Abstract
Objective
To determine the clinical efficacy of an ankle robotic rehabilitation protocol for patients with cerebral palsy.
Design
The clinic cohort was identified from a retrospective chart review in a before-after intervention trial design and compared to a previously published prospective research cohort.
Setting
Urban rehabilitation hospital outpatient clinic.
Participants
Children (n=28, 8.2 ± 3.62 years) with Gross Motor Function Classification System level I, II or III who were referred for ankle stretching and strengthening used an ankle rehabilitation robot in the clinic setting. Clinic results were compared to a previously published cohort of 12 participants (7.8 ± 2.91 years) seen in a research laboratory-based intervention protocol.
Interventions
Patients in the clinic cohort were seen 2 times per week for 75 minute sessions for a total of 6 weeks. The first 30 minutes of the session was spent using the robotic ankle device for ankle stretching and strengthening and the remaining 45 minutes were spent on functional movement activities. There was no control group.
Main Outcome Measures
We compared pre- and post-intervention measures of plantarflexor and dorsiflexor range of motion, strength, spasticity, mobility (timed up and go, 6-minute walk, 10-meter walk), balance (Pediatric Balance Scale), Selective Motor Control Assessment of the Lower Extremity (SCALE), and the Gross Motor Function Measure (GMFM).
Results
Significant improvements were found for the clinic cohort in all main outcome measures except for the GMFM. These improvements were equivalent to those reported in the research cohort, except for larger SCALE test changes in the research cohort.
Conclusion
These findings suggest that translation of repetitive, goal directed biofeedback training into the clinic setting is both feasible and beneficial for patients with cerebral palsy.
Keywords: cerebral palsy, robotics, ankle, resistance training, muscle stretching exercises
Cerebral palsy (CP) is caused by an injury to the immature central nervous system that presents with symptoms such as spasticity, muscle weakness, and reduced selective motor control.1, 2 When these impairments occur in the growing child, reduced range of motion at a joint may also occur, creating abnormal biomechanical alignment that reduces function.
Muscle stretching and strengthening have been long-used in the clinic, but are considered to have uncertain treatment effects in a recent review paper3 due to low systematic review evidence for use of those interventions in isolation. Novak et al.’s comprehensive review3 elegantly compiles the highest level evidence available for treatment of CP across multiple areas the World Health Organization’s International Classification of Function (ICF) system. Stretching was considered to be ineffective for the purposes of contracture management, and strength training was found effective for improving muscle strength short term. Among the recommended interventions for gross motor control was goal directed training, but there remains no consensus how that might be applied in a treatment setting.
The lack of consistent evidence in the areas of stretching in particular reflects the variability in structure and rigor of protocols used in published studies, as well as an emerging understanding of the underlying physiology of muscle tissue in upper motor neuron lesions.4 The rapid evolution of rehabilitation robotics in research laboratories presents the potential to increase our knowledge in this area. Robotic instrumentation can measure the ease or difficulty with which a joint is moved,5-8 or the extent to which a person can volitionally activate muscles at a joint. Quantitative results have the potential to increase sensitivity of measures to guide treatment approaches and track patient progress over time. For the specific goals of stretching and strengthening, robotics can be utilized to give repeatable and quantifiable stretch, and assistance or resistance as needed for motor training.9 Recent advances in haptic feedback and gaming have also been applied to increase motivation.10-13
Despite the strong promise of robotics shown in the research environment, few of the many robotic devices invented in the past decade have been widely adopted into clinic environments. There could be many reasons for reduced translation into clinical practice including safety regulations, prohibitive cost of devices, or clinician bias, but it leaves a gap in our understanding of the efficacy of robotic devices in a typical clinic setting.
The purpose of the present study was to evaluate the effectiveness of a portable robotic device used for stretching and movement training in children with CP as part of their physical therapy treatment. We chose to focus on the ankle joint because of its importance in patient goals, such as gait improvement. The robotic device (the commercially available IntelliStretcha) has previously been shown to be effective in a research laboratory setting,13 and has been adopted into several physical therapy clinics. Physical therapists designed an intervention protocol based on that of Wu et al.13 and also included goal-directed training after time spent working with the IntelliStretcha device. We objectively evaluated performance of patients using this robotic device was used in an outpatient clinic with a diverse CP population.
Methods
This study is a retrospective review of patients seen for clinical indication of ankle and gait rehabilitation. They used the IntelliStretcha robotic device in a defined protocol and were compared with participants of a previously published research study.13
Setting
The clinic cohort was treated in an outpatient pediatric clinic located within an urban rehabilitation hospital. Scheduling accommodated the patient’s school day, and there were typically additional patient-therapist pairs working in the gym. Stairs, a treadmill, adaptive bikes, mats, therapy balls, weights, trampolines, games and toys were available for use, and were selected by the physical therapist as indicated for each patient. The research cohort13 was seen in a research laboratory in the same hospital with no other children present.
Participants
The clinic cohort included 28 participants (19 male, 9 female) with an average age of 8.2 years. They included 11 individuals with diplegia, 16 with hemiplegia, and 1 with triplegia. Characteristics of the clinic cohort are enumerated in Table 1.
Table 1.
Clinic cohort patient characteristics
| ID | Gender | Age (years) |
GMFCS | Diagnosis | # treatment sessions |
|---|---|---|---|---|---|
| 1 | M | 4 | I | Diplegia | 13 |
| 2 | M | 6 | II | Diplegia | 12 |
| 3 | F | 9 | II | Hemiplegia | 12 |
| 4 | F | 7 | Hemiplegia | 12 | |
| 5 | M | 12 | II | Hemiplegia | 10 |
| 6 | M | 8 | II | Diplegia | 12 |
| 7 | F | 5 | Hemiplegia | 11 | |
| 8 | M | 14 | II | Hemiplegia | 12 |
| 9 | M | 8 | II | Diplegia | 12 |
| 10 | M | 14 | III | Diplegia | 12 |
| 11 | F | 14 | Diplegia | 11 | |
| 12 | M | 8 | II | Hemiplegia | 11 |
| 13 | F | 13 | Hemiplegia | 11 | |
| 14 | F | 12 | II | Triplegia | 11 |
| 15 | M | 12 | II | Hemiplegia | 12 |
| 16 | M | 5 | Diplegia | 11 | |
| 17 | M | 4 | II | Hemiplegia | 11 |
| 18 | F | 6 | II | Hemiplegia | 11 |
| 19 | M | 14 | Diplegia | 11 | |
| 20 | M | 4 | Hemiplegia | 12 | |
| 21 | F | 7 | II | Hemiplegia | 11 |
| 22 | M | 11 | II | Hemiplegia | 12 |
| 23 | M | 5 | Diplegia | 12 | |
| 24 | M | 5 | Hemiplegia | 12 | |
| 25 | M | 8 | II | Diplegia | 11 |
| 26 | F | 4 | II | Diplegia | 11 |
| 27 | M | 5 | II | Hemiplegia | 12 |
| 28 | M | 5 | II | Hemiplegia | 12 |
|
| |||||
|
Mean ± SD/
Summary |
19 M 9 F |
8.2 ± 3.62 | 10 I | 11 Diplegia | 11.54 ± 0.64 |
| 17 II | 16 Hemiplegia | ||||
| 1 III | 1 Triplegia | ||||
M=male; F=female; GMFCS = Gross Motor Function Classification System
Patients were referred by their physiatrist for IntelliStretcha treatment and received treatment part of their typical outpatient physical therapy. They needed to be able to follow simple directions and reliably signal pain. The ankle chosen for stretching and strengthening was the hemiparetic side in those with hemiplegia, and the more affected side in those with diplegia.
For inclusion in this analysis, patients had a diagnosis of spastic cerebral palsy, a Gross Motor Functional Classification System (GMFCS)14 level of I, II, or III, and clinical indication for range of motion and strengthening of at least one ankle joint. Patients were excluded from this analysis if they had surgical intervention in the previous year, serial casting within the previous 6 months, history of selective dorsal rhizotomy, use of intrathecal baclofen, or lack of palpable dorsiflexor activation. Ten patients used the IntelliStretcha, but were excluded from the present analysis. Chart review and treatment were completed with ethics approval of the Rehabilitation Institute of Chicago’s Institutional Review Board.
The laboratory cohort has been previously reported13 In brief, they included 12 participants (6 male, 6 female) with an average age of 7.8 years. They included 6 participants with diplegia and 6 with hemiplegia.
Intervention protocols
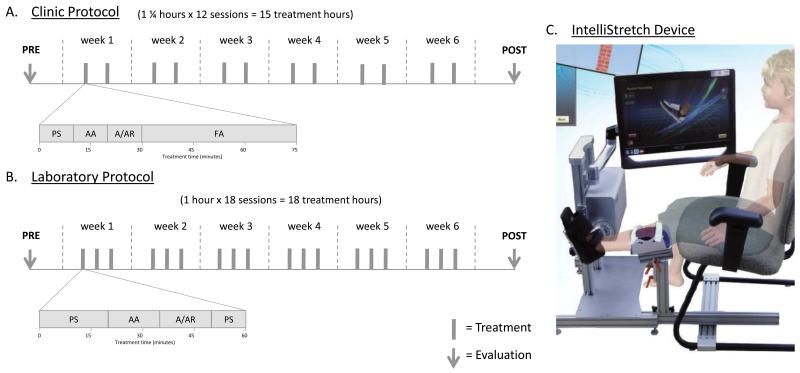
In both cohorts, individuals with CP participated in combined passive stretching and active movement protocol of one ankle joint with the use of the IntelliStretcha rehabilitation robot. A summary diagram of the clinic and laboratory protocols can be found in Figure 1.
Figure 1.
Comparison of clinic (A) and laboratory (B) protocols, with description of time spent for each activity using the IntelliStretch (PS = passive stretching, AA = active assist, A/AR = active/active resist) and outside of the IntelliStretch (FA = functional activities). Each gray bar indicates a treatment session, and arrows indication time of the evaluations. (C) Device setup, showing patient in a seated position with the tested knee in extension and tested ankle strapped to the IntelliStretch footplate. The screen (rotated for photo) shows the feedback the participant would receive during the passive stretching portion of the treatment.
The clinic cohort was seen for 15 treatment hours, 2 times per week for 75-minute sessions (Figure 1A). For the first 30 minutes, participants sat with their knee extended on the leg that was using the IntelliStretcha (Figure 1C). Their foot was strapped to the footplate, and the axis of rotation aligned with their ankle joint. Participants completed passive stretching (10 minutes) followed by active-assisted movement (10 minutes), and then active or active-resisted movements (10 minutes). During passive stretching, the device moved the participant’s ankle through a predetermined range of motion with torque limits set for safety. In active assist mode, the device offered assistance as needed for the participant to play a video game controlled by ankle position; examples of the video game include a helicopter that must move up and down to miss brick walls and a space shuttle that needed to move up and down to shoot asteroids. Therapists could scale the games to the subject’s ankle passive range of motion (greater than the active range of motion) and the time delay for the device to offer assistance. In active-resist mode played in the subject’s active range of motion, therapists could add resistance to movements or change the scale on the game such that higher or lower forces were required to move the cursor. Examples of these games include a brick-breaker game where the patients use their ankle motion to move a paddle across the screen to keep a ball bouncing to remove bricks from a screen, and a tightrope walker whose pole is controlled by ankle motion. All changes to device settings were at the therapist’s discretion, as clinically indicated, and games included a musical accompaniment typical to many video games. The remaining 45 minutes of the session focused on active functional movements, which were individually tailored to each patient by the treating physical therapist. They included a variety of gait (treadmill and overground; forward, backward, and side stepping), balance (single leg stance, use of compliant surfaces, for example), and strengthening (stair climbing, biking, and yoga poses, for example) exercises focused on the use of the range of motion gained during the robotic training. Functional training activities were sometimes completed in pairs or small group settings, depending on scheduling and similarities of ability levels.
As previously reported,13 the research cohort was seen for 18 treatment hours, 3 times per week for 1-hour sessions (Figure 1B). Each session included passive stretching (20 minutes), active-assisted (15 minutes) and active or active-resisted movements (15 minutes), followed by a passive stretching cool down period (10 minutes). Setup of the device was the same as in the clinic.
Outcome measures
Several outcome measures were collected before and after training in both cohorts. They included the 6-minute walk test, the Timed Up-and-Go (TUG), Pediatric Balance Scale (PBS), Selective Control Assessment of the Lower Extremity (SCALE),15 and the Tardieu Scale for spasticity, where R1 and R2 angles (passive range of motion) for the trained ankle were evaluated at a fast stretch speed with the knee extended using a goniometer. In addition, the clinic cohort was evaluated in the flying 10-meter walk (where 2 meters prior to the measured distance is allowed for acceleration), the Gross Motor Function Measure-66 (GMFM-66), the Functional Movement Scale (FMS), and manual muscle tests (MMT) of plantarflexion and dorsiflexion strength. Assessments were performed by the treating physical therapist and were not blinded.
Statistics
First, we evaluated the change scores for each of the outcome measures within the clinic cohort. Due to the non-parametric nature of the data, the Wilcoxon Signed Rank test was used. Secondly, we compared the clinic cohort’s results to the previously published research cohort’s results. A Kruskal-Wallis test was used to evaluate treatment type (clinic versus research) on change scores in the outcome measures that were collected in both settings. A p-value of <0.05 was considered significant in all tests. In additional to statistical comparisons between the clinic and research cohorts, we also sub-divided the clinical cohort into GMFCS levels and diagnosis types, and completed descriptive statistics to compare mean performance.
Results
A modified treatment protocol for a portable robotic stretching device (IntelliStretcha) was piloted in a clinic setting for ankle rehabilitation.
Changes found in the clinic cohort
Within the body structure and function domain of the ICF, there were significant improvements found in the clinic cohort’s passive range of motion of the ankle joint into dorsiflexion (also called R2, p<0.001, Figure 2A). All patients showed improvement in passive range of motion with an average change of 8.6 degrees. Similarly, 27 participants in the clinic cohort showed improvement in Tardieu R1 of the ankle plantarflexors at the fast stretch speed (p<0.001, Figure 2B), with the catch being in a more dorsiflexed position. Over half of the participants demonstrated improved MMT scores by at least a half a grade (16/28 for dorsiflexion, 15/28 for plantarflexion) after intervention. Although more variable, there was a significant improvement in SCALE scores for the clinic cohort (p=0.001, Figure 2C).
Figure 2.
Results from clinical outcome measures, with comparisons to research cohort where available. Average measures with standard deviation error bars are shown for the Pre and Post evaluations both cohorts. Significant changes in the Wilcoxon Signed Rank test (*) were found in all measures in the clinic cohort and all but the TUG in the research cohort. There were no differences between the two cohorts’ change in any test but the SCALE, where the research cohort improved more than the clinic cohort, identified by the Kruskal-Wallis test (†). Note that not all y-axes start with zero.
In the activities domain of the ICF, we found a decrease in the time required to complete the TUG (p<0.001, Figure 2D), with 27 participants showing some improvement (group average of 1.5 seconds improvement). The largest improvement was seen in the individual classified as GMFCS level III, who performed this task 13 seconds faster after training. Similarly, all participants showed gains in their PBS scores (p<0.001, Figure 2E), with an average increase of 3.5 points. In this measure, GMFCS level II improved more than level I, and individuals with diplegia improved more than those with hemiplegia (Table 2).
Table 2.
Average changes scores for selected outcome measures, divided by groups
| Category | Subgroup | PBS (points) |
6-minute walk (feet) |
10-meter walk (sec) |
|---|---|---|---|---|
| GMFCS | I (n=10) | 2.7 | 289.4 | 1.75 |
| II (n=17) | 3.9 | 43.0 | 1.09 | |
| III (n=1) | 6 | 354.0 | NT | |
| Diagnosis | Diplegia (n=11) | 4.3 | 136.0 | 0.61 |
| Hemiplegia (n=16) | 3.0 | 145.5 | 1.80 | |
| Triplegia (n=1) | NT | 155.0 | NT |
GMFCS = Gross Motor Function Classification System; NT = not tested
There was a larger range of responses seen in the 6-minute and 10-meter walking tests, with some participants improving and others declining in performance. On average there was significant improvement in both tests (6-minute walk test average improvement of 142.1 feet, p=0.013, Figure 2F; 10-meter walk test average improvement of 1.35 seconds, p=0.001, Figure 2G). For the 6-minute walk test, GMFCS level I improved more than level II. In the 10-meter test, those with hemiplegia and GMFCS level I improved more than those with diplegia and GMFCS level II. Average change scores for walking tests are shown in Table 2. There were no significant changes found in the GMFM-66 or the FMS.
Changes found in the research cohort
Results for this cohort have been published previously.13 In brief, Wu and colleagues found significant improvements in Tardieu R1, passive range of motion (Tardieu R2), PBS, 6-minute walk. They did not find a significant change in the TUG.
Comparison between clinic and research cohorts
The Kruskal-Wallis test showed statistically similar gains between the clinic and research cohorts in the 6-minute walk test (p=0.215), TUG (p=0.655), PBS (p=0.842), Tardieu Scale R1 (p=0.359), and passive range of motion (p=0.824) between treatment settings. There was greater improvement in the SCALE test in the research cohort (p=0.028).
Discussion
Use of the Intellistretcha for passive stretching and active movement biofeedback resulted in improvements in many of the outcome measures collected in the clinic cohort with results similar to previously published research.13
Passive range of motion improvements in the clinic cohort were larger than previously reported inter-rater differences of 4.86 degrees in lower extremity goniometric measures for children with CP.16 Improvements in PBS scores were higher than the minimal detectable change (MDC) of 1.59 but lower than reported minimal clinically important difference (MCID) of 3.66.17 The clinic cohort as a whole did not exceed the 6-minute walk test MDC of 180 feet,18 but the GMFCS level I subgroup averaged much higher than the MDC. Neither the overall group change nor any subgroup score surpassed the 10-meter walk MDC of 12.2 seconds.18 The one exception was the one patient who was GMFCS level III, but her improvement far surpassed that of any other participant and could be an outlier. Taken together, these results suggest that there may be a differential impact of training related to the GMFCS type, CP diagnosis, or some other criterion.
There has been much discussion about the role of passive stretching in the treatment of children with CP. Manual stretching has been reported to have minimal benefit for reducing contracture,3, 19 but limited information about CP indicates that extrapolation should be cautioned.20 Because of this, daily stretching is still recommended21 to reduce the risk of range of motion regression,22 but should be used as part of an overall treatment plan.23 More work must be done to optimize dosage, modality, and intensity of stretching and its effect. The current study presents one step towards this goal by using a device that delivers repeatable, measurable stretch at a known frequency and duration. For daily intervention, the use of IntelliStretcha or similar small robotic devices in the home environment should be investigated.
Use of robotic systems such as the IntelliStretcha can provide well-controlled stretching for kids with CP, but it is unlikely that changes in range of motion alone can explain the clinical improvements noted in this study. Rather, the critical component of the training described here is more likely the large repetitions of practicing a movement within newly acquired range in isolation or in a functional context. Because of this, another major utility of devices such as the IntelliStretcha is active movement training with total repetitions the same or higher than typically prescribed in resistance training protocols24 and similar to the quantity of repetitions required to instigate motor learning. Goal-directed training is considered a highly effective intervention3 and video games can be a motivating way to engage active participation in a selective movement task,12, 25 also shown to improve motor learning.26 Providing biofeedback of the joint position and effort exerted during active movements may also heighten proprioceptive awareness of the trained joint.27
Treatment effects in the modified protocol applied in the clinic were statistically equivalent to those seen in the research laboratory, with the exception of the SCALE where the research cohort improved more. Distal selective motor control is found to be more impaired than proximal in the lower extremity,28 so focusing training on a distal joint appears to have positive effects across many joints of the lower extremity. Given that the research cohort did spend more time using the Intellistretcha device, they experienced more sensory feedback and practice of isolated plantar- and dorsi-flexion. This may be a reason for the larger improvements and is one potential avenue for further study.
One unique aspect of the setting where this study took place is the proximity of the laboratory to the clinic setting. Early adoption of new devices gave clinicians more tools to improve outcomes for their patients, and findings in the clinic could quickly be translated into improvements in device design and application. The current study demonstrates that the application of a modified research protocol with defined intervention characteristics could be successful in a clinic environment.
The IntelliStretcha has also been implemented in 3 other non-affiliated outpatient clinics. As in the present study, their use of the IntelliStretcha incorporated intensive bouts of 2-3 sessions per week over 6-8 weeks. In addition, those sites have used the device as an ad hoc therapy tool during traditionally-spaced treatment sessions (30-35 minutes use of IntelliStretcha). Despite minor differences in approach, there was agreement on the need for activity-based movement in conjunction with a robotic tool for stretching and joint-specific movement training.29 Use of the IntelliStretcha also extended beyond the diagnosis of CP, and included other neurological impairments, idiopathic toe walking, autism spectrum disorders, incomplete spinal cord injury, and muscular dystrophy. Clinicians reported that the IntelliStretcha offered them an option for stretching and strengthening that was motivating and better tolerated than manual stretching (personal communication with S. Grubich, PT, M. Kelly, PT, and C. Skertich, PT, November 11, 2013)30.
The clinic cohort in this study received less time using the IntelliStretcha and less total intervention time than the research cohort. Both groups improved significantly, indicating further elucidation of the source of improvements is necessary. With appropriate ethical review, de-identified databases of treatment and outcome variables from multiple centers could be combined for a more comprehensive understanding of the relationship between baseline features and improvements (or regressions) seen following treatment with the IntelliStretcha alone or in combination with other activities.
Barriers to transfer of the IntelliStretcha protocol to the clinic included scheduling with consistent therapists for an intensive protocol and authorization and pre-approval from insurance providers for the required number of sessions. Although typical of physical therapy sessions for children, the potentially distracting nature of the clinic environment and patient fatigue after a full day of school may have played a roll compared to the research cohort seen in a quieter setting during school breaks. Nonetheless, the IntelliStretcha promoted significant gains in many outcome measures for children with CP in the clinic cohort.
Limitations
This study only considered clinical measures. Addition of biomechanical measures, such as those reported by Wu et al.,13 could provide more mechanistic information about the source of improvement seen in clinical measures. Because there is no control group in this study, we cannot compare the IntelliStretcha device to any other treatment or lack of treatment, thus the overall clinical benefit remains unclear. Improvements seen in clinical examinations are encouraging and feasibility has been demonstrated, but further work is required to optimize dosage, intensity, and treatment setting.
Conclusion
Improvements across all domains of the ICF were demonstrated in a group of patients using the IntelliStretcha to provide passive stretching and active movement training in a typical outpatient clinic setting. Biofeedback provided through a game interface motivated and challenged patients to actively participate in rehabilitation exercises. This study provides additional support for the role of robotics in the clinic and demonstrated both the feasibility and efficacy of robot-assisted therapy.
Acknowledgements
The authors wish to thank Yi-Ning Wu, PhD for providing individual data points for research cohort to compare with our clinic group. Yupeng Ren, MS and Kai Chen, PhD provided technical support for the IntelliStretch. Sheila Krahl, PT and Tara Egan, PT were additional treating therapists for the clinic cohort, and Margaret Dargan, SLP provided input on scheduling feasibility and supported the development of this protocol for clinic application. The Fellows Editorial Board at the National Institutes of Health provided helpful feedback on the manuscript. This work was funded by the Intramural Program, National Institutes of Health (NIH R42-HD043664), National Science Foundation (NSF SBIR IIP-0750515), and the National Institute on Disability and Rehabilitation Research (NIDRR H133E100007).
Financial support
TSM is supported by the Intramural Program at NIH. The IntelliStretch device was created with grants from the National Institutes of Health (NIH R42-HD043664), National Science Foundation (NSF SBIR IIP-0750515), and National Institute on Disability and Rehabilitation Research (NIDRR H133E100007).
Abbreviations
- CP
cerebral palsy
- ICF
nternational Classification of Function
- GMFCS
Gross Motor Function Classification System
- TUG
Timed Up-and-Go
- PBS
Pediatric Balance Scale
- SCALE
Selective Control Assessment for the Lower Extremity
- GMFM-66
Gross Motor Function Measure – 66 items
- FMS
Functional Mobility Scale
- MMT
manual muscle test
- MDC
minimal detectable change
- MCID
minimal clinically important difference
Footnotes
Suppliers List
Rehabtek LLC. Wilmette, IL 60091.
Acknowledgement of presentation of material:
1. AACPDM annual meeting, October 2013, Milwaukee, WI.
2. APMR annual meeting, October 2013, Washington, DC.
Conflicts of Interest Li-Qun Zhang, PhD holds an equity position in Rehabtek LLC, which received funding from the National Institutes of Health and National Institute on Disability and Rehabilitation Research in developing the rehabilitation robot used in this study.
Contributor Information
Theresa Sukal-Moulton, Rehabilitation Institute of Chicago; National Institutes of Health, Clinical Center, Rehabilitation Medicine, Functional and Applied Biomechanics.
Theresa Clancy, Rehabilitation Institute of Chicago.
Li-Qun Zhang, Rehabilitation Institute of Chicago; Northwestern University, Biomedical Engineering; Northwestern University, Orthopaedic Surgery.
Deborah Gaebler-Spira, Rehabilitation Institute of Chicago; Northwestern University, Physical Medicine and Rehabilitation.
References
- 1.Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):e89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 2.Deon LL, Gaebler-Spira D. Assessment and treatment of movement disorders in children with cerebral palsy. The Orthopedic clinics of North America. 2010;41(4):507–17. doi: 10.1016/j.ocl.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Developmental medicine and child neurology. 2013;55(10):885–910. doi: 10.1111/dmcn.12246. [DOI] [PubMed] [Google Scholar]
- 4.Lieber RL. Skeletal muscle structure, function & plasticity : the physiological basis of rehabilitation. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 2002. [Google Scholar]
- 5.de Gooijer-van de Groep KL, de Vlugt E, de Groot JH, van der Heijden-Maessen HC, Wielheesen DH, van Wijlen-Hempel RM, et al. Differentiation between non-neural and neural contributors to ankle joint stiffness in cerebral palsy. Journal of neuroengineering and rehabilitation. 2013;10:81. doi: 10.1186/1743-0003-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu YN, Ren Y, Goldsmith A, Gaebler D, Liu SQ, Zhang LQ. Characterization of spasticity in cerebral palsy: dependence of catch angle on velocity. Developmental medicine and child neurology. 2010;52(6):563–9. doi: 10.1111/j.1469-8749.2009.03602.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Wu YN, Hwang M, Ren Y, Gao F, Gaebler-Spira D, et al. Changes of calf muscle-tendon biomechanical properties induced by passive-stretching and active-movement training in children with cerebral palsy. J Appl Physiol. 2011;111(2):435–42. doi: 10.1152/japplphysiol.01361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross SA, Foreman M, Engsberg JR. Comparison of 3 different methods to analyze ankle plantarflexor stiffness in children with spastic diplegia cerebral palsy. Archives of physical medicine and rehabilitation. 2011;92(12):2034–40. doi: 10.1016/j.apmr.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang M, Davies TC, Xie S. Effectiveness of robot-assisted therapy on ankle rehabilitation--a systematic review. Journal of neuroengineering and rehabilitation. 2013;10:30. doi: 10.1186/1743-0003-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdea GC, Cioi D, Kale A, Janes WE, Ross SA, Engsberg JR. Robotics and gaming to improve ankle strength, motor control, and function in children with cerebral palsy--a case study series. IEEE transactions on neural systems and rehabilitation engineering : a publication of the IEEE Engineering in Medicine and Biology Society. 2013;21(2):165–73. doi: 10.1109/TNSRE.2012.2206055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cioi D, Kale A, Burdea G, Engsberg J, Janes W, Ross S. Ankle control and strength training for children with cerebral palsy using the Rutgers Ankle CP: a case study. IEEE International Conference on Rehabilitation Robotics : [proceedings] 2011;2011:5975432. doi: 10.1109/ICORR.2011.5975432. [DOI] [PubMed] [Google Scholar]
- 12.Fehlings D, Switzer L, Findlay B, Knights S. Interactive computer play as “motor therapy” for individuals with cerebral palsy. Seminars in pediatric neurology. 2013;20(2):127–38. doi: 10.1016/j.spen.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu YN, Hwang M, Ren Y, Gaebler-Spira D, Zhang LQ. Combined passive stretching and active movement rehabilitation of lower-limb impairments in children with cerebral palsy using a portable robot. Neurorehabilitation and neural repair. 2011;25(4):378–85. doi: 10.1177/1545968310388666. [DOI] [PubMed] [Google Scholar]
- 14.Palisano RJ, Hanna SE, Rosenbaum PL, Russell DJ, Walter SD, Wood EP, et al. Validation of a model of gross motor function for children with cerebral palsy. Physical therapy. 2000;80(10):974–85. [PubMed] [Google Scholar]
- 15.Fowler EG, Staudt LA, Greenberg MB, Oppenheim WL. Selective Control Assessment of the Lower Extremity (SCALE): development, validation, and interrater reliability of a clinical tool for patients with cerebral palsy. Developmental medicine and child neurology. 2009;51(8):607–14. doi: 10.1111/j.1469-8749.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 16.Mutlu A, Livanelioglu A, Gunel MK. Reliability of goniometric measurements in children with spastic cerebral palsy. Medical science monitor : international medical journal of experimental and clinical research. 2007;13(7):CR323–9. [PubMed] [Google Scholar]
- 17.Chen CL, Shen IH, Chen CY, Wu CY, Liu WY, Chung CY. Validity, responsiveness, minimal detectable change, and minimal clinically important change of Pediatric Balance Scale in children with cerebral palsy. Research in developmental disabilities. 2013;34(3):916–22. doi: 10.1016/j.ridd.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Thompson P, Beath T, Bell J, Jacobson G, Phair T, Salbach NM, et al. Test-retest reliability of the 10-metre fast walk test and 6-minute walk test in ambulatory school-aged children with cerebral palsy. Developmental medicine and child neurology. 2008;50(5):370–6. doi: 10.1111/j.1469-8749.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- 19.Katalinic OM, Harvey LA, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane database of systematic reviews (Online) 2010;(9):CD007455. doi: 10.1002/14651858.CD007455.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Wallen M, Stewart K. The evidence for abandoning upper limb stretch interventions in paediatric practice. Developmental medicine and child neurology. 2013;55(3):208–9. doi: 10.1111/dmcn.12000. [DOI] [PubMed] [Google Scholar]
- 21.Pin T, Dyke P, Chan M. The effectiveness of passive stretching in children with cerebral palsy. Developmental medicine and child neurology. 2006;48(10):855–62. doi: 10.1017/S0012162206001836. [DOI] [PubMed] [Google Scholar]
- 22.Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P, et al. The evidencebase for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the International Classification of Functioning, Disability and Health as a conceptual framework. Journal of rehabilitation medicine : official journal of the UEMS European Board of Physical and Rehabilitation Medicine. 2012;44(5):385–95. doi: 10.2340/16501977-0983. [DOI] [PubMed] [Google Scholar]
- 23.Gorter JW, Becher J, Oosterom I, Pin T, Dyke P, Chan M, et al. To stretch or not to stretch in children with cerebral palsy. Developmental medicine and child neurology. 2007;49(10):797–800. doi: 10.1111/j.1469-8749.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Physical therapy. 2005;85(11):1208–23. [PubMed] [Google Scholar]
- 25.Sandlund M, Dock K, Hager CK, Waterworth EL. Motion interactive video games in home training for children with cerebral palsy: parents’ perceptions. Disability and rehabilitation. 2012;34(11):925–33. doi: 10.3109/09638288.2011.626489. [DOI] [PubMed] [Google Scholar]
- 26.Snapp-Childs W, Casserly E, Mon-Williams M, Bingham GP. Active prospective control is required for effective sensorimotor learning. PloS one. 2013;8(10):e77609. doi: 10.1371/journal.pone.0077609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fasoli SE, Ladenheim B, Mast J, Krebs HI. New horizons for robot-assisted therapy in pediatrics. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2012;91(11 Suppl 3):S280–9. doi: 10.1097/PHM.0b013e31826bcff4. [DOI] [PubMed] [Google Scholar]
- 28.Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Developmental medicine and child neurology. 2010;52(3):264–9. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 29.Grubich S, Trenkle J. Passive stretching and active strengthening through use of robotics, combined with functional strength training, to improve gross motor ability in children diagnosed with cerebral palsy: a clinical approach; Proceedings of the American Academy of Cerebral Palsy and Developmental Medicine; Milwaukee, WI. 2013. [Google Scholar]
- 30.Grubich S, Trenkle J. Passive stretching and active strengthening through use of robotics, combined with Functional strength training, to improve gross motor ability in children diagnosed with cerebral Palsy: a clinical approach; Proceedings of the 67th Annual Meeting of the AACPDM; Milwaukee. 2013. [Google Scholar]