Abstract
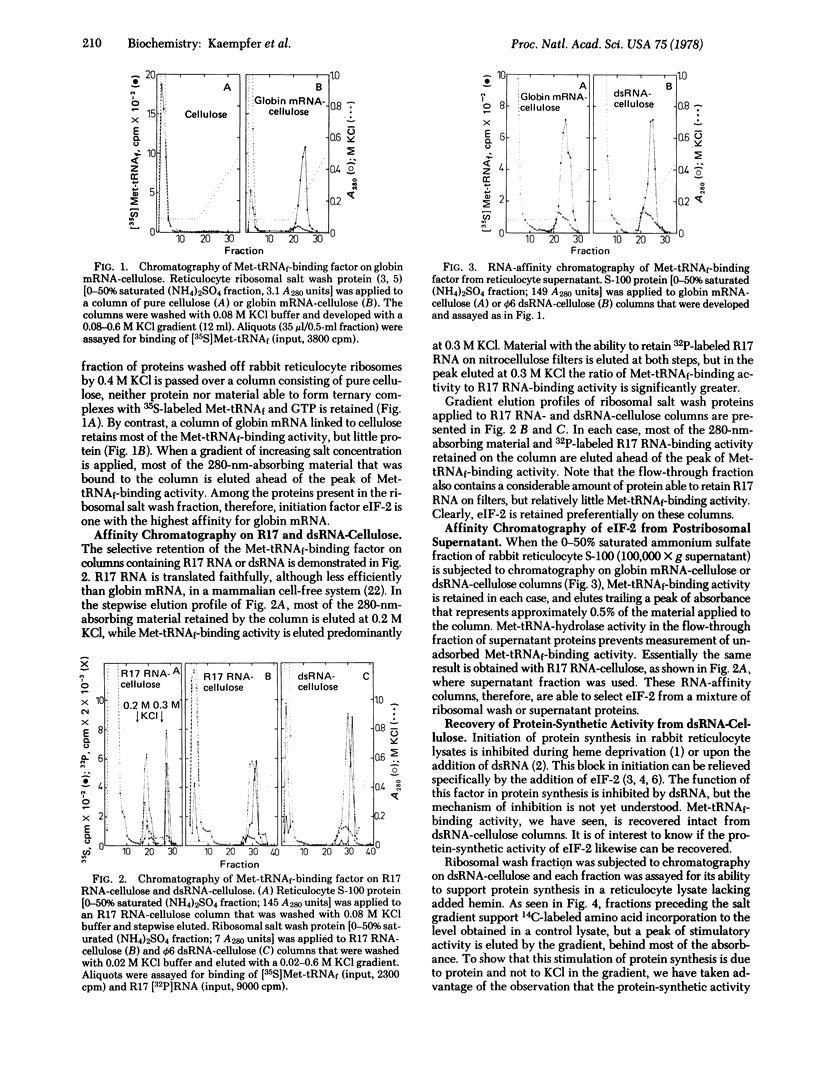
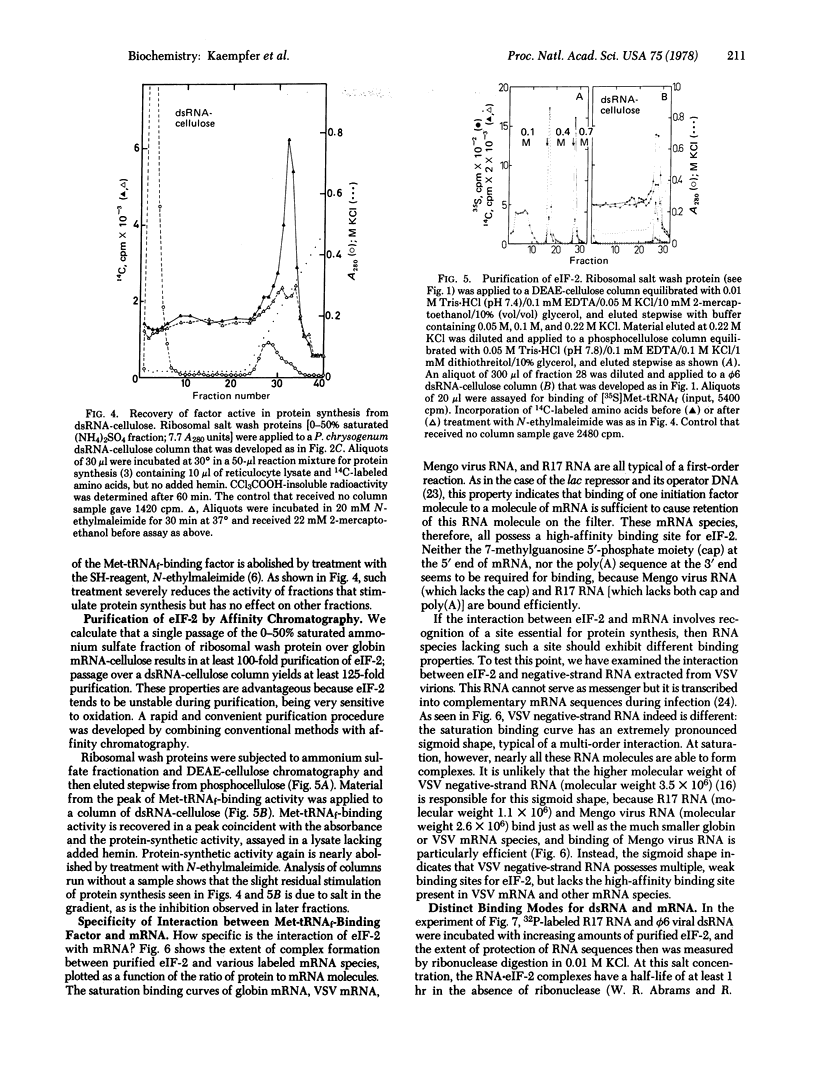
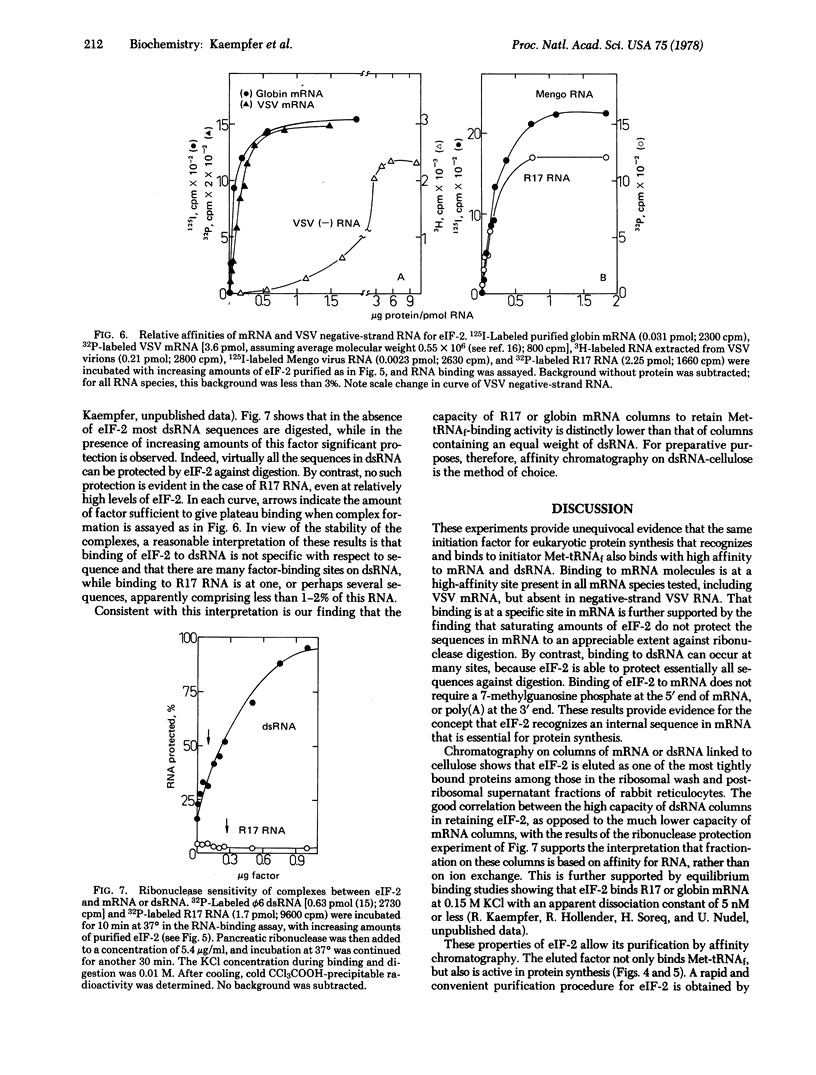
Affinity chromatography on columns containing globin mRNA, R17 phage mRNA, or double-stranded RNA linked to cellose is used to demonstrate unequivocally that the eukaryotic initiation factor (eIF-2) that forms a ternary complex with Met-tRNAf and GTP also binds tightly to these RNA species. Affinity chromatography of reticulocyte ribosomal wash yields over 100-fold purification of Met-tRNAf-binding factor. This factor is eluted as one of the most tightly bound proteins, and is active in protein synthesis even after passage over a column of double-stranded RNA-cellulose. eIF-2 binds mRNA and double-stranded RNA in distinctly different modes, protecting essentially all sequences in double stranded RNA, but very few in mRNA, against digestion with ribonuclease. Apparently, eIF-2 recognized the A conformation of double-stranded RNA, but not its sequence. By contrast, globin, Mengo virus, R17 and vesicular stomatitis virus mRNA are shown to possess a high-affinity binding site for eIF-2 that is absent in negative-strand RNA of vesicular stomatitis virus, an RNA that cannot serve as messenger. The results support the concept that eIF-2, the initiation factor that binds Met-tRNAf, recognizes an internal sequence in mRNA essential for protein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Safer B., Merrick W. C., Anderson W. F., London I. M. Inhibition of protein synthesis in rabbit reticulocyte lysates by double-stranded RNA and oxidized glutathione: indirect mode of action on polypeptide chain initiation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1286–1290. doi: 10.1073/pnas.72.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Dettman G. L., Stanley W. M., Jr Recognition of eukaryotic initiator tRNA by an initiation factor and the transfer of the methionine moiety into peptide linkage. Biochim Biophys Acta. 1972 Nov 16;287(1):124–133. doi: 10.1016/0005-2787(72)90336-x. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Zinder N. D. Structure of the poly(G) polymerase component of the bacteriophage f2 replicase. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1838–1843. doi: 10.1073/pnas.68.8.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerman J. G., Shafritz D. A. Interaction of poly(A) and mRNA with eukaryotic initiator met-tRNA-f binding factor: identification of this activity on reticulocyte ribonucleic acid protein particles. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1021–1025. doi: 10.1073/pnas.72.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Jay G., Abrams W. R., Kaempfer R. Resistance of bacterial protein synthesis to double-stranded RNA. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1357–1364. doi: 10.1016/0006-291x(74)90347-7. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Host interference with viral gene expression: mode of action of bacterial factor i. J Mol Biol. 1974 Jan 15;82(2):193–212. doi: 10.1016/0022-2836(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Initiation of protein synthesis. Binding of messenger RNA. J Biol Chem. 1975 Aug 10;250(15):5742–5748. [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Sequence of events in initiation of translation: a role for initiator transfer RNA in the recognition of messenger RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3199–3203. doi: 10.1073/pnas.71.8.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Inhibition of cellular protein synthesis by double-stranded RNA: inactivation of an initiation factor. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1222–1226. doi: 10.1073/pnas.70.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Rose J. K., Lodish H. F. Translation of individual species of vesicular stomatitis viral mRNA. J Virol. 1975 Apr;15(4):1004–1011. doi: 10.1128/jvi.15.4.1004-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Protein initiation in eukaryotes: formation and function of a ternary complex composed of a partially purified ribosomal factor, methionyl transfer RNA, and guanosine triphosphate. Proc Natl Acad Sci U S A. 1973 Jan;70(1):41–45. doi: 10.1073/pnas.70.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Raffel C., Stein S., Kaempfer R. Role for heme in mammalian protein synthesis: activation of an initiation factor. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4020–4024. doi: 10.1073/pnas.71.10.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Knipe D. Nucleotide sequence complexities, molecular weights, and poly(A) content of the vesicular stomatitis virus mRNA species. J Virol. 1975 Apr;15(4):994–1003. doi: 10.1128/jvi.15.4.994-1003.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safer B., Anderson W. F., Merrick W. C. Purification and physical properties of homogeneous initiation factor MP from rabbit reticulocytes. J Biol Chem. 1975 Dec 10;250(23):9067–9075. [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T., Gesteland R. F., Spahr P. F. Translation of bacteriophage R17 and Qbeta RNA in a mammalian cell-free system. J Mol Biol. 1973 Apr 15;75(3):575–578. doi: 10.1016/0022-2836(73)90462-2. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of eukaryotic protein synthesis: (Met-tRNA f -40S ribosome) initiation complex catalysed by purified initiation factors in the absence of mRNA. Nat New Biol. 1973 Mar 14;242(115):35–38. doi: 10.1038/newbio242035a0. [DOI] [PubMed] [Google Scholar]
- Stanley W. M., Jr Preparation and analysis of L-( 35 S)methionine labeled transfer ribonucleic acids from rabbit liver. Anal Biochem. 1972 Jul;48(1):202–216. doi: 10.1016/0003-2697(72)90183-2. [DOI] [PubMed] [Google Scholar]


