Abstract
Nausea and vomiting are ubiquitous as drug side effects and symptoms of disease; however, the systems that determine these responses are arguably designed for protection against food poisoning occurring at the level of the gastrointestinal (GI) tract. This basic biological pathway using GI vagal afferent communication to the brain is not well understood. Part of this lack of insight appears to be related to current experimental approaches, such as the use of experimental drugs, including systemic chemotherapy and brain penetrant agents, which activate parts of the nausea and vomiting system in potentially unnatural ways. Directly related to this issue is our ability to understand the link between nausea and vomiting, which are sometimes argued to be completely separate processes, with nausea as an unmeasurable response in animal models. An argument is made that nausea and emesis are the efferent limbs of a unified sensory input from the GI tract that is likely to be impossible to understand without more specific animal electrophysiological experimentation of vagal afferent signaling. The current paper provides a review on the use of animal models and approaches to define the biological systems for nausea and emesis and presents a potentially testable theory on how these systems work in combination.
Keywords: nausea, emesis, vomiting, vagus, animal model
Introduction
Nausea and vomiting greatly reduce nutritional balance, appetite, quality of life, and adherence to therapy in patients with gastrointestinal (GI) disorders (Chia and Egan 2008; Murakami et al. 2008; Cherian and Parkman 2012). Antiemetic drugs are often potent for controlling emesis, but largely ineffective for treating nausea (Sanger and Andrews 2006). The design of effective anti-nausea drugs depends on a better understanding of the neuronal and behavioral relationships between the biology of nausea and emesis. To date, many studies in this research domain are difficult to interpret because of the use of non-specific emetic stimuli that activate multiple levels of the gut-brain axis (e.g., chemotherapy), as well as using animals, such as rats and mice, which lack an emetic reflex (Horn 2013).
A recent conference at the University of Pittsburgh, Biology and Control of Nausea and Vomiting 2013 (October 3 and 4), highlighted diverse views on the use of non-human animal models to study physiological substrates of nausea and vomiting. Researchers at this meeting, and elsewhere in the literature, either supported the use of or advocated for replacing or reducing animal experimentation in nausea and emesis research. This review presents the view that animal research studies on the biology of nausea and emesis are essential to understand the mechanisms of these systems; the focus here is to provide a brief overview of the use of animal models and methods in this research domain. I will then end this report by presenting a testable theory, based on the potential for gastrointestinal (GI) vagal afferent fibers to provide a unifying input for both nausea and vomiting, with the essential idea that nausea and vomiting operate on a continuum.
What is the human experience of nausea and vomiting?
Humans report nausea as a highly negative perception that involves a feeling of gastric discomfort and an urge to vomit (Stern et al. 2011). Nausea can be described as a specialized warning system, not unlike pain, that derives from signals from the epigastric region; however, people report this symptom separately from abdominal pain (e.g., Hammer and Vogelsang 2007). Nausea does not appear to be a unitary percept; there are differing descriptions of this experience between people and, consequently, survey tools are sometimes focused on capturing its multi-dimensionality (e.g., somatic, GI, and emotional components, Muth et al. 1996; Gianaros et al. 2001). Disgust and the avoidance of food intake are closely associated with nausea (Bjorklund and Hursti 2004), and the feeling of nausea almost always proceeds activation of the emetic reflex. Indeed, most cases of nausea could be defined as an awareness of low intensity emetic activation that fails to reach sufficient intensity to activate the reflex; this suggests that nausea and vomiting fall on a continuum of activation of the emetic system, with low intensity emetic stimulation triggering nausea. Based on experimental psychology, nausea might be more appropriately described as a perception because it is assigned meaning and shaped by experience as opposed to the simple awareness of sensation (Coren et al. 2004).
Nausea is provoked by an extraordinary range of stimuli and conditions, including medical treatments such as cancer chemotherapy, radiotherapy, opioid analgesics, and general anesthetics (Meyer 1999; Kreis 2006; Urba 2007; Chia and Egan 2008; Hesketh 2008). Nausea is also common in chronic diseases; GI disease, late-stage cancer, and AIDS (Glare et al. 2004; Norval 2004; Murakami et al. 2008). Many of these treatments and diseases are believed to provoke nausea by affecting sensory signals arising from the GI tract. Due to the complexity of these various nausea-provoking situations, it is often difficult to determine the precise mechanisms that underlie the instigation and/or maintenance of the resulting nausea, thereby limiting our ability to develop targeted and effective therapies.
In contrast to nausea, vomiting (emesis) can be assessed objectively, independent of reports from the subject. An emetic episode is often composed of several retches leading up to a final vomit (fluid expulsion). Retches are considered to be preparatory events to position the contents in the gastric compartment for efficient vomiting (Andrews et al. 1990b). Although emesis can be directly observed and even recorded continuously via video and detected with computer vision algorithms in animal experiments (Huang et al. 2010), it is sometimes based on non-objective self-reports of daily experience in clinical research studies.
Can we measure nausea in non-human animals?
Nausea, as a self-reported perception in humans, cannot be directly measured in animal models. Nausea-like measures using animal models have been extensively reviewed (Andrews and Horn 2006; Stern et al. 2011; Andrews and Sanger 2013; Horn 2013); here, I will briefly discuss these assays. Animal studies of nausea either focus on behavior or biological indicators. Behavioral metrics include conditioned taste avoidance (CTA), pica, and general measures of behavior. CTA is sometimes distinguished from conditioned taste aversion because a true taste aversion is believed to be associated with a disgust reaction when fluid is infused into the oral cavity (Parker 2003). CTA is generally believed to reflect a conditioned nausea response in humans and preclinical models (Schwartz et al. 1996; Scalera 2002; Parker 2006). Counter-intuitively, rats – but not musk shrews – form a CTA to chemicals with rewarding properties, like morphine and amphetamine (Parker 2006). Pica is the ingestion of a non-nutritive substance, like kaolin clay. Rats and mice ingest kaolin clay in response to injection of toxins (Takeda et al. 1993; Yamamoto et al. 2002); a response that is inhibited by antiemetic drugs (Saeki et al. 2001; Malik et al. 2007). Clay ingestion could be an adaptive response to toxicosis, because silicate clays can bind to toxins in the GI tract, and therefore, limit absorption (Phillips et al. 1995; Phillips 1999). Lastly, changes in ongoing behavioral activity have been used as measure of nausea. Humans report postural change, lying down, and avoidance of eating when experiencing nausea (Stern et al. 2011). In general, animal species with an emetic reflex show postural change and reduced movement and eating (e.g., reduced eating, rearing, and rotation in musk shrews, and reduced eating and increased curling up and lying flat in ferrets) (Bermudez et al. 1988; Watson et al. 1995; Horn et al. 2011; Stern et al. 2011). Reports from my laboratory have explored the possibility of analyzing these patterns of behavior using temporal pattern analysis (Horn et al. 2011; Horn et al. 2013c), a statistical approach to grouping the timing of behavioral events (Magnusson 2000).
Other metrics of nausea-like responses in animal models are the physiological correlates of nausea in humans (Stern et al. 2011). Salivation (Furukawa et al. 1998), gastric dysrhythmia (Percie du Sert et al. 2009a; Percie du Sert et al. 2010), and systemic vasopressin release (Billig et al. 2001) have also been reported in humans experiencing nausea (Koch 1997; Stern et al. 2011), but these parameters can be difficult to measure and require invasive procedures in animal studies (Lau et al. 2005; Percie du Sert et al. 2009a; Percie du Sert et al. 2010).
On one level, the use of the word “nausea” is an approximation of a human perception, which is not only difficult to measure in animals but also a challenge to accurately record in humans. Indeed, the word traces its roots to a connection with seasickness (i.e., nautical), which appears to not apply to most of its current usage, for example, chemotherapy-induced nausea. Should we simply discard the word nausea? If we do, we can simply redefine nausea as the “detection of emetic system activation.” In this manner, it would be easier to define this response in animal experiments. For example, it appears unequivocal that animals are detecting an emetic stimulus in conditioning experiments, even when not vomiting, based on studies of CTA. Low intensity activation of the emetic system (i.e., below the threshold for triggering the reflex) might also be related to the idea of “illness behavior” or “visceral malaise” (Kent et al. 1992).
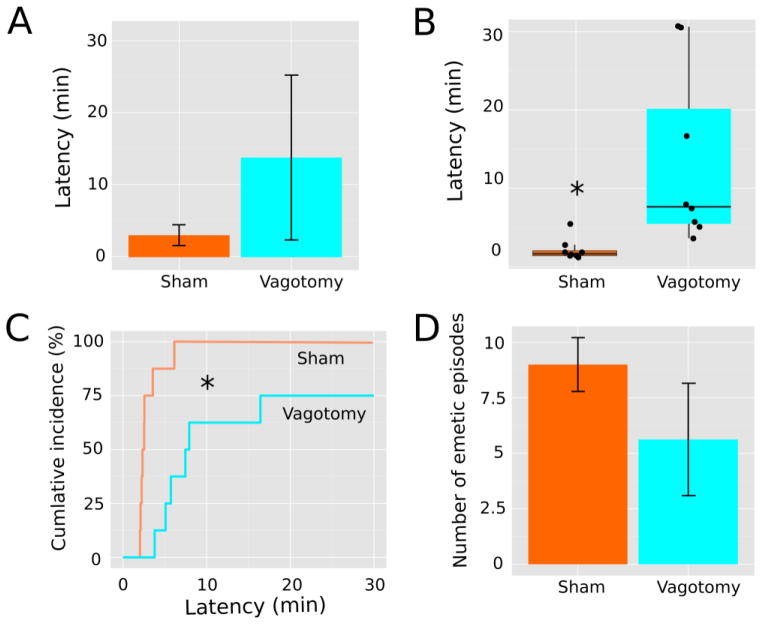
Can emesis be used as a marker of nausea? It would seem that emesis has at least as much validity as other nausea-like measures used in animal studies. Indeed, in the domain of pain research, nociceptive reflexes in non-human animal models are used as measures of pain sensitivity (Gregory et al. 2013). It is reasonable to consider analogies with the field of pain research as we proceed to develop the field of nausea and vomiting research (see review Horn 2014). The possibility exists to refine measures of emesis to achieve greater sensitivity. In a recent paper from my laboratory, we tried to accomplish this in musk shrews by assessing measures of emesis duration (the time from the first to the last emetic episode) and rate of responding; but the latency to the first emetic response was the most notable success (Horn et al. 2013c). It is often difficult to decide how to analyze behavioral latency data because they often lack a normal distribution with censored values (non-responders); therefore, parametric statistical tests are not an appropriate choice. We chose to use the an approach similar to survival analysis on emesis latency data (Horn et al. 2013c), which can be analyzed by Cox regression and does not require assumptions about the nature of sampling distributions (Jahn-Eimermacher et al. 2011). A follow-up report showed that this type of analysis could be more sensitive than parametric statistics (i.e., total number of emetic episodes) when comparing groups (Horn et al. 2014). Using one of our datasets from a prior study (Horn et al. 2014), Figure 1 illustrates an example of this approach to the analysis of emetic latency. These data show that a high dose of copper sulfate (CuSO4; 120 mg/kg) administered intragastrically to musk shrews produced a statistically significant effect on latency to emesis but not on the total number of emetic responses following vagotomy; the effects of lower doses of CuSO4 on the total number of emetic episodes are more dependent on an intact abdominal vagus (Horn et al. 2014).
Fig. 1.
Analysis of latency to the first emetic response. This data set is derived from a prior published report showing the effects of intragastric administration of 120 mg/kg CuSO4, n = 8 sham-operated and n = 8 abdominal vagotomized animals (Horn et al. 2014). A) The mean and standard deviation of emesis latency for each group. Normality tests indicate that both distributions are non-normal (Shapiro-Wilk test, p < 0.05). B) Box and whisker plots of the same data showing the median, 1st and 3rd quartiles, and whiskers representing 1.5 times the inter-quartile range. A scatterplot of all latency values is overlayed. A Mann-Whitney U test between groups is statistically significant, * p = 0.003. C) Cumulative incidence plots of the same data. Cox regression indicates statistical significance, p = 0.003. D) Number of emetic episodes from these two groups plotted as mean ± standard error of the mean. A two-sample t-test or Mann-Whitney U test was not statistically significant (p > 0.05).
Should we take a step back from the outputs of nausea and emesis?
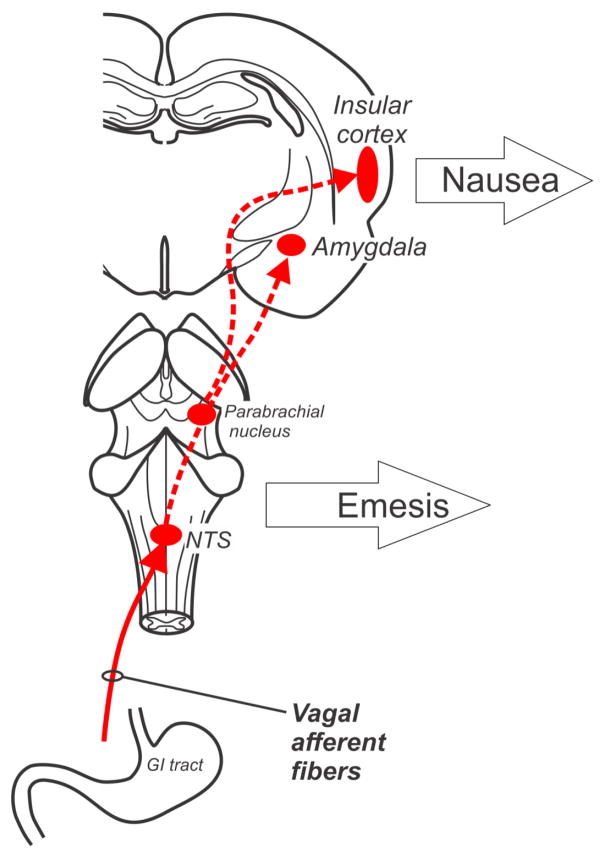
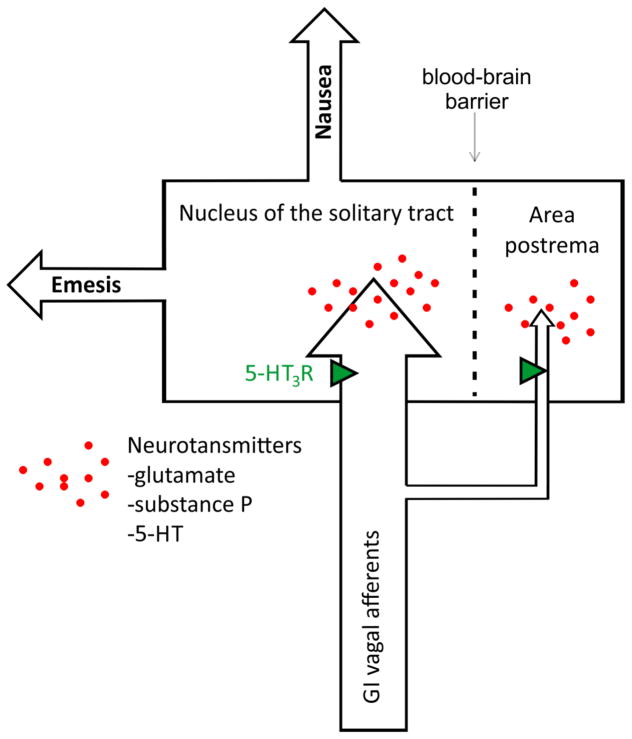
An exclusive focus on only measuring the responses of nausea and vomiting (and modeling such effects in animals) will potentially provide little insight into the sensory processes that lead to these events. Figure 2 shows the division of this system into potential nausea and vomiting efferent pathways. Indeed, a general property of neurosensory systems is divergence within the CNS, which also appears to occur with the system of nausea and vomiting. Focusing on downstream effects (nausea and vomiting) can potentially lead to a more complex problem. Figure 3 shows the critical divergence point of these systems in the nucleus of the solitary tract (NTS) and area postrema (AP), ultimately producing respective efferent pathways for nausea and emesis. Primary afferent signaling to the hindbrain by vagal input (Fig. 3) involves several neurotransmitters, including glutamate, substance P, and serotonin. Here, I only show neurotransmitters that participate in the first stage of this GI vagal input for nausea and vomiting; the reader is refered to several pharmacological reviews of the nausea and vomiting for indepth coverage (Sanger and Andrews 2006; Andrews and Sanger 2013; Horn et al. 2013b). The AP is often called the “chemoreceptor trigger zone” for emesis; a brain region that is outside the blood-brain barrier and potentially capable of detecting circulating toxins. It is a matter of faith that this functional role applies since it is very difficult to unambiguously confirm its function. Notably, the AP also receives vagal afferent input and lesions to this area affect these signals and potentially damage the adjacent NTS (Andrews et al. 1990a).
Fig. 2.
Vagal afferent pathway for nausea and emesis. NTS = nucleus of the solitary tract. Brain areas potentially involved in nausea are included, such as the insular cortex and amygdala, but other regions potentially play role (Vandenberghe et al. 2007; Catenoix et al. 2008; Mulak et al. 2008; Wang et al. 2008; Napadow et al. 2012).
Fig. 3.
Gastrointestinal (GI) vagal afferent projections to the hindbrain and the divergence of the pathways for nausea and vomiting. Neurotransmitters that play a role in signaling from vagal afferents to hindbrain sites are indicated; and, presynaptic 5-HT3 receptors, located on vagal afferents potentially modulate these GI inputs.
An example of the quagmire that can exist when focusing only on downstream effects can be drawn from research on the controls of food intake. In this regard, there are many variables and systems that are associated with producing feeding behavior but primarily these are guided by the sensing of metabolic events (e.g., Friedman 1997), and it is these signaling processes that determine the downstream effects on food intake and energy metabolism. Similarly, nausea and vomiting are the efferent responses and we have only scratched the surface as to what the sensory coding and transduction are for these outputs. It is reasonable to assume that some range of nausea and vomiting in people is produced by GI vagal sensory stimulation, and decoding (and inhibiting) this input might be a more tractable problem than trying to determine the CNS targets for the control of nausea and vomiting. If recent history provides predictive value, identifying and targeting higher brain functions is a huge challenge.
What is the role of gastrointestinal vagal afferent fibers in nausea and vomiting?
Although activation of GI vagal afferent input arguably makes a significant contribution to nausea and emesis, a mechanistic understanding of this GI-to-brain neural pathway is lacking; two common research problems present barriers. First, rodents have significant functional differences compared to humans and other animals, limiting their translational value to studies of nausea and vomiting. Rodents lack a emetic reflex due to a potentially absent hindbrain circuit (Horn et al. 2013a); this removes the opportunity to compare the systems of nausea and emesis in these species. Indeed, without an emesis endpoint in the continuum of stimulation it is less clear that nausea has been generated by a specific stimulus. Second, the use of chemical agents in preclinical studies that act on multiple sites along the GI-to-forebrain axis makes it difficult to determine the contribution of vagal signaling on nausea and emesis. Chemotherapeutic agents have been used extensively to define the biology of nausea and vomiting, but they exert combined actions on vagal signaling and directly on the brain (Andrews et al. 1990a; Percie du Sert et al. 2009b). Oddly, cytotoxic chemotherapy agents, such as cisplatin, produce an acute phase of emesis (up to 24 h) and a delayed phase (the next several days) of emesis in humans (e.g., Hesketh et al. 2003) and animal models (Sam et al. 2001; Sam et al. 2003; Huang et al. 2011). Although the role of an intact vagus seems supported in the acute phase of cisplatin-induced emesis (Hawthorn et al. 1988; Sam et al. 2003), the impact of the vagal ablation on the delayed reponse is generally not supported in few studies that have attempted this testing (Percie du Sert et al. 2009b). It should also be recognized that the vagotomy procedure leads to plasticity of neural responses (e.g., enhanced sensitivity of abdominal spinal afferents) and it is possible that the vagus does indeed play a role in chemotherapy-induced delayed emesis in the nerve intact animal (Andrews et al. 1990a; Hillsley and Grundy 1999).
Provocative or illusionary motion can also produce nausea and emesis. There is no generally accepted explanation for the adaptive role for these responses, which seem to be ill-designed for protection against ingested toxins. Motion-induced emesis also does not appear to require an intact abdominal vagus (e.g., Horn et al. 2014), but as noted above it is difficult to rule out plasticity following nerve ablation. Illusionary motion-induced nausea is associated with gastric dysrthmia in humans and animals (Stern et al. 2011), and vagotomy can disrupt motion-induced CTA in rats (Fox and McKenna 1988). A review in the current special edition by Bill Yates and his colleagues focuses on the potential for integration of vestibular and emetic GI signals to produce nausea and vomiting (Yates et al. 2014).
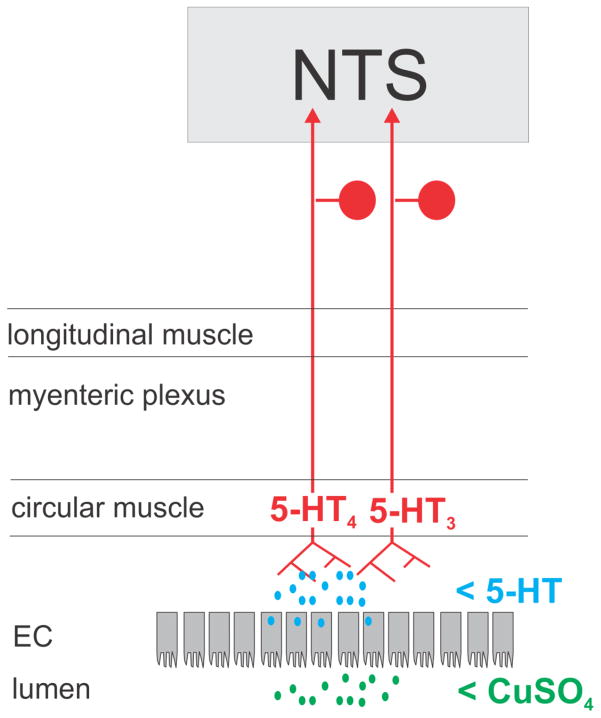
The research limitations noted above (i.e., lack of emetic reflex in rodents and non-specific stimuli) can be overcome by using animal models with an emetic reflex, like the musk shrew (Suncus murinus), and testing specific GI stimuli. In humans and preclinical models with an emetic reflex, application of gastric stretch or toxins to the gastric lumen produce nausea and emesis (Blackshaw et al. 1987; Ladabaum et al. 1998; Araya et al. 2001; Olivares et al. 2001; Hu et al. 2007; Uchino et al. 2008). Preclinical research using intragastric CuSO4 (a mucosal irritant) has provided significant insights into this GI pathway: 1) CuSO4 stimulates release of 5-HT from enteroendocrine cells in ferrets; 2) intragastric CuSO4 increases vagal afferent activity in ferrets; 3) abdominal vagotomy markedly inhibits intragastric CuSO4-induced emesis in dogs, ferrets, and musk shrews; and, 4) systemic antagonism of 5-HT4 receptors blocks CuSO4-induced emesis in dogs, ferrets, and musk shrews (Bhandari and Andrews 1991; Makale and King 1992; Fukui et al. 1994; Endo et al. 1995; Reynolds et al. 1995; Hu et al. 2007). In contrast, 5-HT3 antagonists fail to inhibit intragastric CuSO4-induced emesis in ferrets, dogs, and musk shrews (Bhandari and Andrews 1991; Yoshida et al. 1992; Ito et al. 1995). Although 5-HT3 signaling has been a dominant target in the biology of nausea and emesis, antagonists of this receptor can produce effects by action at central sites (Marazziti et al. 2001); and, potentially at presynaptic sites on vagal afferents in the hindbrain (Fig. 3). The role of the 5-HT4 receptor in nausea and vomiting represents an under-developed potential therapeutic target (Sanger 2009; Mawe and Hoffman 2013). One hypothesized mechanism for CuSO4-induced nausea and emesis signaling by vagal afferents is shown in Figure 4. This model also shows the ubiquitous 5-HT3 receptors on vagal afferent fibers. 5-HT4 and 5-HT3 receptors do not necessarily require locations on separate populations of vagal afferent fibers for differential signaling to the hindbrain; there is evidence that 5-HT4 and 5-HT3 receptors mediate distinct slow and fast phases of depolarization, respectively (Bley et al. 1994).
Fig. 4.
Proposed mechanism for CuSO4-induced nausea and emesis signaling in gastrointestinal (GI) vagal afferent fibers. EC = enteroendocrine cells in the mucosal layer of the GI tract.
How can we understand the emetic and nauseogenic signaling by gastrointestinal vagal afferent fibers?
To more accurately assess vagal afferent communication it will be necessary to record multiple single fiber responses in each experimental animal. Some work using the chemotherapy agent cisplatin and recording primary vagal and spinal single-unit afferent responses has been conducted (Hillsley and Grundy 1999; Horn et al. 2004), but these investigations were limited to a few fibers in the rat, a non-vomiting species. Vagal afferent fibers are heterogeneous and sampling a population of single-fiber responses is an important goal. We must also move beyond traditional one fiber per experiment methodology, and bring forth computational approaches to decoding vagal information (Horn 2009). Analysis of spike trains and the pattern of responses should be implemented.
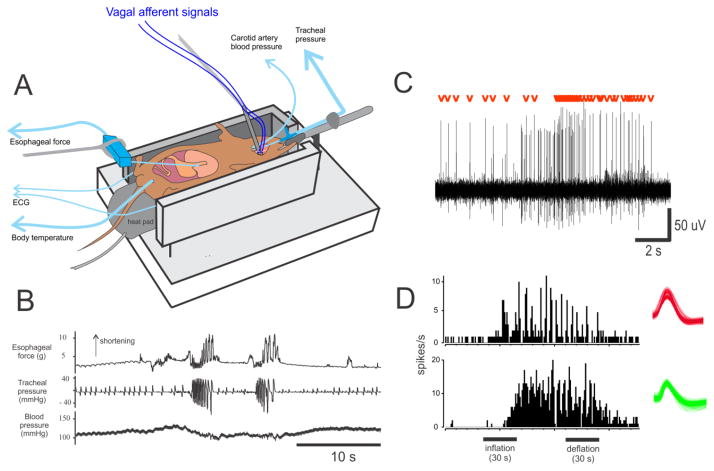
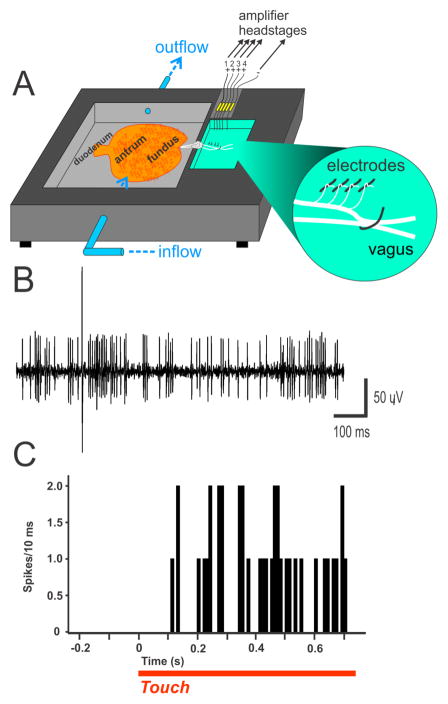
A small animal model is beneficial because these studies of neurophysiological signaling will likely require a significant number of animals that may be cost prohibitive in the larger models (ferrets, cats, and dogs). The musk shrew appears to be a good candidate. I have had success with in vivo and in vitro preparations using this animal (Fig. 5 and 6); and, another research group has also reported nerve recordings in vitro using the musk shrew stomach (Javid et al. 2013). Figures 5 and 6 illustrate a multi-electrode technique to make a high throughput recording that was previously developed for recording vagal afferents in the rat (Horn and Friedman 2003; Horn and Friedman 2004; Horn et al. 2004; Horn 2009). Both approaches lead to robust recordings of single-unit vagal afferent responses; however, each has advantages and disadvantages. For the in vivo preparation (Fig. 5), the brainstem is included and emetic responses are readily measured, but this preparation uses urethane anesthesia and it is unclear what effects this has on the coding of sensory signals. Conversely, the in vitro preparation (Fig. 6) does not have the effects of anesthesia but it lacks an emetic response. The in vitro approach is often applied in the field of nociception research to mechanistically determine spinal afferent coding (e.g., Feng et al. 2013); and, this could be a major direction of nausea and emeis research, as more information is acquired from the in vivo preparation. Importantly, these preparations have the requisite “hardware” of vagal afferents and other cells types that will allow a detailed analysis of sensory function related to emetic and nauseogenic stimulation.
Fig. 5.
In vivo electrophysiology of single afferent fibers in the musk shrews. A) An in vivo anesthetized preparation. B) Mechanical stimulation of the gastric antrum triggers two emetic episodes (esophageal shortening and closely spaced intratracheal pressure changes; retches). C) Activation of GI vagal afferents by gastric distension (1ml balloon). Signal-to-noise ratio of spikes; red marks show the single-unit with the red waveform from section B. D) Simultaneous recording of two (of 6) vagal fibers from one shrew that were sensitive to gastric distension. Unit waveforms are shown on the right.
Fig. 6.
In vitro electrophysiology of single afferent fibers in the musk shrew. Mechosensitive vagal afferent activity from the musk shrew stomach. A) Recording chamber. B) Representive recording showing the signal-to-noise ratio. (C) Vagal afferent activity after gentle touch is applied to gastric antrum (spikes/10 ms).
All representative experiments shown in the figures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care international-accredited animal care facility.
Summary
It is clearly too soon to discard the use of animal experimentation to determine the mechanistic underpinnings of nausea and emesis; much of this work cannot be conducted in human studies. Very limited physiological research on the gut-brain axis can occur in human research, and there are limited ethical ways to use electrophysiology to decode emetic signals from the human vagus. We are also appropriately hampered by limits on the types and intensities of stimuli that can be used in these studies; for example, the use of emetogenic chemicals outside the context of patient care is mostly forbidden. Furthermore, it is common that human studies require many more subjects than animal experiments for statistical power; and, indeed, the variability of humans (e.g., genetics, personal history) often precludes the study of effects that are obscured by baseline subject variability, even with a large pool of human subjects. The disadvantages to using animal models are that they cannot communicate directly their internal states, such as nausea, and secondly, they are not humans; and, therefore, physiological processes from other mammals might not translate to humans. I think the first disadvantage is not as large as it might seem since human and patient reports are not always factual, and it is sometimes unclear what they are reporting since opinions can differ on what constitutes “nausea” between people. The second aspect leads to an open question; is mammalian physiology with regard to emetic (and nausea-like) signaling similar across species? I believe that the answer is generally yes, specifically at the level of sensory inputs. Conversely, where there is likely less commonality in these systems between species is in what occurs further along the neuro-axis through the process of interpretation of sensory signals by higher levels of the CNS.
The sensory processes that trigger emesis and nausea-like responses are critically important; GI vagal afferent communication can potentially provide a significant level of insight into the triggering of these events. Although several emesis capable animal models could be used in these studies, musk shrews provide an efficient approach. The musk shrew has a distinct advantage, similar to mice, as a research model; they are a small animal (40 to 80 g) and can be efficiently used in large numbers. There is also a developing database on the molecular genetics of the GI tract in this species, notably ghrelin and motilin (Ishida et al. 2009; Suzuki et al. 2012; Sugino et al. 2014). Here, I have shown in vivo and in vitro preparations in this species to perform single-unit electrophysiological recordings of GI vagal afferent fibers. Ultimately, by understanding the transduction and coding of vagal sensory information, we will be able to move towards in vitro testing of cell types and possibly in silico experiments (Holmes et al. 2009; Rojas et al. 2010a; Rojas et al. 2010b; Stern et al. 2011), all of which can lead to the rationale development of new therapies to control nausea and vomiting.
Lastly, I present a testable model of how this system functions in preclinical animals, as shown in Figure 7. The model suggests that intense activation of GI vagal afferents produces immediate responses; for example, the ejection of toxins (emesis) or ingestion of clay to dilute the effects of toxins on the organism (pica; as observed in rodent studies) (Phillips et al. 1995; Andrews and Horn 2006). Low intensity activation of this pathway is predicted to support a CTA (Garcia et al. 1974), a longer term strategy to prevent the ingestion of toxins in the future. This simple model is indeed testable with a combination of electrophysiological and behavioral methods.
Fig. 7.
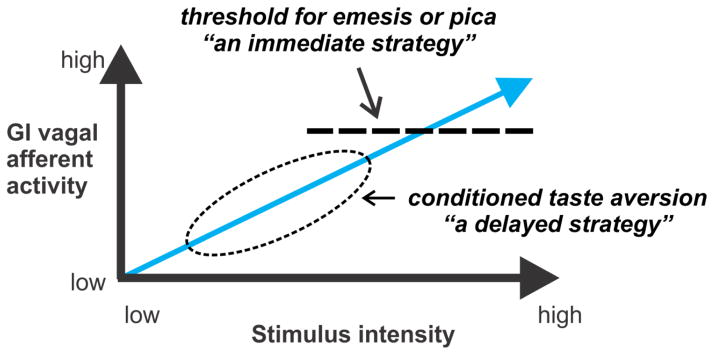
Proposed model of emetic stimulation of gastrointestinal (GI) vagal afferent fibers. A threshold is shown for the emetic reflex, for those animals with an emetic reflex (human, cat, dog, ferret, shrew, etc.) and pica in rodents (e.g., rat). This threshold triggers an immediate response to a potential threat (e.g., GI poison). Less immediate dangers (i.e., low concentraions of toxins) can trigger a delayed strategy that supports a conditioned taste aversion, which leads to avoidance of a toxin in the future.
Acknowledgments
The electrophysiological data were acquired using the support of NIH Grant P30 CA047904 (Cancer Center Support Grant; CCSG). I thank the University of Pittsburgh, Division of Laboratory Animal Research, especially Dawn Everard, Katie Leschak, Megan Lambert, and Dr. Joseph Newsome for excellent care of the musk shrew colony at the University of Pittsburgh Cancer Institute (UPCI).
References
- Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990a;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. S1566-0702(06)00011-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Nausea and the quest for the perfect anti-emetic. European journal of pharmacology. 2013 doi: 10.1016/j.ejphar.2013.09.072. [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Bhandari P, Garland S, et al. Does retching have a function?: An experimental study in the ferret. Pharmacodynamics Therapeutics. 1990b;9:135–152. [Google Scholar]
- Araya M, McGoldrick MC, Klevay LM, et al. Determination of an acute no-observed-adverse-effect level (NOAEL) for copper in water. Regulatory toxicology and pharmacology: RTP. 2001;34:137–145. doi: 10.1006/rtph.2001.1492. [DOI] [PubMed] [Google Scholar]
- Bermudez J, Boyle EA, Miner WD, Sanger GJ. The anti-emetic potential of the 5-hydroxytryptamine3 receptor antagonist BRL 43694. Br J Cancer. 1988;58:644–650. doi: 10.1038/bjc.1988.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P, Andrews PL. Preliminary evidence for the involvement of the putative 5-HT4 receptor in zacopride- and copper sulphate-induced vomiting in the ferret. European journal of pharmacology. 1991;204:273–280. doi: 10.1016/0014-2999(91)90852-h. [DOI] [PubMed] [Google Scholar]
- Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1243–1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- Bjorklund F, Hursti TJ. A Swedish translation and validation of the Disgust Scale: a measure of disgust sensitivity. Scand J Psychol. 2004;45:279–284. doi: 10.1111/j.1467-9450.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D, Scratcherd T. Involvement of gastrointestinal mechano- and intestinal chemoreceptors in vagal reflexes: an electrophysiological study. Journal of the autonomic nervous system. 1987;18:225–234. doi: 10.1016/0165-1838(87)90121-4. [DOI] [PubMed] [Google Scholar]
- Bley KR, Eglen RM, Wong EH. Characterization of 5-hydroxytryptamine-induced depolarizations in rat isolated vagus nerve. European journal of pharmacology. 1994;260:139–147. doi: 10.1016/0014-2999(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Isnard J, Guenot M, Petit J, Remy C, Mauguiere F. The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. S1525-5050(08)00205-9 [pii] [DOI] [PubMed] [Google Scholar]
- Cherian D, Parkman HP. Nausea and vomiting in diabetic and idiopathic gastroparesis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24:217–222. e103. doi: 10.1111/j.1365-2982.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- Chia CW, Egan JM. Incretin-Based Therapies in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2008;93:3703–3716. doi: 10.1210/jc.2007-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S, Ward LM, Enns JT. Sensation and perception. J. Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- Endo T, Nemoto M, Minami M, Yoshioka M, Saito H, Parvez SH. Changes in the afferent abdominal vagal nerve activity induced by cisplatin and copper sulfate in the ferret. Biogenic Amines. 1995;11:399–407. [Google Scholar]
- Feng B, Kiyatkin ME, La JH, Ge P, Solinga R, Silos-Santiago I, Gebhart GF. Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci. 2013;33:9831–9839. doi: 10.1523/JNEUROSCI.5114-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RA, McKenna S. Conditioned taste aversion induced by motion is prevented by selective vagotomy in the rat. Behavioral and neural biology. 1988;50:275–284. doi: 10.1016/s0163-1047(88)90954-5. [DOI] [PubMed] [Google Scholar]
- Friedman MI. An energy sensor for control of energy intake. Proc Nutr Soc. 1997;56:41–50. doi: 10.1079/pns19970008. [DOI] [PubMed] [Google Scholar]
- Fukui H, Yamamoto M, Sasaki S, Sato S. Possible involvement of peripheral 5-HT4 receptors in copper sulfate-induced vomiting in dogs. Eur J Pharmacol. 1994;257:47–52. doi: 10.1016/0014-2999(94)90692-0. [DOI] [PubMed] [Google Scholar]
- Furukawa N, Fukuda H, Hatano M, Koga T, Shiroshita Y. A neurokinin-1 receptor antagonist reduced hypersalivation and gastric contractility related to emesis in dogs. Am J Physiol. 1998;275:G1193–1201. doi: 10.1152/ajpgi.1998.275.5.G1193. [DOI] [PubMed] [Google Scholar]
- Garcia J, Hankins WG, Rusiniak KW. Behavioral regulation of the milieu interne in man and rat. Science. 1974;185:824–831. doi: 10.1126/science.185.4154.824. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Muth ER, Mordkoff JT, Levine ME, Stern RM. A questionnaire for the assessment of the multiple dimensions of motion sickness. Aviat Space Environ Med. 2001;72:115–119. [PMC free article] [PubMed] [Google Scholar]
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Support Care Cancer. 2004;12:432–440. doi: 10.1007/s00520-004-0629-y. [DOI] [PubMed] [Google Scholar]
- Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14:1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer J, Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol Motil. 2007;19:279–287. doi: 10.1111/j.1365-2982.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- Hawthorn J, Ostler KJ, Andrews PL. The role of the abdominal visceral innervation and 5-hydroxytryptamine M-receptors in vomiting induced by the cytotoxic drugs cyclophosphamide and cis-platin in the ferret. Q J Exp Physiol. 1988;73:7–21. doi: 10.1113/expphysiol.1988.sp003124. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. 358/23/2482 [pii] [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van Belle S, Aapro M, et al. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. European journal of cancer. 2003;39:1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Grundy D. Plasticity in the mesenteric afferent response to cisplatin following vagotomy in the rat. J Auton Nerv Syst. 1999;76:93–98. doi: 10.1016/s0165-1838(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Holmes AM, Rudd JA, Tattersall FD, Aziz Q, Andrews PL. Opportunities for the replacement of animals in the study of nausea and vomiting. British journal of pharmacology. 2009;157:865–880. doi: 10.1111/j.1476-5381.2009.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC. Electrophysiology of vagal afferents: amino acid detection in the gut. Ann N Y Acad Sci. 2009;1170:69–76. doi: 10.1111/j.1749-6632.2009.03907.x. NYAS03907 [pii] [DOI] [PubMed] [Google Scholar]
- Horn CC. The Medical Implications of Gastrointestinal Vagal Afferent Pathways in Nausea and Vomiting. Current pharmaceutical design. 2013 doi: 10.2174/13816128113199990568. in press. [DOI] [PubMed] [Google Scholar]
- Horn CC. Overlapping Phenotypes: Genetic Contribution to Nausea and Pain. In: Belfer I, Diatchenko L, editors. Pain Genetics: Basic to Translational Science. Wiley; 2014. pp. 115–125. [Google Scholar]
- Horn CC, Friedman MI. Detection of single unit activity from the rat vagus using cluster analysis of principal components. J Neurosci Methods. 2003;122:141–147. doi: 10.1016/s0165-0270(02)00304-7. [DOI] [PubMed] [Google Scholar]
- Horn CC, Friedman MI. Separation of hepatic and gastrointestinal signals from the common “hepatic” branch of the vagus. Am J Physiol Regul Integr Comp Physiol. 2004;287:R120–126. doi: 10.1152/ajpregu.00673.200300673.2003. [DOI] [PubMed] [Google Scholar]
- Horn CC, Henry S, Meyers K, Magnusson MS. Behavioral patterns associated with chemotherapy-induced emesis: A potential signature for nausea in musk shrews. Front Neurosci. 2011;5:88. doi: 10.3389/fnins.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Kimball BA, Wang H, et al. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS ONE. 2013a;8:e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Meyers K, Lim A, Dye M, Pak D, Rinaman L, Yates BJ. Delineation of vagal emetic pathways: Intragastric copper sulfate-induced emesis and viral tract tracing in musk shrews. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2014 doi: 10.1152/ajpregu.00413.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. S1566-0702(04)00197-3 [pii] [DOI] [PubMed] [Google Scholar]
- Horn CC, Wallisch WJ, Homanics GE, Williams JP. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. European journal of pharmacology. 2013b doi: 10.1016/j.ejphar.2013.10.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn CC, Wang H, Estival L, Meyers K, Magnusson M. Novel dynamic measures of emetic behavior in musk shrews. Autonomic Neuroscience: Basic and Clinical. 2013c doi: 10.1016/j.autneu.2013.07.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DL, Zhu G, Mori F, et al. Staphylococcal enterotoxin induces emesis through increasing serotonin release in intestine and it is downregulated by cannabinoid receptor 1. Cell Microbiol. 2007;9:2267–2277. doi: 10.1111/j.1462-5822.2007.00957.x. CMI957 [pii] [DOI] [PubMed] [Google Scholar]
- Huang D, Meyers K, De la Torre F, Horn CC. Automatic detection of cancer chemotherapy-induced vomiting in musk shrews. Society for the Study of Ingestive Behavior; Pittsburgh, PA: 2010. [Google Scholar]
- Huang D, Meyers K, Henry S, De la Torre F, Horn CC. Computerized detection and analysis of cancer chemotherapy-induced emesis in a small animal model, musk shrew. J Neurosci Meth. 2011;197:249–258. doi: 10.1016/j.jneumeth.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Sakahara S, Tsutsui C, Kaiya H, Sakata I, Oda S, Sakai T. Identification of ghrelin in the house musk shrew (Suncus murinus): cDNA cloning, peptide purification and tissue distribution. Peptides. 2009;30:982–990. doi: 10.1016/j.peptides.2009.01.006. S0196-9781(09)00024-2 [pii] [DOI] [PubMed] [Google Scholar]
- Ito C, Isobe Y, Kijima H, et al. The anti-emetic activity of GK-128 in Suncus murinus. Eur J Pharmacol. 1995;285:37–43. doi: 10.1016/0014-2999(95)00372-r. [DOI] [PubMed] [Google Scholar]
- Jahn-Eimermacher A, Lasarzik I, Raber J. Statistical analysis of latency outcomes in behavioral experiments. Behavioural brain research. 2011;221:271–275. doi: 10.1016/j.bbr.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javid FA, Bulmer DC, Broad J, Aziz Q, Dukes GE, Sanger GJ. Anti-emetic and emetic effects of erythromycin in Suncus murinus: role of vagal nerve activation, gastric motility stimulation and motilin receptors. Eur J Pharmacol. 2013;699:48–54. doi: 10.1016/j.ejphar.2012.11.035. [DOI] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Koch KL. A noxious trio: nausea, gastric dysrhythmias and vasopressin. Neurogastroenterol Motil. 1997;9:141–142. doi: 10.1046/j.1365-2982.1997.d01-44.x. [DOI] [PubMed] [Google Scholar]
- Kreis ME. Postoperative nausea and vomiting. Auton Neurosci. 2006;129:86–91. doi: 10.1016/j.autneu.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Koshy SS, Woods ML, Hooper FG, Owyang C, Hasler WL. Differential symptomatic and electrogastrographic effects of distal and proximal human gastric distension. The American journal of physiology. 1998;275:G418–424. doi: 10.1152/ajpgi.1998.275.3.G418. [DOI] [PubMed] [Google Scholar]
- Lau AH, Rudd JA, Yew DT. Action of ondansetron and CP-99,994 on cisplatin-induced emesis and locomotor activity in Suncus murinus (house musk shrew) Behav Pharmacol. 2005;16:605–612. doi: 10.1097/00008877-200512000-00002. [DOI] [PubMed] [Google Scholar]
- Magnusson MS. Discovering hidden time patterns in behavior: T-patterns and their detection. Behav Res Methods Instrum Comput. 2000;32:93–110. doi: 10.3758/bf03200792. [DOI] [PubMed] [Google Scholar]
- Makale MT, King GL. Surgical and pharmacological dissociation of cardiovascular and emetic responses to intragastric CuSO4. Am J Physiol. 1992;263:R284–291. doi: 10.1152/ajpregu.1992.263.2.R284. [DOI] [PubMed] [Google Scholar]
- Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol. 2007;555:164–173. doi: 10.1016/j.ejphar.2006.10.043. S0014-2999(06)01209-X [pii] [DOI] [PubMed] [Google Scholar]
- Marazziti D, Betti L, Giannaccini G, et al. Distribution of [3H]GR65630 binding in human brain postmortem. Neurochem Res. 2001;26:187–190. doi: 10.1023/a:1010939530412. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nature reviews Gastroenterology & hepatology. 2013;10:473–486. doi: 10.1038/nrgastro.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. Palliative care and AIDS: 2--Gastrointestinal symptoms. Int J STD AIDS. 1999;10:495–505. quiz 506-497. [PubMed] [Google Scholar]
- Mulak A, Kahane P, Hoffmann D, Minotti L, Bonaz B. Brain mapping of digestive sensations elicited by cortical electrical stimulations. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2008;20:588–596. doi: 10.1111/j.1365-2982.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Murakami N, Nakagawa K, Yamashita H, Nagawa H. Palliative radiation therapy for advanced gastrointestinal cancer. Digestion. 2008;77(Suppl 1):29–35. doi: 10.1159/000111485. [DOI] [PubMed] [Google Scholar]
- Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: the Nausea Profile (NP) J Psychosom Res. 1996;40:511–520. doi: 10.1016/0022-3999(95)00638-9. [DOI] [PubMed] [Google Scholar]
- Napadow V, Sheehan JD, Kim J, et al. The Brain Circuitry Underlying the Temporal Evolution of Nausea in Humans. Cerebral cortex. 2012;23:806–813. doi: 10.1093/cercor/bhs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norval DA. Symptoms and sites of pain experienced by AIDS patients. S Afr Med J. 2004;94:450–454. [PubMed] [Google Scholar]
- Olivares M, Araya M, Pizarro F, Uauy R. Nausea threshold in apparently healthy individuals who drink fluids containing graded concentrations of copper. Regul Toxicol Pharmacol. 2001;33:271–275. doi: 10.1006/rtph.2000.1440. S0273-2300(00)91440-5 [pii] [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA. The role of nausea in taste avoidance learning in rats and shrews. Auton Neurosci. 2006;125:34–41. doi: 10.1016/j.autneu.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Reduced normogastric electrical activity associated with emesis: a telemetric study in ferrets. World J Gastroenterol. 2009a;15:6034–6043. doi: 10.3748/wjg.15.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol. 2010;95:768–773. doi: 10.1113/expphysiol.2009.052001. expphysiol.2009.052001 [pii] [DOI] [PubMed] [Google Scholar]
- Percie du Sert N, Rudd JA, Moss R, Andrews PL. The delayed phase of cisplatin-induced emesis is mediated by the area postrema and not the abdominal visceral innervation in the ferret. Neurosci Lett. 2009b;465:16–20. doi: 10.1016/j.neulet.2009.08.075. S0304-3940(09)01181-1 [pii] [DOI] [PubMed] [Google Scholar]
- Phillips TD. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci. 1999;52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Sarr AB, Grant PG. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat Toxins. 1995;3:204–213. doi: 10.1002/nt.2620030407. [DOI] [PubMed] [Google Scholar]
- Reynolds DJM, Andrews PLR, Davis CJ. Serotonin and the scientific basis of anti-emetic therapy. Oxford Clinical Communications; Oxford; Philadelphia: 1995. [Google Scholar]
- Rojas C, Li Y, Zhang J, et al. The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. Journal of pharmacology and experimental therapeutics. 2010a;335:362–368. doi: 10.1124/jpet.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. European journal of pharmacology. 2010b;626:193–199. doi: 10.1016/j.ejphar.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Saeki M, Sakai M, Saito R, et al. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol. 2001;86:359–362. doi: 10.1254/jjp.86.359. [DOI] [PubMed] [Google Scholar]
- Sam TS, Chan SW, Rudd JA, Yeung JH. Action of glucocorticoids to antagonise cisplatin-induced acute and delayed emesis in the ferret. Eur J Pharmacol. 2001;417:231–237. doi: 10.1016/s0014-2999(01)00915-3. [DOI] [PubMed] [Google Scholar]
- Sam TS, Cheng JT, Johnston KD, et al. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) Eur J Pharmacol. 2003;472:135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- Sanger GJ. Translating 5-HT receptor pharmacology. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2009;21:1235–1238. doi: 10.1111/j.1365-2982.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutr Neurosci. 2002;5:159–188. doi: 10.1080/10284150290013059. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Jacobsen PB, Bovbjerg DH. Role of nausea in the development of aversions to a beverage paired with chemotherapy treatment in cancer patients. Physiol Behav. 1996;59:659–663. doi: 10.1016/0031-9384(95)02096-9. [DOI] [PubMed] [Google Scholar]
- Stern RM, Andrews PLR, Koch KL. Nausea: Mechanisms and Management. Oxford University Press; New York: 2011. [Google Scholar]
- Sugino S, Horn CC, Janicki PK. De novo assembly of musk shrew hindbrain transcriptome and comparison of gene expressions in animals with high and low incidence of postanesthesia nausea and vomiting. Am. Soc. Anesthesiologists Annual meeting; New Orleans, LA. 2014. [Google Scholar]
- Suzuki A, Ishida Y, Aizawa S, et al. Molecular identification of GHS-R and GPR38 in Suncus murinus. Peptides. 2012;36:29–38. doi: 10.1016/j.peptides.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Uchino M, Ito K, Kuwahara M, Ebukuro S, Tsubone H. Interactions of carotid sinus or aortic input with emetic signals from gastric afferents and vestibular system. Auton Neurosci. 2008;144:36–42. doi: 10.1016/j.autneu.2008.09.001. S1566-0702(08)00166-5 [pii] [DOI] [PubMed] [Google Scholar]
- Urba S. Radiation-induced nausea and vomiting. J Natl Compr Canc Netw. 2007;5:60–65. doi: 10.6004/jnccn.2007.0008. [DOI] [PubMed] [Google Scholar]
- Vandenberghe J, Dupont P, Van Oudenhove L, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684–1693. doi: 10.1053/j.gastro.2007.03.037. S0016-5085(07)00561-6 [pii] [DOI] [PubMed] [Google Scholar]
- Wang GJ, Tomasi D, Backus W, et al. Gastric distention activates satiety circuitry in the human brain. NeuroImage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Watson JW, Gonsalves SF, Fossa AA, McLean S, Seeger T, Obach S, Andrews PL. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br J Pharmacol. 1995;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol. 2002;24:135–138. doi: 10.1358/mf.2002.24.3.802297. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Catanzaro M, Miller D, McCall A. Integration of Vestibular and Emetic Gastrointestinal Signals that Produce Nausea and Vomiting: Potential Contributions to Motion Sickness. Experimental Brain Research. 2014 doi: 10.1007/s00221-014-3937-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Omoya H, Ito T. DAT-582, a novel serotonin3 receptor antagonist, is a potent and long-lasting antiemetic agent in the ferret and dog. J Pharmacol Exp Ther. 1992;260:1159–1165. [PubMed] [Google Scholar]