Abstract
Integrated Vector Management (IVM) strategies are intended to protect communities from pathogen transmission by arthropods. These strategies target multiple vectors and different ecological and socioeconomic settings, but the aggregate benefits of IVM are limited by the narrow focus of its approach; IVM strategies only aim to control arthropod vectors. We argue that IVM should encompass environmental modifications at early stages, for instance, infrastructural development and sanitation services, to regulate not only vectors but also nuisance-biting arthropods. An additional focus on nuisance-biting arthropods will improve public health, quality of life, and minimize social disparity issues fostered by pests. Optimally, IVM could incorporate environmental awareness and promotion of control methods in order to proactively reduce threats of serious pest situations.
Keywords: IVM, vector control, biting density, pest management, social disparities
Integrated vector management to enhance the environment
Responsible for 17% of the global burden of communicable disease, vector-borne diseases (VBDs) (see Glossary) [1-3] induce significant morbidity and mortality and have major effects on the socioeconomic development of affected countries [4]. Use of indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) are among the most crucial measures used to protect humans from vectors [1]. As intensified control measures decrease VBD incidence in certain areas, herd immunity declines, and the severity of arthropod transmitted diseases increases as they spread to new regions [5,6]. Weakened political resolution to continue funding for vector control dissolves successful vector control organizations and community awareness [5] and further propagates the public health problem caused by VBDs. Lack of awareness by decision-makers on the impact of VBDs on public health has limited research in this area and caused complacency towards development of new vector control methods [7]. Alterations in population dynamics and climate changes that modify the habitats of arthropods have led to the re-emergence and spread of VBDs to new regions [8].
The World Health Organization has widely promoted vector control for the prevention and elimination of VBDs [9] and recommends the pragmatic use of both non-chemical and chemical strategies encompassed in Integrated Vector Management (IVM) [10,11]. Through the use of a rational evidence based decision-making process to utilize available resources in an optimal manner, IVM aims to make vector control more effective by use of economical and ecologically sound strategies [11]. IVM encourages integrative and multi-disease approaches to promote collaborative interventions [12] that better combat the spread of VBDs [13], but fails to address nuisance-biting arthropods that affect inherent quality of life. Furthermore, its common reactive application following vector outbreaks limits the entirety of potential preventative benefits; an additional environmental enhancement component would significantly improve IVM strategies’ capabilities to control all arthropods.
Goals of integrated vector management
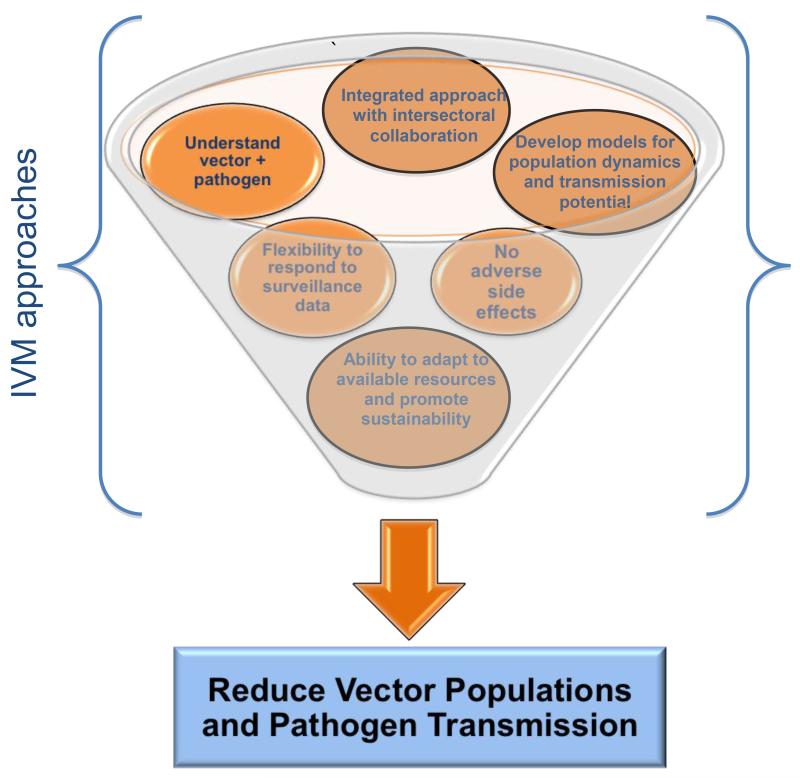
Through the use of evidence-based collaborative approaches such as governmental and community involvement, IVM promotes capacity building to further vector control [12] and uses dynamic strategies to incorporate the best use of tools to reduce vector populations and pathogen transmission (Figure 1). Given that arthropod vectors impact the health, agricultural, and environmental sectors, control through IVM strategies obliges local government and community involvement to implement goal-achieving interventions [13]. Vector control is an essential factor in improving public health and has the potential to alleviate poverty if fully exploited because disability-adjusted life years (DALYs) can be reduced [11]. The 2006 Chikungunya epidemic in India burdened the country with an estimated 25 588 DALYs from infection [14]. Lack of proper vector control and environmental maintenance exacerbated spread of the pathogen and caused extensive suffering to residents. Increasing productivity by limiting workdays lost to illness benefits the public and private sector.
Figure 1.
Multiple approaches used in IVM to control vectors. The graphic illustrates how IVM strategies, each depicted as separate circular images, encompass a wide array of comprehensive and versatile approaches used for the achievement of a common goal: the reduction of arthropod vectors and their consequential transmission of harmful pathogens.
Increased insecticide resistance [1] augments the need for effective non-chemical and environmental control strategies. Pesticides, even when used correctly, can be hazardous to human and environmental health [15]. Medical literature records the negative impacts of consistently applied chemical control for agricultural pest management on the eyes, skin, and respiratory, neurologic, and gastrointestinal systems [16]. A study of 152 Philippine rice farmers using levels of pesticides recommended by the US Environmental Protection Agency recorded a number of abnormalities: 36% for eye effects; 50% for skin effects; 30% for respiratory effects for farmers who did not smoke; 24% for neurologic effects for farmers who did not drink; and 27% for gastrointestinal effects [16]. Furthermore, a study assessing the effects of household pesticides, which are frequently applied incorrectly and at incorrect doses during pest outbreaks, correlated with pesticide levels found in plasma with decreased birth weight in children [17]. Insecticide exposure through use of indoor household pesticides was also associated with increased leukemia risk [18].
Given the aforementioned effects on human health as unintended consequences of pesticides, new complementary strategies beyond pesticide application may be warranted. Through a promotion of multiple, evidence-based strategies to reduce VBDs, for instance, IRS and LLINs, along with the collaboration of various sectors including government agencies and community stakeholders [19], incorporation of IVM in early stages of community development is the safest and most affordable approach to reduce host-vector contact, pathogen transmission, and prevent serious vector and pest situations [11].
Many approaches, one target: what is missing?
The damaging effects of VBDs can impact human and animal health, outdoor recreation, and tourism [20]. Notwithstanding, nuisance-biting arthropods such as non-vector mosquitoes, bed bugs, cockroaches, fleas, and head lice, which are exceedingly disregarded in public health research, play a role in quality of life [21]. A US study found that asthmatics sensitized to cockroach allergens and exposed to more than 8 units/g of allergen had more severe asthma symptoms than those asthmatics with a lesser degree of cockroach allergen exposure [22]. Economically, pests can also reduce crop yield by billions of dollars annually, clog water intakes, and impact landscape and housing infrastructure [23]. Invasive pest species alter ecosystem services and affect populations, community interactions, and habitats [24].
A quantitative relationship exists between vector population densities and pathogen transmission [25], yet there is no measure of mosquito population density and quality of life for individuals exposed to mosquitoes; the mosquito density in populated areas is rarely a concern for international authorities. High mosquito biting levels are not tolerated in the US, yet globally, tens of millions suffer and die from arthropod-transmitted pathogens [26]. The US has up-to-date mosquito control programs that target control of mosquitoes in larval and adult stages to reduce mosquitoes as nuisances, provided that pathogens are monitored through ongoing sentinel programs [27]. Residents in New Jersey are willing to pay close to $8 per person per week to be able to spend mosquito-free time in their backyards [28]. In developing countries, however, access to and accountability for mosquito control is not a choice [27].
Environmental changes that reduce human-vector contact will take commitment from stakeholders and government institutions. National leadership and suitable resources are needed, but governments and international organizations must ensure that afflicted countries have adequate capital for development and vector control [4]. In underdeveloped areas where malaria is endemic, such as in Africa, the biting intensity is highly variable with annual entomological inoculation rates (EIR) ranging from <1 to >1000 infective bites per person per year [29]. Addressing components of neighborhood and community development that affect quality of life is a key responsibility of governing institutions [30]. Sanitary facilities and a safe water supply can decrease the incidence of cholera by 68% and 73%, respectively [31]; while piped water may be inaccessible owing to limited availability of resources in developing countries, water treatments and safe storage interventions are economical and accessible methods of reducing disease risk [32, 33]. Although lack of intersectoral collaboration and secure funding limit IVM implementation in many unindustrialized nations [12], the Zambia Malaria Control Programme with the Roll Back Malaria Partners have successfully applied IVM and established environmental protection strategies that have reduced malaria in Zambia [34].
Studies show that certain environmental characteristics may make communities more susceptible to poor health outcomes, without specific regard to a particular virus or toxic substance (see: http://www.mosquito.org/control). The presence or absence of a pathogen is less indicative of disease prevalence than is a community’s environmental characteristics - for example, socioeconomic characteristics or proximity to standing water - linking health with exposure to the outdoor environment [35]. A lack of bed nets and a porous built environment infrastructure, that is, poorly constructed edifices for human activity, can also link the indoor environment to disease. The built environment that has previously been linked to the social health of community members [35, 37-41] is probably a major contributing factor of certain diseases, including not only obesity and related chronic diseases [42-43] but also infectious diseases [39, 40, 44]. Negative characteristics, such as the presence of nuisance mosquitoes and pests will reduce the time individuals spend outdoors, limiting outdoor exercise and community building activities [45-46].
A controlled study created mosquito free environments that showed a positive impact on children’s outdoor physical activities [45]; the presence of Aedes albopictus is a barrier promoting indoor, sedentary behavior. Physical activity is critically important for managing health, and while nutrition and sedentary behaviors have decreased activity, pests produce a similar effect [35, 47]. Their presence reduces social cohesion, as people are less likely to interact if they are limiting their outdoor experience [48, 49] to reduce human-pest contact. Although IVM strategies target and reduce pathogen transmission, by not focusing on environmental components in all stages of development that may harbor nuisance-biting arthropods, they miss the mark on many aspects of social and public health, such as obesity, that have been growing in importance [48].
IVM benefits are constrained by its narrow focus (Figure 2). With the escalation of urbanization and the creation of artificial habitats that facilitate the reproduction of mosquitoes and pests [50], environmental management strategies incorporated in all stages of vector development are critical. Sustainable and economically sound strategies such as adequate sewage and trash disposal can combat the corollary of rapid and unplanned population growth: unmanaged pest prevalence and increased biting densities. Early, economical, and lasting control strategies will be needed to combat the socio-ecological factors that affect arthropod development and pathogen exposure in complex urban areas [50-51]. Otherwise, invasive species such as Ae. albopictus will continue to have economic, environmental, and social consequences [9].
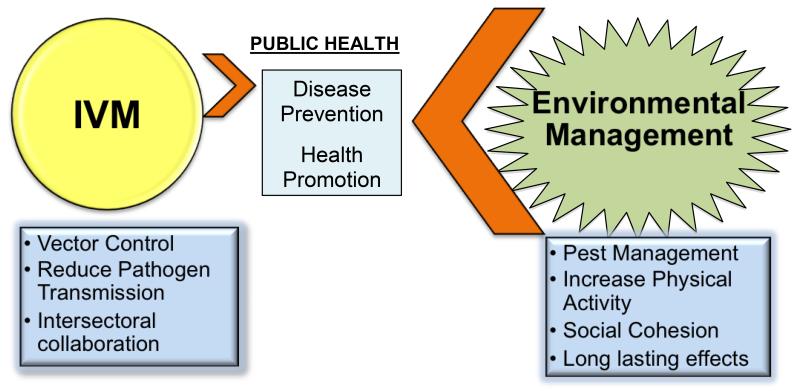
Figure 2.
Environmental management and IVM to improve public health. This graphic shows the differences between IVM and Environmental Management (EM) approaches. IVM is limited in its approach, as it only aims to prevent disease. EM approaches have a greater impact in their potential to both prevent disease and promote healthy behaviors through the modification of communities. Although good IVM includes EM, on its own EM can encompass vector and pest control through important infrastructural development that can ensure healthy environments with adequate sewage systems, waste disposals, and safe water access.
Environmental awareness to lessen social disparities
Mosquito infestation and species abundance differ significantly with economic conditions, furthering the gap between socioeconomic groups and intensifying social disparities [50]. Cockroaches, in particular, are ranked as the most common insects encountered by low-income homeowners in the US, rely on human activities to spread, and proliferate in areas with poor sanitation and dilapidated structures [15]. Environmental risk factors for bed bugs and tick-borne diseases include low socioeconomic status (SES), high-density neighborhoods, and human activities [52-53]. The social environment influences exposure levels and disease pathways [54] and is linked with racial and ethnic environmental health disparities [55] where economically disadvantaged groups endure environmental hazards and associated illness [56]. Particularly, the severe gap between socioeconomic groups in developing countries leads toward significant health disparities such as malnutrition, diarrheal diseases, and other outcomes attributed to unfavorable environmental exposure [57]. Necessarily, the elimination of health disparities is the fundamental goal of public health in developing and industrialized nations [58].
Adequate arthropod control that compensates for poor planning and biased budget allocations becomes paramount to improving health conditions. The human rights perspective is pertinent to VBD control as individuals willing to pay for vector-control tools are of higher SES, leaving lower income households under-protected. Arthropods affect communities and weaken economic development through crop damage, disturbance of mental health such as the psychological effects caused by head lice and bed bugs [52-53], and damaged housing infrastructure [59]. As higher SES individuals have better health because of cumulative protective early-life behaviors such as perinatal care, adequate nutrition, and positive health outcomes of wearing sunscreen and insecticide [55-60], it is preferable to eliminate environmental disparities to prevent onset of illness to lower SES individuals that have limited protective health benefits [59, 61].
There is a stark relationship between lower SES and unequal environmental regulation follow-through, including inadequate response to community complaints [55, 61]. Sanitary and physical conditions, such as peeling paint and water damage, greatly affect the probability of pest infestations [15]. The dilapidated housing present in many developing cities also offer limited protection against nuisance pests [33]. In developing countries such as Malaysia, common arthropod pests, including mosquitoes, cockroaches, and houseflies, are regarded an important health concern [33]. A study of asthmatics from Connecticut and Massachusetts found that low SES and minority status had a positive association with high levels of cockroach allergens in house dust [62]. Additionally, children who are allergic to and exposed to high levels of cockroach allergens were hospitalized for asthma 3.4 times more than other children, had 78% more unscheduled health care visits, and missed significantly more days of school than other children [63]. This preventable onset of illness creates strenuous situations for low SES children that potentially limit their future success. Additionally, asthma causes an estimated $14.5 billion burden in the US, and medical and societal costs associated with pest-related asthma is likely a sizeable contributor to that cost [64]. Globally, arthropods damage up to 10% and 25% of industrialized and developing nations’ gross products, respectively (see: http://www.cals.ncsu.edu/course/ent425/text01/impact1.html), and are a significant economic burden.
Structural factors, such as the local economy, determine the ability of a community to mobilize resources to make positive changes [55]. Neighborhood conditions, particularly building code violations and deteriorated housing, affect communities and make them more vulnerable to environmental hazards [55]. Environmental pollutants, structural processes, and neighborhood resources all contribute to community stress, a state of vulnerability that can lead to individual stress and increase the susceptibility to illness [55]. Policy level changes that address infrastructure and maintenance can therefore improve public health [55, 65]. Public access to information and participation in the development of interventions and in policy decisions are necessary to eliminate health disparities [56]. Health parity is a global overarching goal [56], and IVM strategies can aid in this aim to reduce the repercussions of unjust and inadequate services and remedy a facet of the social disparity concern through the prevention and mitigation of residual VBD impacts.
Recommendations for government led environmental modification
To reduce harmful effects of inadequately maintained communities, government involvement is crucial to confront issues of poverty reduction [4, 40]. Institutions and mechanisms must be persuaded to enable vulnerable citizens and have their preferences represented [66]. Good governance encompasses a reevaluation of primary issues, public problems, and allocation of resources [30]. Improvements to dilapidated environments, especially to settings that propagate unhealthy living conditions, are primary issues that fall under government responsibility. Afflicted communities must undergo a needs assessment to evaluate the conditions of drainage schemas, vegetation levels, infrastructure, and quality of municipal services. These community concerns will require the intersectoral collaboration of governing bodies and global associations to ensure that developing nations can address core environmental facets that directly affect health. Evaluation of these fundamental components for neighborhood health and the appropriation of resources to improve deficient conditions are essential for the promotion of healthy environments. Furthermore, the knowledge gained from needs assessments must be retained and implemented in future development projects to prevent the reoccurrence of unhealthy conditions.
Incorporating a policy and structural level of control will enhance the goals of IVM and Integrated Pest Management (IPM) (Box 1). Control strategies that are hindered by poor conditions reduce beneficial and perdurable outcomes. Importantly, stagnant water due to undeveloped or unmaintained sewage systems will continue to exacerbate arthropod proliferation. Poor sanitation and garbage disposal will also damage environmental health and the achievement of social parity. As IVM strategies are generally considered best management practices for the control of arthropod vectors and IPM is likewise known for most adequately controlling pests, jointly utilizing the fundamental methods of both strategies, along with increased governmental and non-governmental organizations’ responsibility for infrastructure and sanitation, can result in rapid and effective improvements.
Box 1. Use of Integrated Pest Management for pest control.
Integrated Pest Management (IPM) is an ecological approach to pest control based on sound biological principles [69]. It requires the ability to manipulate host plants, arthropods, and the environment to reduce pest populations [70]. Although there is not one single definition for IPM, its comprehensive goal is to use available resources and approaches to maintain pest populations below economically damaging levels [71]. A strong element of IPM is the use of nonchemical control measures. These measures can include changes in cultural practices to diminish open water containers, and mechanical or physical barriers to stop pests from entering buildings [72]. Additionally, resistant and tolerant plants or other biological but environmentally sound control methods are supported by IPM. The stress on nonchemical control tools is due to the increase in insecticide resistance and the side effects sublethal concentrations of chemicals have at dispersing pests into nearby uninfested areas [73]. Pests can compete with people for valuable resources, such as crops, and directly affect health; in order to keep pests below economically damaging levels IPM uses appropriate measures to reduce pest populations while minimizing detrimental human and environmental effects [72]. IPM uses control tactics that influence plants, pests, and the environment to create safer and more effective pest management (Figure I). IPM is widely used because it is an adaptable strategy that can adjust to evolving situations [51]. IPM was developed under the acceptance that pests cannot be entirely eliminated and therefore, its focus is on controlling pest populations. IPM does have disadvantages, such as requiring extensive efforts to implement and manage, as many of its control measures are labor-intensive and require frequent reapplication [71]. Additionally, much of the success of interventions can be weather dependent [71], so IPM needs to focus on perdurable programs that can have lasting effects without requiring continual management.
As individual responsibility for vector control becomes standard practice worldwide [67], educating individuals on culturally accepted control practices becomes an important component of arthropod control implementation. However, empowering communities and individuals through education and access to resources and services is not enough to reduce negative social, structural, and physical factors in the environment [68]. As risk factors such as poor sanitation, education, and housing are beyond individual control [68], the public sector, governing nations, and international organizations must work to improve the environment, which will in turn improve health. In doing this and empowering individuals through educative strategies, the harmful consequences of vectors and pests will be reduced.
Concluding remarks and future perspectives
Regulations that take into account the risk of pest infestation and disease transmission caused by development, as well as ensuring new buildings are protected against infestation are necessary implementations during rapid urbanization. Reduced urban and rural borders caused by urban sprawl increases the susceptibility of all areas to disease agents [4, 17, 33]. If integrated vector and pest management strategies successfully incorporated environmental modifications as methods of controlling pests, communities would be cleaner, structural soundness would increase, and health disparities that arise from external hazards would decrease. Realization of the importance of vector and pest management will increase understanding of the economic and social benefits of expanding comprehensive municipal services in developing and low SES neighborhoods in industrialized nations. These services are crucial to ensure the effectiveness of nonchemical control strategies, and require the dedicated efforts and awareness of the long-term health benefits of private and non-profit organizations. The aggregate benefits of healthier environments such as the promotion of outdoor activities, social cohesion, and reduced DALYs are driving forces that urge IPM and IVM to incorporate environmental modifications in their control strategies. This approach would make arthropod control more cost effective, because of reduced pesticide reapplications and decrease the onset of health conditions. Overall, a comprehensive approach towards pest and vector control where structural, policy, and environmental issues are addressed will produce long-lasting effects that reduce disease, create healthier environments, and improve quality of life.
Highlights.
Integrated Vector Management fails to address nuisance-biting arthropods.
Vector control must include an early environmental management component.
Enhancing the environment will reduce social disparity issues created by pests.
Control measures must prevent pest and vector outbreaks to improve quality of life.
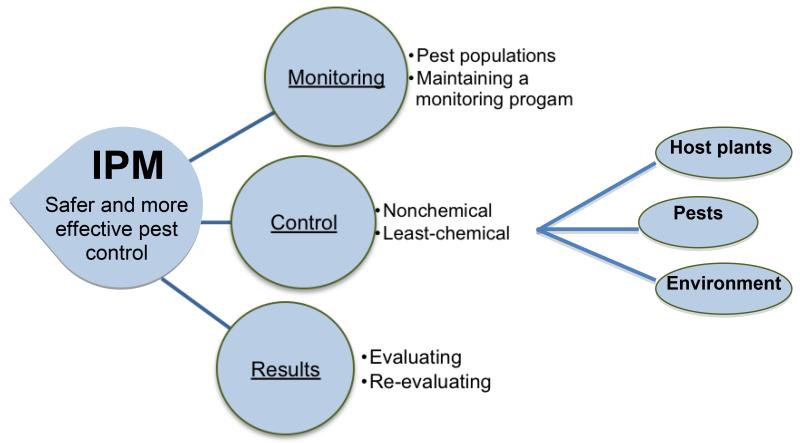
Figure I.
The three components of integrated pest management. Integrated Pest Management (IPM) depends upon: (i) monitoring pest populations; (ii) using nonchemical and limited chemical control methods; and (iii) evaluating and re-evaluating results to maintain effective program standards. While all three major components of IPM are equally important, control measures are the active component of the management strategies. Manipulation of host plants, pests, and the environment require specific measures such as physical and biological control or behavior modification of pests, and alternating between heat and cold, irrigating and flooding, or the planting of refugia for beneficial organisms. All strategies have the goal of leading to safer and more effective pest control.
Acknowledgements
We would like to offer special thanks to Dr. Bruce Christensen and Dr. Ali Hassan for their invaluable expert recommendations provided in the development of this paper. This research was supported by grant R01 GM093345 and R01 AI100968 from the National Institute of Health.
Glossary
- Aedes albopictus
highly anthropogenic mosquito species and most well-known for transmitting dengue and Chikungunya viruses but has also been found naturally infected with West Nile virus, eastern equine encephalitis, and Japanese encephalitis.
- Arthropod
an invertebrate animal of the large phylum Arthropoda, for example, insects, ticks and mites, spiders, or crustaceans.
- Built environment
the surroundings established by humans for settlement.
- Disability adjusted life years (DALYs)
the number of healthy years lost to illness, morbidity, or death; an assessment of the overall disease burden.
- Entomological inoculation rate (EIR)
the number of infectious bites an individual is exposed to in a given time period.
- Herd Immunity
a form of immunity that arises from the majority of individuals in a community being resistance to a disease and limiting the presence of the disease for the minority.
- Indoor residual spraying (IRS)
the practice of spraying areas where people reside with an insecticide to kill mosquitoes.
- Insecticides
a mixture of a toxic active ingredient used specifically for killing insects.
- Integrated pest management (IPM)
a comprehensive strategy that incorporates best practices for controlling pests.
- Integrated vector management (IVM)
a process of optimizing decisions to utilize resources effectively for vector control.
- Long lasting insecticidal nets (LLINs)
a fine net or screen treated with insecticide used to keep out mosquitoes.
- Nuisance-biting arthropods
arthropods that do not transmit pathogens but affect quality of life owing to nuisance biting.
- Pesticide
a mixture of a toxic active ingredient used for killing arthropods or other pests destructive to crops or animals.
- Vector-borne disease (VBD)
a disease diffused by blood-feeding arthropods and caused by their transfer of infectious microbes.
- Vector
an organism, typically a biting insect or tick that transmits a pathogen from one animal or plant to another.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews K, et al. World Malaria Report 2013. World Health Organization; 2013. [Google Scholar]
- 2.Guidelines for control of vectors of public health importance in Pakistan. Department of Zoonotic and Vector-Borne Diseases, Epidemic Investigation Cell, Public Health Laboratories Division, National Institute of Health, Ministry of Health, Government of Pakistan; 2010. [Google Scholar]
- 3.Guidelines on public health pesticide management policy for the WHO African Region. World Health Organization (WHO) Regional Office for Africa and the WHO Department of Control of Neglected Tropical Diseases; 2011. [Google Scholar]
- 4.Global Strategic Framework for Integrated Vector Management. World Health Organization; 2004. [Google Scholar]
- 5.Mendis K, et al. Global Malaria Control and Elimination: Report of a Technical Review. World Health Organization; 2008. [Google Scholar]
- 6.Lemon SM, et al. Understanding the Environmental, Human Health, and Ecological Connections, Workshop Summary. National Academies Press; 2008. [PubMed] [Google Scholar]
- 7.Gubler D. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends in Microbio. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 8.Townson HN, et al. Exploiting the potential of vector control for disease prevention. Policy and Practice. 2005;93:942–947. [PMC free article] [PubMed] [Google Scholar]
- 9.Series WTR. Malaria Vector Control and Personal Protection. World Health Organization; 2006. p. 936. [PubMed] [Google Scholar]
- 10.Mutero CM, et al. Integrated vector management for malaria control in Uganda: knowledge, perceptions and policy development. Malar J. 2012;11:21. doi: 10.1186/1475-2875-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg HM, et al. Guidance on policy-making for Integrated Vector Management. World Health Organization; 2012. [Google Scholar]
- 12.Beier JC, et al. Integrated vector management for malaria control. Malar J. 2008;7:S4. doi: 10.1186/1475-2875-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich JN, et al. How much vector control is needed to achieve malaria elimination? Trends Parasitol. 2013;29:104–109. doi: 10.1016/j.pt.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamoorthy K, et al. Burden of Chikungunya in India: estimates of disability adjusted life years (DALY) lost in 2006 epidemic. J of Vector Borne Diseases. 2009;46:26–35. [PubMed] [Google Scholar]
- 15.Bonnefoy X, et al. Public health significance of urban pests. World Health Organization; 2008. [Google Scholar]
- 16.Prabhu LP, et al. Pesticides and Philippine Rice Farmer Health: A Medical and Economic Analysis. Am J of Agri Econ. 1994:76. [Google Scholar]
- 17.Garry VF. Pesticides and children. Special Ped. 2004;198:152–163. doi: 10.1016/j.taap.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Alavanja MCR, et al. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annual Review of Pub Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- 19.van den Berg H, et al. Handbook for Integrated Vector Management. World Health Organization; 2012. [Google Scholar]
- 20.Communicating about Effective Mosquito Control. The Association of State and Territorial Health Officials; 2008. [Google Scholar]
- 21.Eldridge B. Pesticide Application and Safety Training for Applicators of Public Health Pesticides. California Department of Public Health; 2008. [Google Scholar]
- 22.Rosenstreich D, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. The New England J of Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 23.Pejchar L, Mooney H. Invasive species, ecosystem services and human well-being. Trends in Ecol & Evol. 2009;24:497–504. doi: 10.1016/j.tree.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Charles HDJ. Impacts of Invasive Species on Ecosystem Services. Ecol Studies. 2007:193. [Google Scholar]
- 25.Woolhouse M, et al. Emerging pathogens: the epidemiology and evolution of species jumps. Trends in Ecol & Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lashari AA, Zaman G. Global dynamics of vector-borne diseases with horizontal transmission in host population. Computers & Math with Applications. 2011;61:745–754. [Google Scholar]
- 27.Impoinvil D, et al. Comparison of mosquito control programs in seven urban sites in Africa, the Middle East, and the Americas. Health Policy (Amsterdam, Netherlands) 2007;83:196–212. doi: 10.1016/j.healthpol.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halasa YA, et al. Quantifying the impact of mosquitoes on quality of life and enjoyment of yard and porch activities in New Jersey. PLOS One. 2014 doi: 10.1371/journal.pone.0089221. DOI: 10.1371/journal.pone.0089221 ( www.plosone.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating J, et al. Anopheles gambiae s.l. and Anopheles funestus mosquito distributions at 30 villages along the Kenyan coast. J of Med Entomol. 2005;42:241–246. doi: 10.1093/jmedent/42.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merilee SG. Good Enough Governance Revisited. Development Policy Review. 2011:29. [Google Scholar]
- 31.Azurin JC, Alvero M. Field evaluation of environmental sanitation measures against cholera. Bull World Health Org. 1974;51:19–26. [PMC free article] [PubMed] [Google Scholar]
- 32.Reller ME, et al. Cholera Prevention With Traditional and Novel Water Treatment Methods: An Outbreak Investigation in Fort-Dauphin, Madagascar. Am J of Public Health. 2001;91:1608–1610. doi: 10.2105/ajph.91.10.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gratz NG. Urbanization, arthropod and rodent pests and human health. J of Vector Ecol. 1999:1291. [Google Scholar]
- 34.Chanda E, et al. Integrated vector management: The Zambian experience. Malaria J. 2008;7:164. doi: 10.1186/1475-2875-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butterworth I. The Relationship Between the Built Environment and Wellbeing: a Literature Review. Victorian Health Promotion Foundation; 2000. [Google Scholar]
- 36.Brown S, et al. The relationship of built environment to perceived social support and psychological distress in Hispanic elders: The role of “eyes on the street.”. J of Gerontology: Social Sciences. 2009 doi: 10.1093/geronb/gbn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szapocznik J, et al. The impact of the built environment on children’s school conduct grades: The role of diversity of use in a Hispanic neighborhood. Am J of Community Psych. 2006;38:299–310. doi: 10.1007/s10464-006-9084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leyden K. Social capital and the built environment: The importance of walkable neighborhoods. Am J of Pub Health. 2003;93:1546–1551. doi: 10.2105/ajph.93.9.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan W, et al. The fruit of urban nature: Vital neighborhood spaces. Environment and Behavior. 2004;36:678–700. [Google Scholar]
- 40.Cohen D, et al. Neighborhood Physical Conditions and Health. Am J of Pub Health. 2003;93:467–471. doi: 10.2105/ajph.93.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alaimo K, et al. Community gardening, neighborhood meetings and social capital. J of Community Psych. 2010:38. [Google Scholar]
- 42.Brown S, et al. Walk Score: Associations with purposive walking in recent Cuban immigrants. Am J of Preventive Med. 2013;45:202–206. doi: 10.1016/j.amepre.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rundle A, et al. Neighborhood food environment and walkability predict obesity in New York City. Env Health Perspectives. 2009;117:442–447. doi: 10.1289/ehp.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown S, et al. The role of settings in family based prevention of HIV/STDs. In: Pequegnat W, Bell CC, editors. Family and HIV/AIDS: Cultural and Contextual Issues in Prevention and Treatment. Springer; New York: 2012. pp. 69–93. [Google Scholar]
- 45.Worobey J, et al. Child Outdoor Physical Activity is Reduced by Prevalence of the Asian Tiger Mosquito, Aedes albopictus. J of the Am Mosquito Control Association. 2013;29:78–80. doi: 10.2987/12-6296R.1. [DOI] [PubMed] [Google Scholar]
- 46.McNeill LH, et al. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011–1022. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Rahman T, et al. Contributions of built environment to childhood obesity. The Mount Sinai J of Med, New York. 2011;78:49–57. doi: 10.1002/msj.20235. [DOI] [PubMed] [Google Scholar]
- 48.Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 49.Gordon-Larsen P, et al. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- 50.LaDeau SL, et al. Higher mosquito production in low-income neighborhoods of Baltimore and Washington, DC: understanding ecological drivers and mosquito-borne disease risk in temperate cities. Int J Environ Res Public Health. 2013;10:1505–1526. doi: 10.3390/ijerph10041505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandler H. Integrated Pest Management. Cranberry Station Best Management Practices Guide; 2010. [Google Scholar]
- 52.Goddard J, de Shazo R. Psychological Effects of Bed Bug Attacks. The Am J of Med. 2012 doi: 10.1016/j.amjmed.2011.08.010. DOI: http://dx.doi.org/10.1016/j.amjmed.2011.08.010 ( www.amjmed.com) [DOI] [PubMed] [Google Scholar]
- 53.Stefanoff P, et al. A national case-control study identifies human socio-economic status and activities as risk factors for tick-borne encephalitis in Poland. PLOS One. 2012 doi: 10.1371/journal.pone.0045511. DOI: 10.1371/journal.pone.0045511 ( www.plosone.org) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yen ISS. The social environment and health-a discussion of the epidemiologic literature. Annu Rev Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287. [DOI] [PubMed] [Google Scholar]
- 55.Gee GC, Payne-Sturges DC. Environmental Health Disparities: A Framework Integrating Psychosocial and Environmental Concepts. Environ Health Perspectives. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Payne-Sturges DC, Gee GC. National environmental health measures for minority and low-income populations: tracking social disparities in environmental health. Environ Res. 2006;102:154–171. doi: 10.1016/j.envres.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Braveman P, Tarimo E. Social inequalities in health within countries: not only an issue for affluent nations. International Health in the 21st Century. 2001;54:1621–1635. doi: 10.1016/s0277-9536(01)00331-8. [DOI] [PubMed] [Google Scholar]
- 58.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 59.Tanzi V. Globalization and the work of fiscal termites. Finance & Development. 2001:38. [Google Scholar]
- 60.Prus S. Age, SES, and health: a population level analysis of health inequalities over the lifecourse. Sociology of Health & Illness. 2007;29:275–296. doi: 10.1111/j.1467-9566.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 61.Venables T. Spatial disparities in developing countries: cities, regions and international trade. Centre for Economic Performance, London School of Economics and Political Science; London: 2003. [Google Scholar]
- 62.Leaderer B, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspectives. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rust M, Reierson DA. Chlorpyrifos resistance in German cockroaches (Dictyoptera: Blattellidae) from restaurants. J of Econ Entomology. 1991;84:736–740. doi: 10.1093/jee/84.3.736. [DOI] [PubMed] [Google Scholar]
- 64.Krieger J. The Seattle-King County healthy homes project: implementation of a comprehensive approach to improving indoor environmental quality for lowincome children with asthma. Environ Health Perspectives. 2002;110:311–322. doi: 10.1289/ehp.02110s2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauh V, et al. Deteriorated housing contributes to high cockroach allergen levels in inner-city households. Environ Health Perspectives. 2002;110:323–327. doi: 10.1289/ehp.02110s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snyder J, Yackovlev I. Political and economic determinants of changes in government spending on social protection programs. Massachusetts Institute of Technology; Cambridge, United States: 2000. Mimeographed document. [Google Scholar]
- 67.Allen W, et al. Benefits of collaborative learning for environmental management: applying the integrated systems for knowledge management approach to support animal pest control. Environmental Management. 2001;27:215–223. doi: 10.1007/s002670010144. [DOI] [PubMed] [Google Scholar]
- 68.Israel B, et al. Health education and community empowerment: conceptualizing and measuring perceptions of individual, organizational, and community control. Health Education Quarterly. 1994;21:149–170. doi: 10.1177/109019819402100203. [DOI] [PubMed] [Google Scholar]
- 69.Dowling Z, et al. Socioeconomic Status Affects Mosquito (Diptera: Culicidae) Larval Habitat Type Availability and Infestation Level. J of Med Entomol. 2013;50:764–772. doi: 10.1603/me12250. [DOI] [PubMed] [Google Scholar]
- 70.Norris RF. Integrated Pest Management. Encyclopedia of Biological Invasions. 2010:353–355. [Google Scholar]
- 71.Ehi-Eromosele C, et al. Integrated Pest Management, Weed and Pest Control - Conventional and New Challenges. Agricultural and Biological Sciences; 2013. [Google Scholar]
- 72.Miller FS, et al. Integrated Pest Management in the Urban Environment. Illinois Research. 1987;29:22–26. [Google Scholar]
- 73.Alpert G. Integrated Pest Management Program for Research Facilities. Biohazards Management Handbook; 2000. pp. 79–90. [Google Scholar]