Abstract
Objective
To examine associations of clinical need, defined by elevated parent ratings of child behavior problems, and utilization of behavioral health services in young children with TBI and in an orthopedic injury (OI) comparison group.
Design
Parents completed outcome measures 18 months after injury and at an extended follow-up conducted an average of 38 months post injury.
Setting
Recruitment was conducted at Level 1 Trauma Centers at three children’s hospitals and one general hospital.
Participants
Participants included parents of three groups of children injured between 3 and 7 years of age: 47 children with complicated mild to moderate TBI, 18 with severe TBI, and 74 with OI.
Interventions
Not applicable.
Main outcome measures
Parents completed ratings of child behavior, mental health symptomology, and family functioning at both visits; at the extended follow-up, they reported utilization of behavior therapy or counseling services since the 18-month visit.
Results
Children with TBI had more behavior problems than those with OI. Although clinical need at both follow-ups was associated with greater service utilization at the extended follow-up, all groups had unmet needs as defined by a clinical need in the absence of services. Lower socioeconomic status was associated with higher rates of unmet need across groups.
Conclusions
The results document unmet long-term behavioral health needs following both TBI and OI in children and underscore the importance of monitoring and treatment of post-injury behavior problems.
Keywords: traumatic brain injury, behavioral therapy, childhood behavior problems, service utilization
Traumatic brain injury (TBI) is a leading cause of acquired disability in children. Approximately 160 per 100,000 U.S. children under the age of 7 experience TBI annually.1 Rates of TBI may be higher in non-western counties; for example, a recent study conducted in New Zealand reported a total incidence rate of 790 per 100,000 persons. Of these cases, approximately 70% were children, adolescents, and young adults.2 However, these rates are based on hospital-treated TBI and may therefore underestimate incidence. Behavior problems are one of the major consequences of pediatric TBI 3, 4 and include post-injury-onset attention deficit hyperactivity disorder (ADHD),5, 6, 7oppositional defiant disorder (ODD),6, 8 and anxiety disorders.4, 9, 10, 11, 12
Consistent with the greater susceptibility of younger children to diffuse brain insult and abnormalities in neurogenesis, younger age at injury is associated with greater long-term functional and behavioral difficulties and an increased risk for mental health disorders.4, 11, 13In an earlier report of findings from the sample assessed in this study, younger age at injury was associated with increased symptoms of ADHD and anxiety.4 In other studies, children injured at less than 7-8 years of age had worse long-term neurocognitive and academic outcomes than those injured at older ages, including poorer attention, language, memory, processing speed, executive functions, and visuo-spatial abilities.14, 15, 16 These studies suggest that children injured at younger ages at are higher risk than older children for deficits across multiple domains and that behavioral health services may thus be critical for those with TBI in early childhood.
Long-term developmental and behavioral problems are not limited to children with severe TBI.17, 18 Although deficits are greater in children with moderate to severe TBI,2 impaired episodic memory and cognitive processing and diminished ability to manage cognitive interference are reported even in children with complicated mild TBI.18 One study found adverse longer-term psychosocial outcomes in some children with mild TBI, especially in those under the age of 5 years at the time of injury.15 Other research suggests that children with mild TBI are at increased risk for clinically significant anxiety,9 depression,16 and hyperactivity.17 Post-injury mental health services may thus benefit children across the spectrum of TBI severity.
Despite the need for behavioral health services for young children with TBI,2, 3, 4, 5, 6 relatively little is known about service utilization. Slomine and colleagues found rates of unmet or unrecognized health care needs in 26% and 31% of children with TBI 3 and 12 months after injury, respectively. Cognitive services were the most frequently reported unmet need.19 In another study examining utilization of health care services by children 1 year post TBI, occupational therapy and mental health services were the most commonly reported unmet need.20
Recent research suggests that family characteristics, such as socioeconomic status (SES), are related to the development of cognitive and socio-emotional problems in children 21 as well as to unmet mental health care needs.22, 23 Specifically examining children with TBI, lower SES is associated with poorer long-term social competence,24 academic achievement, adaptive skills,23 behavioral outcomes , and family functioning.23, 25, 26 Family dysfunction may increase following TBI and have an adverse effect on post-injury behavioral recovery.27, 28 Injury-related family stress and parental psychological distress are higher in families of younger children with TBI than in controls with orthopedic injury (OI).27 Stress levels also diminish over time in families with higher social resources.29 Predictors of greater post-TBI family dysfunction include severe injury, higher levels of parental criticism/coldness, and greater family conflict, and greater child behavior problems.30 Family dysfunction and stress may also be a barrier to obtaining mental health treatment.31 Prior research examining utilization of health care services within the first year post TBI reveals associations of both poorer family functioning and non-white ethnicity with greater rates of unmet need.29 However, we are not aware of research examining utilization of mental health services and the influence of family functioning beyond the first year after TBI.19, 20
The current study examined parent-reported long-term utilization of behavioral therapy and counseling services in young children with mild complicated to severe TBI relative to a comparison group of children with OI. Parents of children who sustained injuries between 3 and 7 years of age were assessed at visit 18-month follow-up visit and again at an extended follow-up an average of more than 3 years post injury. Utilization of behavioral therapy or counseling services was surveyed at the extended follow-up. Based on prior research indicating that TBI increases risks for long-term behavior disorder,2,3,4,5,11 we hypothesized that the children with TBI would have higher rates of behavior impairments at both follow-up visits than the children with OI and that these differences would be evident across the spectrum of TBI severity. We further hypothesized that the children with TBI would have concomitantly higher rates of service utilization relative to children with OI. Because demographic factors are related to service utilization and in view of age differences in long-term behavioral outcomes of TBI,4, 11, 13 comparisons of the TBI and OI groups controlled for gender, race, and age at injury. As family stresses21and SES22may represent barriers to care, a secondary aim was to investigate these factors as additional predictors of utilization. We anticipated that higher family functioning and SES would be associated with greater service utilization independent of injury group. Assuming that families of children with severe TBI experience higher levels of injury-related stress than other families, 29 we also examined the possibility that injury severity moderated the association between family functioning and service utilization.
Methods
Participants
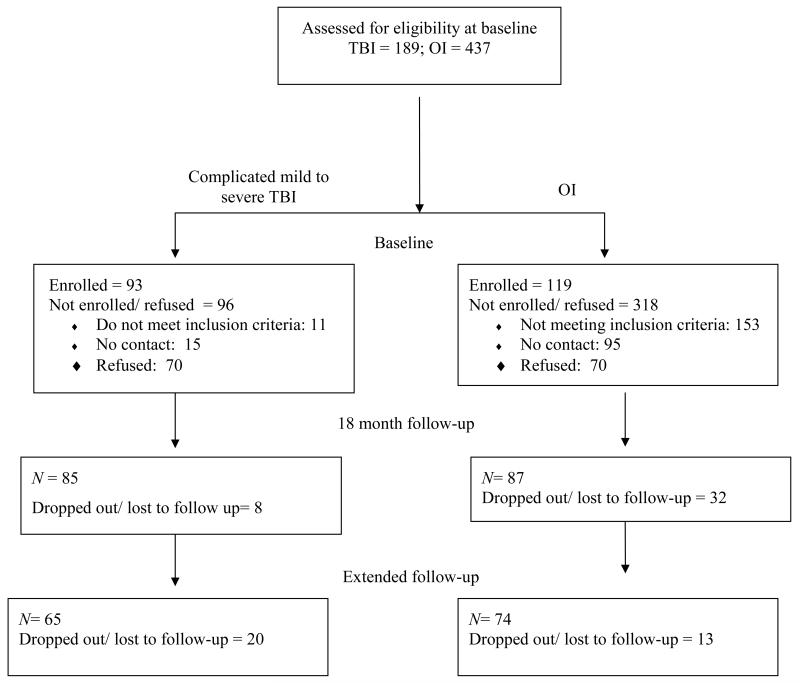
The current study was part of a larger prospective follow-up study of children hospitalized for mild complicated to severe TBI between 3 and 7 years of age.32Children hospitalized for OI within this same age span were recruited as a comparison group to control for the experience of hospitalization for traumatic injury and for child characteristics that predispose children to their injuries.33 Participants were consecutively recruited from Level 1 Trauma Centers at three children’s hospitals and one general hospital. Figure 1 provides information about recruitment and retention. Although participants and non-participants (i.e., those not enrolled because of refusal or inability to be contacted) did not significantly differ with regard to gender or age at injury, non-participants were more likely to be white/ Caucasian and to have OI (p < .001). Parents who agreed to participate were enrolled in the larger project shortly after injury (mean number of days = 40.68; SD = 21.96), at which time baseline data on demographics and information about the children’s post-acute status was obtained. Subsequent assessments were conducted at 18-month and extended follow-up visits, with mean times since injury of 18.34 months (SD = .98, range = 16-21) and 38.34 months (SD = 10.29; range = 25-63) respectively. At each follow-up assessment, parents completed the measures of family functioning and child behavior and clinical symptomology. At the 18-month visit, participants included 87 parents of children with OI, 64 with complicated mild to moderate TBI, and 21 with severe TBI. Of these families, 74 with OI, 47 with complicated mild to moderate TBI, and 18 with severe TBI participated in the extended follow-up visit. Only participants who completed both the 18-month and extended follow-up visits were included in analyses (Figure 1). Compared to children seen at both follow-up visits, proportionally fewer children with TBI than with OI failed to complete follow-up (p < .05). Non-completers were significantly older at the time of injury than those remaining in the study, but these two groups did not differ significantly in age at injury, race, SES, and gender.
Figure 1.
Recruitment and retention of participants.
Based on their injury status, children were classified into three groups: OI, complicated mild to moderate TBI, and severe TBI. The OI group included children who sustained a bone fracture, other than to the skull, and had no alterations in consciousness or other symptoms of head trauma. Children with complicated mild to moderate TBI group had either a lowest Glasgow Coma Scale (GCS) 34 score of 9-12 or a GCS score of 13-15 accompanied by abnormality on imaging (magnetic resonance imaging or computed tomography), whereas those with severe TBI had a GCS score <9. Additional eligibility criteria included hospitalization for at least 1 night for injuries sustained between 36 and 83 months of age, the absence of evidence of child abuse as the cause of the injury or of a history of neurological problems or developmental delays pre-injury, and residence in a home in which English was the primary language. Children with TBI with GCS scores 13-15 who had an isolated skull fracture without abnormality on imaging were excluded.
Table 1 presents baseline characteristics for participants who completed the 18-month and extended follow-up assessments. Although information on family census tract median income and primary caregiver education are listed for descriptive purposes, a composite SES measure defined as the mean of the sample z-scores for these two variables was used in analysis. Comparisons of the injury groups failed to reveal significant differences in the child’s sex or race, caregiver education, age at injury, or family census track income (p > .05). However, the period between injury and the extended follow-up visit was longer for the OI group than the complicated mild to moderate TBI group (t = 4.09, p < .001). Injury Severity Score (ISS)35 for the OI was lower compared to both the complicated mild to moderate TBI group (t = −6.95, p < .001) and the severe TBI group (t = −.35, p < .001), suggesting that the TBI groups sustained more severe injuries than the OI group. The complicated mild to moderate TBI group (t = −3.54, p = .001) and severe TBI group (t = −2.05, p = .05) had more days of post-injury hospitalization compared to the OI group. The severe TBI (χ2 = 15.92, p = .001) and complicated mild to moderate TBI (χ2 = 20.28, p = .001) groups also differed from the OI group when considering mechanism of injury. The TBI groups were more likely to have injuries that resulted from falls or involved a motor vehicle, including being a passenger in a car or being hit as a pedestrian, while the OI group had a larger proportion of participants who were injured while playing sports or on playground equipment.
Table 1.
Demographic information.
| OI | Complicated mild to moderate TBI |
Severe TBI | |
|---|---|---|---|
| N | 74 | 47 | 18 |
| Lowest GCS c | N/A | 13.39 (1.92) Range = 9 – 15 |
3.94 (1.86) Range = 3 – 8 |
| Injury Severity Score (ISS) a, b |
6.97 (2.77) Range = 4.0 – 17.0 |
13.18 (7.76) Range = 1.0 – 34.0 |
13.50 (9.52) Range = 1.0 – 26.0 |
| Mechanism of injury (%)a, b |
12.1 = Involving vehicle 22.9 = Fall 45.9 = Sports or playground injury 19.1 = Other |
31.9 = Involving vehicle 42.5 = Fall 10.6 = Sports or playground injury 15.0 = Other |
50.0 = Involving vehicle 33.3 = Fall 11.0 = Sports or playground injury 5.7 = Other |
| Days hospitalized a, b Age at injury |
1.21 (.52) 4.99 (1.06) Range = 3.15 – 6.88 |
2.26 (1.91) 4.84(1.24) Range = 3.03 – 6.93 |
11.55 (21.38) 4.83 (1.01) Range = 3.64 – 6.62 |
| Child’s race (% white) |
74.3 | 67.7 | 61.1 |
| Child’s gender (% male) |
50.0 | 55.9 | 66.6 |
| Caregiver education, (% high school diploma or less) |
40.5 | 54.2 | 66.6 |
| Time since injury at extended follow-up in months b |
42.24 (9.24) Range = 25.24 – 62.93 |
35.42 (9.22) Range = 25.36 – 61.77 |
39.07 (11.26) Range = 25.24 – 63.27 |
| Median census tract income |
$63,208 ($21,047) | $56,567 ($26,976) | $54,657 ($17,919) |
| Proportion (%) receiving counseling/ behavioral therapy at extended follow-up |
9.5 | 20.0 | 37.5 |
significant difference between OI and severe;
difference between OI and complicated mild to moderate;
significant difference between complicate mild to moderate and severe TBI
Note: Extended = follow-up that occurred at average of 38 months post-injury; median census tract income is based on census information for the zip code of the family’s primary residence.
Procedure
The study was approved by the Institutional Review Boards at each study site and informed consent was obtained from the parent or legal guardian once the child was known to be in stable medical condition. Demographic and injury-related information was collected at the initial assessment and hospitalization data were obtained from medical records at the time of injury and from parent self-report at the follow-up assessments. At both the 18-month and extended follow-up assessments, primary caregivers completed standardized measures assessing family and the child’s behavioral and emotional functioning. Utilization of counseling and mental health services was assessed at the extended follow-up by asking parents if the child had these services since their last research visit.
Parent report measures
Family functioning was assessed using the Global Functioning Scale of the McMaster Family Assessment Device (FAD-GF)36, a self-report measure of family functioning with established reliability and validity. FAD-GF scores range from 1 to 4 with higher scores indicating poorer family functioning. Consistent with previous work, a raw score of > 2.17 was used to define significant family dysfunction37, with the binary trait of presence/absence of dysfunction considered in analysis.
The Child Behavior Checklist (CBCL),38, 39 a parent rating of child behavior problems, identified children who, by virtue of having elevated ratings of behavior problems, would be expected to benefit from counseling or behavioral interventions. Parents with children younger than 6 years received the CBCL 11/2-5 version and those with children 6 years or older received the CBCL 6–18 version. The CBCL has high test-retest reliability and criterion validity40, 41 and has been shown to be sensitive to behavior problems following TBI.3, 4, 5 Consistent with previous research on behavioral outcomes of pediatric TBI, 2-11 standard scores from the Total Problems subscale was used as a summary measure of behavior problems and the DSM-oriented Affective, Anxiety, ADHD, and ODD subscales were used to assess specific types of problems. Clinically significant elevations in ratings were defined as T-scores ≥ 63.3, 4, 5 A clinical need was defined as the presence of an elevation on Total Problems or one of the DSM-oriented subscales. An unmet need was defined as a clinical need that was not accompanied by service utilization.
Statistical analysis
Group comparisons using one-way ANOVA were conducted to examine group differences in demographics, injury-related characteristics, and the behavioral measures. Correction for multiple comparisons were made by dividing the significance value by the number of CBCL subscale considered (i.e., .05 / 5 = p <.01). χ2 analyses were used to examine group differences in rates of clinical need and service utilization. Logistic regression was used to examine associations of service utilization with clinical need at each of the two follow-ups controlling for gender, race, SES, age at injury, and the presence/absence of family dysfunction on the FAD-GF. The two-way interactions of group with SES and family dysfunction and the three-way interaction of group, SES, and FAD-GF were included to examine potential moderating effects of injury severity on family adversity and utilization. Non-significant interactions were trimmed from final models (p = 0.05 used as threshold for significance), but group, clinical need, and the control variables (gender, race, SES, age at injury, and FAD-GF) were retained even if non-significant. Logistic regression was also used to examine associations of clinical need with injury status, gender, race, SES, age at injury, and family functioning.
Results
Group differences in rates of impairments
Table 2 presents means and standard deviations for the behavioral measures and the proportion of children with clinically significant problems. Group differences at the 18-month visit were found for CBCL Total Behavior Problems, F (166) = 7.93, p = .001, as well as for the Affective, F (166) = 5.82, p = .004, and ADHD, F (166) = 7.31, p = .001, subscales. Follow-up tests indicated higher scores (more problems) for the severe TBI group than for the OI group for Total Behavior Problems (t = −2.91, p= .008) and ADHD subscale (t = −2.78, p = .01). Using the adjusted alpha level (p < .01), differences between the complicated mild to moderate and OI groups and the severe and complicated mild to moderate TBI groups were non-significant. Examining the subscales that comprise Total Behavior Problems at the 18-month assessment, 5.8 % of children with OI reported clinically significant elevations on the Internalizing subscale and 4.7% were elevated on the Externalizing subscale. Within the complicate mild to moderate TBI group, 6.5% were elevated on the Internalizing subscale and 14.7% were elevated on the Externalizing subscale. In the severe TBI group, 23.8% were elevated on the Internalizing subscale and 28.5% were elevated on the Externalizing subscale. Of the total sample, 70% had no clinical elevations, 13% were elevated on one subscale, 8% on two subscales, 3% on three subscales, 3% on four subscales, and 3% on all five subscales. Sixty-one percent of children with severe TBI, 44% with complicated mild to moderate TBI, and 16% with OI met criteria for clinical need. These proportions were significantly higher for the severe TBI group compared to OI group (χ2 = 9.57, p = .002) and for the complicated mild to moderate TBI compared to the OI group (χ2 = 7.76, p = .005).
Table 2.
Means and standard deviations for behavioral measures at extended follow-up and proportion meeting clinical cutoff.
| OI | Complicated mild to moderate TBI | Severe TBI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 18 month | Extended | 18 month | Extended | 18 month | Extended | |||||||
|
| ||||||||||||
| Measure | Mean (SD) | % elevated |
Mean (SD) | % elevated |
Mean (SD) | % elevated |
Mean (SD) | % elevated |
Mean (SD) | % elevated |
Mean (SD) | % elevated |
| FAD-GF | 1.55 (.52) | 8.33 | 1.61 (.46) | 11.26 | 1.62 (.40) | 5.55 | 1.62 (.47) | 9.43 | 1.72 (.56) | 17.64 | 1.72 (.46) | 5.88 |
| CBCL Totala, b |
45.04 (10.32) | 11.42 | 47.56 (12.37) | 9.72 | 49.75 (12.12) | 18.86 | 51.27 (14.08) | 16.94 | 55.41 (13.74) | 35.29 | 59.00 (13.70) | 38.88 |
| CBCL Affective |
52.74 (5.17) | 5.71 | 53.90 (6.76) | 10.95 | 54.62 (7.13) | 18.86 | 54.62 (8.57) | 11.86 | 57.88 (9.36) | 35.29 | 60.55 (11.03) | 44.44 |
| CBCL Anxiety |
52.75 (5.09) | 8.57 | 53.42 (6.17) | 10.95 | 53.28 (4.87) | 7.54 | 54.44 (7.00) | 11.86 | 55.94 (9.07) | 23.52 | 55.61 (7.68) | 22.22 |
| CBCL ADHD a, b |
53.15 (5.50) | 8.57 | 54.58 (6.77) | 16.43 | 55.20 (7.14) | 18.86 | 56.20 (8.09) | 18.64 | 57.76 (6.25) | 17.64 | 61.33 (8.39) | 38.88 |
| CBCL ODD b |
53.28 (4.85) | 8.57 | 54.60 (6.36) | 17.80 | 55.35 (7.21) | 18.86 | 56.74 (8.93) | 20.33 | 56.35 (8.21) | 17.64 | 60.55 (8.97) | 33.33 |
significant difference between OI and severe at 18- months;
OI and severe at the extended follow-up
Note: Adjusted alpha level of p < .01 used to determine significance; extended = follow-up that occurred at average of 38 months post-injury CBCL Total = CBCL Total Behavior Problems subscale; % elevated = CBCL subscales t ≥ 63 or raw score > 2.17 for the FAD-GF.
At the extended follow-up, group differences were found for CBCL Total Behavior Problems, F (152), = 5.95, p = .003, as well as for the Affective, F (152) = 6.00, p = .003, ADHD, F (152) = 7.01, p = .001, and ODD, F (152) = 3.98, p = .02, subscales. Follow-up tests indicated higher scores for the severe TBI group compared to the OI group for Total Behavior Problems (t = −3. 22, p= .004), as well as for the ADHD (t = −3.16, p = .004), and ODD (t = −2.65, p = .01) subscales. Using the adjusted alpha level (p < .01), differences between both the severe TBI and complicated mild to moderate TBI groups and the complicated mild to moderate TBI and OI groups were non-significant. Examining the subscales that comprise the Total Behavior Problems scale, 15.0 % of children with OI reported clinically significant elevations on the Internalizing subscale and 10.9% evidenced elevations on the Externalizing subscale. Within the complicated mild to moderate TBI group, 11.6% were elevated on the Internalizing subscale and 20.0% on the Externalizing. In the severe TBI group, 36.8% were elevated on the Internalizing subscale and 36.8% were elevated on the Externalizing. Of the total sample, 62% had no clinical elevations on the subscales used to define clinical need, 15% were elevated on one subscale, 10% on two subscales, 5% on three subscales, 3% on four subscales, and 5% on all five subscales. Comparison of the groups on rates of clinical need revealed a significant difference (χ2 = 6.62, p = .01), with 61% of the severe TBI group, 46% of the complicated mild to moderate TBI group, and 28% of OI group meeting criteria for clinical need.
Group differences in service utilization
Behavioral therapy or counseling services between the 18-month and extended follow-up assessments were utilized by 9.5% of the OI group, 20.0% of the complicated mild to moderate TBI group, and 37.5% of the severe TBI group (Table 1). Chi square analyses indicated a non-significant trend for higher utilization in the TBI groups compared to the OI group (χ2 = 5.36, p = .06).
Factors associated with service utilization
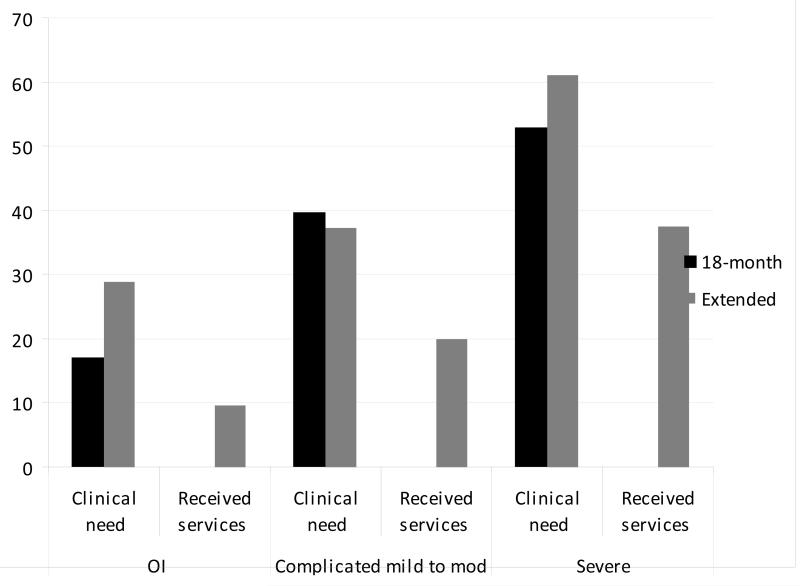
Clinical need at the 18-month follow-up was associated with service utilization at the extended follow-up (p = .01). Main effects for group, race, SES, gender, age at injury, and FAD-GF were not significant. Because analysis also failed to reveal interactions of group with SES and family dysfunction, these factors were trimmed from the final model. Of the subset of children with clinical need at the 18-month follow-up, 66% with severe TBI, 57% with complicated mild to moderate TBI, and 50% of the OI group had unmet needs (Figure 2), a non-significant group difference (p > .05). Logistic regression failed to reveal associations of group or individual factors (race, SES, gender, age at injury, or FAD-GF) with unmet clinical need.
Figure 2.
Proportion that met criteria for clinical need at the 18-month and extended follow-up visits and reported receiving behavioral therapy or counseling services at the extended follow-up.
Clinical need at the extended follow-up was also associated with receipt of services (p = .01), with non-significant main effects for group, race, SES, gender, age at injury, and FAD-GF (Table 3). The two-way interactions of group with SES and family dysfunction were non-significant (p > .05) and thus trimmed from the final model. Group differences in the proportions of children meeting criteria for clinical need at the extended follow-up were not significant, with 72% with severe TBI, 59% with complicated mild to moderate TBI, and 71% of the OI group reporting no utilization of services (Figure 2). Results of logistic regression revealed that lower SES was associated with higher risk for unmet clinical need [odds ratio (95% confidence interval) = .58 (.34 – .98), p = .04].
Table 3.
Logistic regression examining clinical need as a predictor of receipt of behavioral therapy or counseling services.
| Association of 18 month clinical need and utilization of services at extended |
Association of clinical need at extended and utilization of services at extended |
|||
|---|---|---|---|---|
|
| ||||
|
Adjusted Odds Ratio
(95% Confidence Interval) |
p |
Adjusted Odds Ratio
(95% Confidence Interval) |
p | |
| Group | 2.08 (.81 – 5.33) | .12 | 2.25 (.83 – 6.09) | .10 |
| Race | 3.47 (.90 – 13.51) | .07 | 2.20 (.52 – 9.15) | .27 |
| Z-combined | 1.01 (.51 – 1.99) | .96 | 1.18 (.60 – 2.31) | .62 |
| Gender | .79 (.20 – 3.13) | .74 | 1.52 (.41 – 5.60) | .52 |
| Age at injury | .89 (.52 – 1.50) | .66 | .93 (.54 – 1.62) | .93 |
| FAD- GF | 1.00 (.12 – 7.86) | >.99 | 4.22 (.86 – 20.71) | .07 |
| Clinical need | 5.05 (1.37 – 18.63) | .01* | 7.24 (1.61 – 32.58) | .01* |
significant at.05 alpha level
Note: extended = follow-up that occurred at average of 38 months post-injury; z-combined = composite measure based on family income and caregiver education; FAD-GF= Family Assessment Devise- Global Functioning
Discussion
This study described long-term utilization of behavioral therapy or counseling services following TBI in early childhood and investigated the correspondence of service utilization with clinical need. At both an 18-month follow-up and a more extended follow-up a mean of 38 months post injury, children with TBI had higher rates of clinical need than the OI group and clinical need was associated with higher rates of service utilization. There was also a trend for higher service utilization in children with TBI. Although group differences in unmet need were not significant at either follow-up visit, lower SES was associated with a higher risk for unmet need at the extended follow-up. As the children were injured in early childhood, the findings are consistent with evidence that younger children with TBI experience persistent, long-term behavioral concerns post-injury.4-12 The findings are also consistent with evidence that even children with less severe TBI often experience persistent difficulties with behavior and social competence17, 18and that unmet post-injury clinical needs are common in these children.19, 20, 40This study adds to the literature by documenting the high rates of unmet clinical need in these children.
We confirmed the hypothesis that parent-reported clinical need would be related to greater service utilization, even when controlling for injury severity and demographic factors (gender, race and SES). We also confirmed the hypothesis that unmet needs would be more common in lower SES families.24, 25, 26Contrary to expectations, we failed to document greater unmet need in children with TBI than in those with OI; rather, unmet need remained high in all groups at both follow-up visits. These findings suggest that a substantial proportion of children with traumatic injuries who have clinical needs fail to receive services for these needs whether or not these injuries are to the head.
Study limitations
One limitation of this study is that we could not determine the extent to which children had behavior problems prior to injury. The high rate of unmet needs in all groups is consistent with prior research suggesting increased psychological distress related to hospitalization for other traumatic injuries in children,19, 41 but we do not know how much this distress contributed to problems in our sample. A second limitation is that our comparison group of children with OI may have had less significant injuries that the children with TBI, especially in view of the cognitive consequences of TBI. While the OI group was recruited to control for behavioral predispositions to injury33 group differences may not be due to brain insult alone, but may also reflect the different traumatic experiences of children with TBI compared to OI.
Additional limitations pertain to measurement issues and sample representativeness. We defined service utilization as occurring at any point between the 18-month and extended follow-up visit, rather than concurrently, and we failed to account for frequency and duration of treatment. Our measures of behavior problems and clinical need are likewise limited in scope and precision, as we relied on parent ratings to define clinical need and could have over-identified these needs by including children whose problems were well managed by their parents or teachers. With regard to the sample, attrition was higher in the OI group than in the TBI groups and in children injured at older compared to younger ages. Finally, post-injury utilization of behavioral therapy or counseling services in the United States may vary from other counties (i.e., access to mental health care services may be more restricted compared to countries with universal health care systems). In Australia, for example, a longitudinal follow-up of children with TBI indicated that 28% of children with moderate TBI and 54% of those with severe TBI were still receiving psychological services 10 years post injury.42 Caution is thus advised in generalizing these findings to other populations.
Conclusions
Despite these limitations, this is to our knowledge one of the few studies to examine utilization of behavioral therapy and counseling services following pediatric TBI. The sample was followed prospectively for several years post-injury and employed a standardized parent-report measure to assess multiple domains of behavior problems. Structured diagnostic interviews, observational measures, or teacher report measures may be useful in future studies to obtain more in-depth information about behavior across settings and contexts and track other types of clinical services. Further research is also needed to examine other factors that may relate to clinical need and service utilization that were not considered in this study, such as family history of mental health disorders and mental health recommendations made by providers at the time of hospitalization.
In summary, these results support previous findings of persistent behavior problems in children after early childhood TBI relative to those with OI but suggest that unmet clinical needs are common following both types of traumatic injuries. TBI is a chronic health condition with behavioral effects that often persist over time,43 and many children with OI also have mental health needs.42 The findings support the need for monitoring of behavior and clinical symptomology by health care providers and referral of children for behavioral health services as appropriate.
Acknowledgements
The authors wish to acknowledge the contributions of Christine Abraham, Andrea Beebe, Lori Bernard, Anne Birnbaum, Beth Bishop, Tammy Matecun, Karen Oberjohn, Elizabeth Roth, Elizabeth Shaver, and Mary Ann Toth in data collection and Amy Cassedy in data management. The Cincinnati Children’s Medical Center Trauma Registry, Rainbow Pediatric Trauma Center, Rainbow Babies & Children’s Hospital, Nationwide Children’s Hospital Trauma Program, and MetroHealth Center Department of Pediatrics and Trauma Registry provided assistance with recruitment.
Conflict of interest and funding
The research reported here was supported by grant R01 HD42729 from NICHD, in part by USPHS NIH Grant M01 RR 08084, and by Trauma Research grants from the State of Ohio Emergency Medical Services, all to Dr. Wade. The project described was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077-04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Kurowski received support from NIH career development grant K12 HD001097-16. Dr. Yeates received support from career development grant K02 HD44099 from NICHD. For the remaining authors, none were declared.
Glossary
Abbreviations
- OI
orthopedic injury
- TBI
traumatic brain injury
- ADHD
Attention Deficit/ Hyperactivity Disorder
- ODD
Oppositional Defiant Disorder
- SES
socioeconomic status
- CBCL
Child Behavior Checklist
- SD
standard deviation
- GCS
Glasgow Coma Scale
- FAD-GF
Family Assessment Device- Global Functioning
- DSM
Diagnostic and Statistical Manual
- ANOVA
Analysis of variance
- AOR
Adjusted Odds Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christine L. Karver, University of Cincinnati, Department of Psychology, and the Cincinnati Children’s Hospital, Department of Physical Medicine and Rehabilitation Cincinnati, OH
Brad Kurowski, Cincinnati Children’s Hospital, Department of Physical Medicine Rehabilitation, Cincinnati, OH
Erin A. Semple, University of Toledo, College of Medicine
Terry Stancin, Case Western Reserve University and MetroHealth Medical Center, Department of Psychiatry, Cleveland, OH
H. Gerry Taylor, Case Western Reserve University/University Hospitals Cleveland, OH
Keith O. Yeates, Ohio State University/Nationwide Children’s Hospital, Columbus, OH
Nicolay C. Walz, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH
Shari L. Wade, Cincinnati Children’s Hospital, Department of Physical Medicine and Rehabilitation, and University of Cincinnati, College of Medicine, Cincinnati, OH
References
- 1.Langlois JA, Rutland-Brown W, Thomas KE. The Incidence of traumatic brain injury among children in the United States: Differences by race. J Head Trauma Rehabil. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, et al. Incidence of traumatic brain injury in New Zealand: A populations-based study. Lancet. 2013;12:53–64. doi: 10.1016/S1474-4422(12)70262-4. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz L, Taylor HG, Drotar D, Yeates KO, Wade SL, Stancin T. Long-term behavior problems following pediatric traumatic brain injury: Prevalence, predictors, and correlates. J Pediatr Psychol. 2003;28:251–263. doi: 10.1093/jpepsy/jsg013. [DOI] [PubMed] [Google Scholar]
- 4.Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood Traumatic Brain Injury. Rehabil Psychol. 2010;55:48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, Yeates KO, Walz NC. Age at injury and long-term behavior problems following traumatic brain injury in young children. Rehabil Psychol. 2012;57(3):256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassenburg R, Max JE, Lindgren SD, Schatz A. Sustained attention in children and adolescents after traumatic brain injury: relation to severity of injury, adaptive functioning, ADHD and social background. Brain Injury. 2004;2004;18(8):751–764. doi: 10.1080/02699050410001671775. [DOI] [PubMed] [Google Scholar]
- 7.Bloom DR, Levin HS, Ewing-Cobbs L, Saunders AE, Song J, Fletcher JM, Kowatch RA. Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Psy. 2001;40(5):572–579. doi: 10.1097/00004583-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Max JE, et al. Oppositional Defiant Disorder symptomology after traumatic brain injury: A prospective study. J Nerv Ment Dis. 1998;186(6):325–332. doi: 10.1097/00005053-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Max JE, et al. Posttraumatic stress symptomology after childhood traumatic brain injury. J Nerv Ment Dis. 1998;186(10):589–596. doi: 10.1097/00005053-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Levi RB, Drotar D, Yeates KO, Taylor HG. Posttraumatic stress symptoms in children following orthopedic or traumatic brain injury. J Clin Child Psy. 1999;28(2):232–243. doi: 10.1207/s15374424jccp2802_10. [DOI] [PubMed] [Google Scholar]
- 11.Grados MA, Vasa RA, Riddle MA, Slomine BS, Salorio C, Christensen J, Gerring J. New onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depression and Anxiety. 2008;25(5):398–407. doi: 10.1002/da.20398. [DOI] [PubMed] [Google Scholar]
- 12.Vasa RA, Gerring JP, Grados M, Slomine B, Christensen JR, Rising W, Denckla MB, Riddle MA. Anxiety after severe pediatric closed head injury. J Am Acad Child Adol Psy. 2002;41(2):148–156. doi: 10.1097/00004583-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Verger K, Junque C, Jurado MA, Tresserras P, Bartumeus F, Nogues P, Poch JM. Age effects on long-term neuropsychological outcomes in paediatric traumatic brain injury. Brain Injury. 2000;14(6):495–503. doi: 10.1080/026990500120411. [DOI] [PubMed] [Google Scholar]
- 14.Anderson V, Catroppa C, Godfrey C, Rosenfeld G. Intellectual ability 10 years after traumatic brain injury in infancy and childhood: What predicts outcomes? J Neurotraum. 2012;29:143–153. doi: 10.1089/neu.2011.2012. [DOI] [PubMed] [Google Scholar]
- 15.Anderson V, Jacobs R, Spencer-Smith M, Coleman L, Anderson P, Williams J, Greenham M, Leventer R. Does early age at brain insult predict worse outcome? Neuropsychological implications. J Pediatr Psychol. 2010;35(7):716–727. doi: 10.1093/jpepsy/jsp100. [DOI] [PubMed] [Google Scholar]
- 16.McKinlay A, Darlymple-Alford JC, Horwood LJ, Fergusson DM. Long term psychosocial outcomes after mild head injury in early childhood. J Neurol Neurosurg Ps. 2002;73:281–288. doi: 10.1136/jnnp.73.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massagli TL, Fann JR, Burington BE, Jaffe KM, Katon WJ, Thompson RS. Psychiatric illness after mild traumatic brain injury in children. Arch Phys Med and Rehab. 2004;85:1428–1434. doi: 10.1016/j.apmr.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Levin HS, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slomine BS, McCarthy ML, et al. Health care utilization and needs after pediatric traumatic brain injury. Pediatrics. 2006;117(4):663–674. doi: 10.1542/peds.2005-1892. [DOI] [PubMed] [Google Scholar]
- 20.Greenspan AL, MacKenzie EJ. Use and need for post-acute services following paediatric head injury. Brain Injury. 2000;14(5):417–429. doi: 10.1080/026990500120529. [DOI] [PubMed] [Google Scholar]
- 21.Bradley RH, Corwyn RF. Socioeconomic status and child development. Ann Rev Psy. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 22.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: Results from the National Comorbidity Survey Replication. JAMA Psych. 2005;62(6):629–640. doi: 10.1001/archpsyc.62.6.629. [DOI] [PubMed] [Google Scholar]
- 23.Snells-Johns J, Mendez JL, Smith BH. Evidence-based solutions for overcoming access barriers, decreasing attrition, and promoting change with underserved families. J Family Psychol. 2004;18:19–35. doi: 10.1037/0893-3200.18.1.19. [DOI] [PubMed] [Google Scholar]
- 24.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsych Soc. 2001;7:755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 25.Catroppa C, Anderson VA, Morse SA, Haitou F, Rosenfeld JV. Outcomes and predictors of functional recovery 5 years following pediatric Traumatic Brain Injury (TBI) J Ped Psy. 2008;33(7):707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- 26.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsych. 2002;16:514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- 27.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO. Family burden and adaptation during the initial year after traumatic brain injury in children. Pediatrics. 1998;102:110–116. doi: 10.1542/peds.102.1.110. [DOI] [PubMed] [Google Scholar]
- 28.Stancin T, Wade SL, Walz NC, Yeates KO, Taylor HG. Family adaptation 18 months after traumatic brain injury in early childhood. J Dev Behav Pediatr. 2010;310:317–325. doi: 10.1097/DBP.0b013e3181dbaf32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Burant C. Bidirectional child-family influences on outcomes of traumatic brain injury in children. J Int Neuropsych Soc. 2001;7:755–767. doi: 10.1017/s1355617701766118. [DOI] [PubMed] [Google Scholar]
- 30.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO, Minich M. A prospective study of long-term caregiver and family adaptation following brain injury in children. J Head Trauma Rehabil. 2002;17:96–111. doi: 10.1097/00001199-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Kazdin AE, Holland L, Crowley M. Family experience of barriers to treatment and premature termination from child therapy. J Consult Clin Psychol. 1997;65(3):453–463. doi: 10.1037//0022-006x.65.3.453. [DOI] [PubMed] [Google Scholar]
- 32.Kurowski BG, Taylor HG, Yeates KO, Walz NC, Stancin T, Wade SL. Caregiver ratings of long-term executive dysfunction and attention problems after early childhood traumatic brain injury: family functioning is important. PM&R. 2011;3:836–845. doi: 10.1016/j.pmrj.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstrohm SL, Arffa S. Preschool children with mild to moderate traumatic brain injury: An exploration of immediate and post-acute morbidity. Arch Clin Neuropsychol. 2005;20:675–695. doi: 10.1016/j.acn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 35.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 36.Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. J Mart Family Ther. 1983;9(2):171–180. [Google Scholar]
- 37.Wade SL, Taylor HG, Drotar D, Stancin T, Yeates KO. Family burden and adaptation during the initial year after traumatic brain injury in children. Pediatrics. 1998;102:110–116. doi: 10.1542/peds.102.1.110. [DOI] [PubMed] [Google Scholar]
- 38.Achenbach TM, Rescorla LA. Manual for ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2000. [Google Scholar]
- 39.Achenbach TM, Rescorla LA. Manual for ASEBA School-Aged Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- 40.Jensen PS, et al. Overlooked and underserved: “Action signs” for identifying children with unmet mental health needs. Pediatrics. 2011;128:970–979. doi: 10.1542/peds.2009-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masel BE, DeWitt DS. Traumatic brain injury: A disease process, not an event. J Neurotraum. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 42.Catroppa C, Godfrey C, Rosenfeld JV, Hearps SSJC, Anderson VA. Functional recovery ten years after pediatric traumatic brain injury: outcomes and predictors. J Neurotrauma. 2012;29:2539–2947. doi: 10.1089/neu.2012.2403. [DOI] [PubMed] [Google Scholar]