very few pixels surpassed the 30 grayscale unit threshold in the subtracted image, even during the normally-active L4 and adult stages. This result was expected because the animal makes no significant movement in a 0.1 second period. As a result, the fraction of quiescence is close to or at 1.0 during lethargus and significantly greater than 0 outside of lethargus. In contrast, at long Δt=20 seconds, a high number of pixels moved at every time point, even during the normally quiescent lethargus period, and this results in a fraction of quiescence close to or at 0 even during lethargus. This result too was expected because most quiescent bout durations were shorter than 20 seconds [5]. In practice, the Δt should be selected to clearly distinguish lethargus from surrounding periods but without creating a ceiling effect during lethargus. Another factor that impacts the choice of Δt is whether one desires to analyze the quiescence bout architecture. Since the durations of short quiescent bouts are on the scale of seconds, for bout analysis, we recommend using at most Δt=0.5 seconds.
3.3 Detecting quiescence using body posture dynamics
The most detailed, accurate, and computationally intensive method for evaluating quiescence and motion relies on identifying the posture of the nematode in each frame and analyzing the dynamics of head and body bends. Typically such an analysis requires high spatial and temporal resolutions. Due to the prolonged nature of the behavior in question, the resulting datasets can be on the terabyte scale and analyzing them requires the use of methods specifically catered to the analysis of large data sets. We developed an adaptable suite of analysis tools, compatible with high performance computing resources, called PyCelegans [15].
We begin by defining the body of the animal in a single image using standard thresholding methods [20]. When thresholding was insufficient for obtaining (complex) postures, a more sophisticated statistical model of anatomical features was used for the object recognition step [21]. PyCelegans extracts the midline and boundaries information of the body, as well as the positions of the head and the tail. These data, which are much smaller in size than the original movie, were further processed and analyzed to characterize the dynamics of locomotion and quiescence.
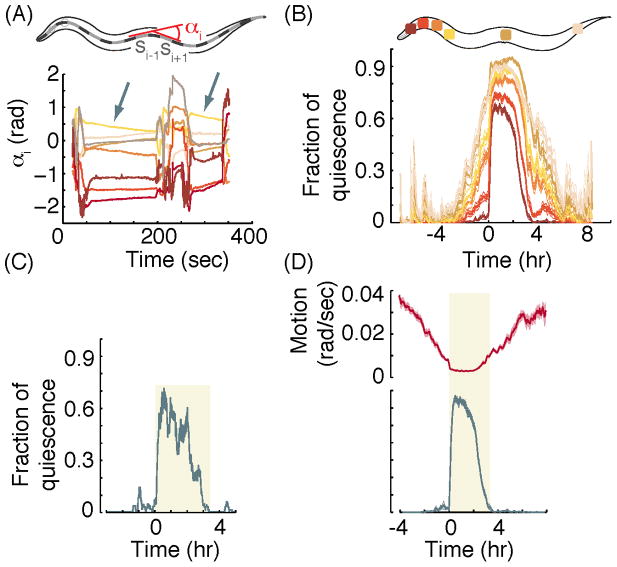
The angle at an individual position along the midline can be defined as the angle between the intervals of the midline flanking the target position (Fig. 3A–B). Quiescence can be defined locally for a target position along the midline by setting a threshold rate of angular change, ω. If the angle between the flanking intervals changes at a rate that falls below ω, the target position is defined as “quiescent”. Whole-animal quiescence can be defined as the simultaneous quiescence of a sufficient sample of positions along the body. In practice this strict definition is relaxed to account for experimental noise, e.g., due to the difficulty of precisely identifying the tip of the tail of the animal. The data shown in Fig. 3C–E were obtained using an imaging rate of 10 frames per second, a pixel size corresponding to 1.54×1.54 μm2 (using a 4.5X magnification), a threshold angular change at each position of ω=0.01 rad/sec, and by considering 18 equally-spaced local angles along the midline (adapted from [15]). Interestingly, we found that the most sensitive part of the animal for detecting movement is in the nose region and that analysis of ω in this region alone (as compared to analysis of the ω at each of the 18 segments of the worm) was sufficient for distinguishing quiescence from motion bouts (Fig 3C). The sensitivity of the nose for detecting movement has been previously exploited by Bringmann and colleague in their studies of quiescence [6].
Figure 3. The fraction of quiescence and overall motion detected using the posture-based method.
(adapted from [15]). (A) Top: a schematic representation of the body of C. elegans. The midline of the worm was divided into 20 equi-length intervals (black/grey dashes) and the local angles at position i along the body, αi, was defined as the angle formed between the intervals si−1 and si+1. Bottom: the temporal dynamics of eight angles during early L4leth. Quiescence at the localized position along the body was detected when αi was constant or slowly relaxed at a sub-threshold rate (grey arrows). (B) Mean fraction of local quiescence at four anterior positions, one mid-body position and one posterior position. Quiescence is the lowest when tracking the most anterior position. (C) The QF of a single wild-type animal, determined based on the absence of motion of individual body-bends as detailed in [15]. (D) Top: the sum of the absolute rates of change of 18 angles, α1…α18, defined as in (A), corresponding to the overall motion of the animal. Bottom: the mean QF of wild-type animals. In panels (B) and (D) traces from different animals were aligned at the onset of lethargus quiescence, identified manually based on the posture data, as the start of a 30-minute period in which the QF increased at least 0.3. Shaded areas depict ± s.e.m., N=37 wild-type animals.
3.4 Detecting the distribution of bouts of quiescence using the body posture-based method
Two features of quiescent behavior become immediately apparent: (i) the quiescence onset is abrupt, allowing for precise definition of the start of lethargus, and (ii) There are at least two micro-states within lethargus, characterized by bouts of quiescence and bouts of motion [5]. Continuous and accurate measurements of quiescence using the posture-based method improve on previous assays of the microarchitecture of lethargus. Within the 2–3 hour period of L4leth, larvae exhibit alternating bouts of quiescence and motion of durations of 1–100 sec. Although the nature and function of motion bouts remain unclear, we used a posture-based method to measure the distribution of quiescence bouts in wild-type L4leth larvae. Since we previously reported that early L4leth period might be regulated differently from late L4lth [5], we considered the distribution of quiescence bouts in each of these tentative phases separately (Fig. 4). We used the Akaike Information Criteria (AIC) for model selection to compare three candidate statistical models for these distributions: a single exponential distribution, a double exponential distribution, and a spike and slab distribution. The spike and slab distribution was observed in an analysis of rapid eye movement (REM) and Non-REM sleep in mice and suggested that mouse sleep is a mixture of two sub-populations of shorter and longer bouts [22]. The double exponential model, shown in Fig. 4, achieved the lowest AIC score of the three and was selected as the best fit to the data (p<0.01). The spike and slab model for our data, not shown in Fig. 4, achieved a higher (worse) AIC score than the double exponential model, but was visually similar. During the first half of L4leth (Fig. 4A) the mean durations of the two sub-populations were 47.9 and 2.3 sec and the double exponential model was required to capture both the “spike” of short bouts and the long tail of the distribution. In contrast, during the second half of L4leth (Fig. 4B) the mean durations of the two sub-populations were 19.1 and 6.2 sec and the advantage of the double exponential model was predominantly more accurate at describing the tail of durations that exceeded 30 seconds. Although at this point we do not yet know the biological significance of sub-populations of quiescence bouts, this analysis provides a convenient approach for describing the data.
3.5 The importance of continuous measurements and a controlled sensory environment
External stimuli such as touch, light, vibrations, and odors, have been shown to influence behavior during lethargus. In particular, the fraction of quiescence during the few minutes following the stimulus shifts from its baseline level. The genetic background of the animal can affect responses to an external cue, e.g., by affecting the amplitude of the response, the duration of the response, or the probability of the animal to respond in the first place [9, 13, 23, 24]. Importantly, the efficacy of numerous combinations of environmental conditions and sensory stimuli for disrupting quiescence in a mutant or transgenic animal of interest cannot be accurately predicted. Thus, it is important to perform the experiment under controlled conditions that minimize sensory stimulation of the animals.
For instance, the NPR-1 neuropeptide Y receptor-like protein effects the responses to a variety of external cues, including ethanol, oxygen, carbon dioxide, pheromones, and mechanical stimuli [25] [26–30]. Mutants in the npr-1 gene were shown to be hypersensitive to mechanical stimuli and periodic sampling periods of 30–75 seconds each during L4leth suggested that these mutants have greatly reduced quiescence in comparison to wild-type animals [31].
To test npr-1 mutants with continuous recordings and with minimal sensory perturbation, we analyzed their behavior in the artificial dirt microfluidics chambers. These continuous recordings showed that the fraction of quiescence of npr-1 mutants was mildly smaller than wild-type (Fig. 5A–C) and the duration of lethargus was not changed. Exposing these animals to a mild mechanical stimulus – 15 second pulses of weak vibrations every 15 minutes – was sufficient to reduce the total quiescence fraction of npr-1 mutants by 20% as compared to wild-type animals under identical conditions. The difference between the responses of wild-type and mutant animals was even more pronounced during the first five minutes after each stimulus: the QF of the mutants was reduced by up to 50% as compared to wild-type during this period. Correspondingly, the onset of the first long quiescence bout occurred on average four minutes after the stimulus in the mutants, as compared to two minutes in wild-type animals (Fig. 5C). The different phenotypes obtained under these different conditions emphasize the need to assess quiescence through continuous, uninterrupted recordings, where external disturbances are minimized.
3.6 Animals may show reduced quiescence yet preserved postural dynamics
Locomotion quiescence stands out as the most noticeable phenotype during lethargus, but additional behavioral changes during this period have been reported. These include the cessation of feeding (“pharyngeal pumping quiescence”), reduced responses to external stimuli, and adopting distinct postures [2, 5, 8, 9]. Broad locomotion defects may result in changes to the measured fraction of locomotion quiescence, while other behavioral correlates of lethargus may or may not be affected. Relying solely on quiescence could thus exaggerate or mask the perceived effect of a genetic manipulation on lethargus. We posit that assaying distinct, possibly separately regulated, behavioral correlates of lethargus is conducive for informed interpretations.
EGL-30 is an ortholog of the Gq protein alpha subunit that was shown to affect locomotion, viability, egg laying, and pharyngeal pumping [32] [33]. A gain-of-function mutation tg26 in the egl-30 gene results in hyperactive head movements both during and outside of lethargus [34]. We considered the possibility that while quiescence, as defined by a strict threshold, may be nearly abolished in this mutant, the contrast in postural dynamics between L4int and L4leth may be preserved. This could be the result of a different genetic regulation of quiescence and postural dynamics, or, more likely, because measurements of quiescence that involve thresholding can be affected by global changes in locomotion. In contrast to the use of a strict threshold to identify quiescence, continuous behavioral metrics would preserve the contrast between lethargus and other stages under such global changes. Indeed, we found that, unlike quiescence, which was nearly abolished relative to that of wild-type animals, the locomotion of animals carrying the egl-30(tg26) gain-of-function mutation (see methods) was reduced during L4leth relative to L4int (Fig. 6A–B). Similar to wild-type animals, the body curvature of the mutants dropped sharply at the onset of L4leth, remained low for approximately two hours, and gradually returned to its baseline value during late L4leth (Fig. 6C). The contrast between the postures associated with L4int and L4leth was thus largely preserved, suggesting that not all of the behavioral dynamics associated with lethargus are abnormal to the same extent in the mutants. Since a typical posture is considered one of the behavioral hallmarks of sleep in both vertebrates and invertebrates [35], these results suggest that at least some aspects of sleep are retained during lethargus by animals carrying the egl-30(tg26) mutation. Although these results do not demonstrate a clear separation between the regulation of locomotion and posture, they call attention to the possibility that reduced quiescence does not necessarily correlate with other aspects of lethargus. Therefore, when applicable, considering multiple behavioral correlates in addition to the QF, and continuous ones in particular, would be superior to quiescence analysis alone.
Figure 6.
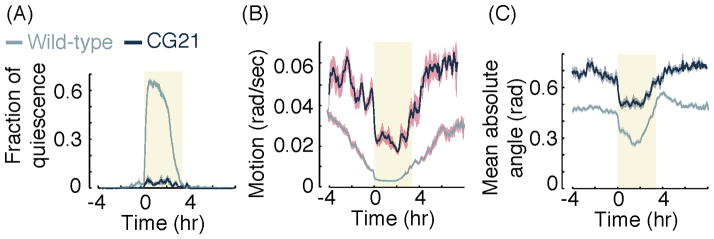
(A) Fraction of quiescence as detected using posture based analysis (adapted from [15]) in wild-type and CG21 animals carrying the egl-30(tg26) gain-of-function mutation. The locomotion quiescence of the mutants is nearly abolished. (B) The sum of the absolute rates of change of 18 angles, α1…α18, corresponding to the overall motion of the animal (see also Fig. 3A) in wild-type and CG21 animals. Although the locomotion rates of the mutants are higher than those of wild-type animals both during and outside of lethargus, they exhibit a sharp drop at the onset of L4leth and gradually return to baseline during late L4leth. (C) The mean absolute angle, averaged over the absolute values of 18 body angles, α1…α18, of wild-type and CG21 animals. Although the mean body curvature of the mutants is higher than that of wild-type animals, the temporal dynamic of the posture of the mutants resembles that of wild-type animals: body curvature drops sharply at the onset of L4leth and gradually returns to baseline during late L4leth. In all panels: N(wild-type) = 37, N(CG21) = 14, shaded areas depict ± s.e.m.
4. Discussion
We provide a summary in Table 1 of key difference between the methods used to analyze locomotion and those used to analyze quiescence. In this paper, we highlighted the importance of these differences and described in detail two methods for detecting quiescence of locomotion.
Table 1.
Comparison of C. elegans locomotion to quiescence studies
| Locomotion studies | Quiescence studies | |
|---|---|---|
| Time scale | Seconds to minutes | Hours to days |
| Machine vision | Centroid tracking posture analysis | Frame subtraction posture analysis |
| Measurement continuity | Not important | very important |
| Sensory environment | important | Very important |
Determining quiescence based on frame-differences has provided the vast majority of insight in into the behavior and the underlying biochemistry associated with lethargus. Similar to centroid-tracking, it is computationally simple, but it appears to perform better than centroid tracking in terms of providing accurate and reproducible data under similar conditions and sample sizes. Identifying the posture of the animal in each frame is more computationally intensive; the timely analysis of the resulting large volumes of data require big data approaches. However, the acquired data is rich in behavioral information: it can be used to quantify several behavioral correlates of lethargus which might be regulated by distinct molecular mechanisms; it can be used to separately examine different body parts; and it reduces the risk of bias associated with manual scoring of subtle phenotypes.
While we demonstrate that centroid tracking has limitations for the accurate identification of quiescence, it has the advantage of being widely available in both commercial and academic machine vision packages. In addition, since it identifies a location for the animal and measures the magnitude of movement, one can learn from centroid tracking the position of the animal relative to some reference point, and its speed outside lethargus relative to lethargus. We note however that magnitude of movement can also be discerned using the frame subtraction approach, by measuring the total number of pixels moved. In practice, the method chosen will be influenced by the biological question posed [8, 31, 36]. For example, if the question is whether or not an animal slows movement during lethargus, or whether it is engaged in dwelling or roaming behavior[37], then centroid tracking, frame subtraction, or postural dynamics analysis would be appropriate. If the question is how much total quiescence occurred during lethargus, then frame subtraction or postural dynamics analysis would be appropriate [5, 9, 13, 15, 24]. If the question centers on the architecture of quiescent bouts, than the frame acquisition rate must be sufficiently small to be able to measure short quiescent bouts[5, 15].
We showed an example of a mutant, egl-30(tg26), which has a reduced overall quiescence during lethargus yet displays posture dynamics changes during lethargus similar to those observed in wild-type animals. In humans, pathological conditions such as periodic limb movement disorder [38] and REM-sleep behavior disorder (RBD) [39] are associated with movements during sleep, resulting in reduced quiescence during sleep. However, other aspects of human sleep, such as reduced responsiveness, appear unimpaired in such patients. Likewise, posture analysis demonstrates that reduced quiescent behavior in C. elegans may not necessarily be coupled to equally severe defects in additional behavioral, developmental, or physiological correlates of lethargus.
In our approaches to understand the microarchitecture of lethargus, we considered the simplest scenario of only two microstates: brief movement bouts and brief quiescence bouts. In mammals, there are three distinct states defined electrophysiologically—wake, REM sleep, and Non-REM sleep—and sub-states defined by bout analysis [22]. In addition, during wakefulness, mammals can have both behavioral and electrophysiologically-defined sub-states. Future analysis of microarchitecture in wild-type and mutant animals, as well as increasing use of physiological measurements of neuronal activity, may allow us to define additional sub-states of both motion and quiescent bouts during lethargus.
Finally, we note that we here focused only on locomotion quiescence and posture dynamics during lethargus. In addition to locomotion quiescence, animals are quiescent for feeding and defecation behaviors; they are also less responsive to stimuli. Finally, forced bouts of locomotion during lethargus, even brief ones making use of the photoavoidant response [40], can affect subsequent quiescence and sensory responsiveness of the animals. These observations have let to the proposal that quiescent behavior during lethargus, like sleep in mammals, is under homeostatic regulation [9]. The development of quantitative methods to study these other behaviors is an important but still on-going endeavor in our labs as well as in other labs.
Acknowledgments
This work was supported by the Burroughs Wellcome Fund (D.B), the Searle Scholars Program (D.B), the National Science Foundation (to D.B. grant No. PHYS-1066293), and the National Institutes of Health (to DMR, grant No. NS064030).
Footnotes
Conflicts of interest: None identified.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh RN, Sulston JE. Some observations on moulting in Caenorhabditis elegans. Nematologica. 1978;24:63–71. [Google Scholar]
- 3.Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode C. elegans. Developmental Biology. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 4.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwanir S, Tramm N, Nagy S, Wright C, Ish D, Biron D. The microarchitecture of C. elegans behavior during lethargus: homeostatic bout dynamics, a typical body posture, and regulation by a central neuron. Sleep. 2013;36:385–395. doi: 10.5665/sleep.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz J, Lewandrowski I, Bringmann H. Reduced activity of a sensory neuron during a sleep-like state in Caenorhabditis elegans. Curr Biol. 2011;21:R983–984. doi: 10.1016/j.cub.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Cho JY, Sternberg PW. Multilevel Modulation of a Sensory Motor Circuit during C. elegans Sleep and Arousal. Cell. 2014;156:249–260. doi: 10.1016/j.cell.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nature Neuroscience. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 9.Raizen DM, Zimmerman JE, Maycock MH, Ta UD, You YJ, Sundaram MV, Pack AI. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 10.Belfer SJ, Chuang HS, Freedman BL, Yuan J, Norton M, Bau HH, Raizen DM. Caenorhabditis-in-drop array for monitoring C. elegans quiescent behavior. Sleep. 2013;36:689–698G. doi: 10.5665/sleep.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turek M, Lewandrowski I, Bringmann H. An AP2 transcription factor is required for a sleep-active neuron to induce sleep-like quiescence in C. elegans. Curr Biol. 2013;23:2215–2223. doi: 10.1016/j.cub.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Yu CC, Raizen DM, Fang-Yen C. Multi-well imaging of development and behavior in Caenorhabditis elegans. Journal of neuroscience methods. 2013;223C:35–39. doi: 10.1016/j.jneumeth.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh K, Chao MY, Somers GA, Komatsu H, Corkins ME, Larkins-Ford J, Tucey T, Dionne HM, Walsh MB, Beaumont EK, et al. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr Biol. 2011;21:825–834. doi: 10.1016/j.cub.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.You YJ, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy S, Wright C, Tramm N, Labello N, Burov S, Biron D. A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by Galphas signaling. eLife. 2013;2:e00782. doi: 10.7554/eLife.00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann H. Agarose hydrogel microcompartments for imaging sleep- and wake-like behavior and nervous system development in Caenorhabditis elegans larvae. Journal of neuroscience methods. 2011;201:78–88. doi: 10.1016/j.jneumeth.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods in Enzymology. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 18.Simonetta SH, Golombek DA. An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. Journal of neuroscience methods. 2007;161:273–280. doi: 10.1016/j.jneumeth.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman JE, Raizen DM, Maycock MH, Maislin G, Pack AI. A video method to study Drosophila sleep. Sleep. 2008;31:1587–1598. doi: 10.1093/sleep/31.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noboyuki O. A Threshold Selection Method from Gray-Level Histograms. IEEE Transactions on Systems, Man and Cybernetics. 1979;9:62–66. [Google Scholar]
- 21.Amit Y. 2D Object Detection and Recognition: Models, Algorithms, and Networks. Boston MA: MIT PressZ; 2002. [Google Scholar]
- 22.McShane BB, Galante RJ, Jensen ST, Naidoo N, Pack AI, Wyner A. Characterization of the bout durations of sleep and wakefulness. Journal of neuroscience methods. 2010;193:321–333. doi: 10.1016/j.jneumeth.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driver RJ, Lamb AL, Wyner AJ, Raizen DM. DAF-16/FOXO regulates homeostasis of essential sleep-like behavior during larval transitions in C. elegans. Current Biology. 2013 doi: 10.1016/j.cub.2013.02.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson MD, Trojanowski NF, George-Raizen JB, Smith CJ, Yu CC, Fang-Yen C, Raizen DM. The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans. Nat Commun. 2013;4:2846. doi: 10.1038/ncomms3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419:899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron. 2004;42:731–743. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Styer KL, Singh V, Macosko E, Steele SE, Bargmann CI, Aballay A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science. 2008;322:460–464. doi: 10.1126/science.1163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, Kruglyak L, Bargmann CI. Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron. 2009;61:692–699. doi: 10.1016/j.neuron.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi S, Chatzigeorgiou M, Taylor KP, Schafer WR, Kaplan JM. Analysis of NPR-1 Reveals a Circuit Mechanism for Behavioral Quiescence in C. elegans. Neuron. 2013;78:869–880. doi: 10.1016/j.neuron.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trent C, Tsuing N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron. 1996;16:999–1009. doi: 10.1016/s0896-6273(00)80123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz J, Bringmann H. Reduced sleep-like quiescence in both hyperactive and hypoactive mutants of the Galphaq Gene egl-30 during lethargus in Caenorhabditis elegans. PLoS One. 2013;8:e75853. doi: 10.1371/journal.pone.0075853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher T, Bjorness T, Greene R, You YJ, Avery L. The geometry of locomotive behavioral states in C. elegans. PLoS One. 2013;8:e59865. doi: 10.1371/journal.pone.0059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujiwara M, Sengupta P, McIntire SL. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron. 2002;36:1091–1102. doi: 10.1016/s0896-6273(02)01093-0. [DOI] [PubMed] [Google Scholar]
- 38.Bliwise D, Petta D, Seidel W, Dement W. Periodic leg movements during sleep in the elderly. Archives of gerontology and geriatrics. 1985;4:273–281. doi: 10.1016/0167-4943(85)90009-3. [DOI] [PubMed] [Google Scholar]
- 39.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 40.Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 2008;6:e198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]