Abstract
Solid tumors typically develop hostile microenvironments characterized by irregular vascularization and poor oxygen (O2) and nutrient supply. While normal cells modulate anabolic and catabolic pathways in response to changes in nutrient availability, cancer cells exhibit unregulated growth even under nutrient scarcity. Recent studies have demonstrated that constitutive activation of growth-promoting pathways results in a dependence on unsaturated fatty acids for survival under O2 deprivation. In cancer cells, this dependence represents a critical metabolic vulnerability that could be exploited therapeutically. Here we review how this dependence on unsaturated lipids is affected by the microenvironmental conditions faced by cancer cells.
Keywords: unsaturated lipids, hypoxia, metabolism, SCD1, ER stress
Metabolic and microenvironmental challenges affecting cancer cells
Oncogenic events, such as the loss of tumor suppressors or activation of oncogenes, imbue cancer cells with a dysregulated growth rate, leading to uncontrolled proliferation. Consequently, solid tumors can quickly outgrow existing vasculature and experience decreased access to blood-borne nutrients and O2. While the induction of angiogenesis is common during cancer progression, the resulting tumor blood vessels are often disorganized and leaky. Despite their vascularity, many solid tumors exhibit high levels of tissue hypoxia [5], which can increase cellular reactive oxygen species and cause endoplasmic reticulum (ER) stress. In addition to hypoxia, abnormal blood vessels limit the delivery of blood-borne nutrients to tumor cells [6-8]. While normal cells can adapt to these circumstances by adjusting their rate of proliferation, neoplastic transformation disrupts the signaling pathways that control cell division and growth. Cancer cells therefore confront the compound challenges of high growth rates and limited and unreliable supply of O2 and nutrients. Recent studies suggest that the metabolic challenges of malignant growth lead to vulnerabilities associated with discontinuous nutrient supply. The results of these investigations could reveal new treatment strategies for targeting poorly vascularized tumor microenvironments.
Compared to non-malignant cells, cancer cells exhibit significant metabolic alterations with respect to several critical nutrients and substrates, including important changes in the metabolism of both glucose and glutamine [12]. Cancer cells also exhibit increased demand for fatty acids, which are derived endogenously from citrate or taken up from exogenous sources. The elevated rates of lipid synthesis occur through increased expression of various lipogenic enzymes. There is ample evidence that increased lipid production is critical for cancer cell survival, while expression of a central lipogenic enzyme, fatty acid synthase (FASN), is strongly correlated with cancer progression [13, 14]. Fatty acids can be incorporated into membranes as phospholipids, stored in lipid droplets and used for the production of signaling lipids. In prostate tumors, which are known to import less glucose than other tumor types [15], ß-oxidation of FAs may be an important alternative energy source [16, 17].
Although the requirement for fatty acids is mostly met by synthesis from glucose-derived carbon, fatty acid uptake can also be an important source of lipids in some settings. To obtain free fatty acids from blood, triglycerides in circulating chylomicrons and very low-density lipoprotein particles are hydrolyzed to fatty acids by lipoprotein lipase (LPL) and then imported via the fatty acid channel protein CD36. Both are widely expressed in clinical breast, liposarcoma and prostate tumor samples [19], indicating that cancer cells may obtain fatty acids from circulating, diet-derived lipoprotein particles. In vitro studies demonstrate that cancer cells can utilize these exogenous lipids and in some cases depend on exogenous lipid for survival [13]. The critical role of lipids in cancer cell proliferation has led to a number of proposed strategies for treating cancer through inhibiting lipid availability [20].
Recent studies have identified unsaturated lipid deprivation as an important challenge for rapidly dividing cancer cells. In this review, we focus on mechanisms that may underlie this vulnerability and discuss the role of different lipid species in mediating this phenotype.
Cancer, ER stress and lipids
To satisfy the requirements of doubling biomass for rapid cell division, cancer cells increase macromolecular synthesis through oncogenic mutations in numerous signaling pathways. One pathway commonly activated in cancer is regulated by the serine/threonine kinase complex mTORC1 [21]. mTORC1 contributes to unrestrained proliferation through its effects on ribosome biogenesis, protein synthesis and lipogenesis via numerous downstream effectors [22, 23]. While mTORC1 activation stimulates growth, hyperactivation of mTORC1 can have negative effects on cell function [24]. In mouse embryonic fibroblasts (MEFs), constitutive mTORC1 activity can be modelled through depletion of the TSC complex, which negatively regulates mTORC1. Loss of either TSC1 or TSC2 leads to an enhanced growth rate, increased protein synthesis and numerous other changes in macromolecular biogenesis. However, elevated rates of mRNA translation also increase ER unfolded protein load, which leads to ER stress and activation of the unfolded protein response (UPR; see Box 1) [24].
Whether the high growth rate of cancer cells affects their survival under complex tumor microenvironmental conditions is central to the study of cancer metabolism. Recently, highly proliferative MEFs exhibiting mTORC1 dysregulation (Tsc2−/−, p53−/−) and grown under tumor-like conditions of serum and O2 deprivation were found to undergo programmed cell death due to a specific deficiency in unsaturated lipid [25]. Indeed, the requirement for unsaturated lipids applies to multiple cancer cell lines and is supported by in vivo data in Tsc2−/− mouse kidney cystic adenomas [22]. This cell death occurred because the desaturation of de novo synthesized lipids by steoryl-coA desaturases, such as SCD1, requires O2. O2 deprivation inhibits this enzymatic reaction, rendering cells dependent on exogenous unsaturated lipids. Restricting the supply of exogenous lipid to hypoxic cells therefore leads to a critical unsaturated lipid deficiency and causes cell death by eliciting ER stress and activating the UPR. UPR-mediated cell death under these conditions is dependent on mTORC1 and is likely caused by an mTORC1-driven increase in protein synthesis [24, 25]. IRE1α is required for apoptosis upon unsaturated lipid deprivation in Tsc2−/− MEFs, indicating that decreased cell survival is a consequence of terminal UPR signaling. Taken together, these findings suggest that proliferating cells need to balance their growth rate and unsaturated lipid availability to prevent ER stress, terminal UPR activation and cell death.
SCD1-mediated lipid desaturation was also found to be a critical determinant of cancer cell survival downstream of SREBP transcription factors [26]. SREBPs have important roles in regulating lipid metabolism. When activated, they induce expression of a lipogenic program thought to play a critical role in cancer cell metabolism [22, 27]. SREBPs are regulated by mTORC1 [22, 28] and thus may be essential effectors of its role in promoting cancer cell growth. SREBP-mediated lipid synthesis is elevated in human glioblastoma multiforme (GBM), for example, and loss of SREBP and lipid synthesis blocks growth of glioblastoma cells in xenograft models [26, 27, 29]. SREBP inhibition results in decreased cellular unsaturated lipid levels and cell death when exogenous lipid supplies are limited [26]. This phenotype can be rescued by addition of unsaturated oleic acid or by re-expressing SCD1 [26, 29], indicating that the effects of SREBP loss are attributable to the regulation of SCD1 expression by SREBP. SREBP ablation is also accompanied by significant ER stress and activation of the IRE1α and PERK branches of the UPR. Moreover, UPR induction is abrogated by the addition of exogenous unsaturated lipids. These data confirm that loss of SCD1 activity, which can occur through hypoxia or loss of Scd1 gene expression after SREBP inhibition, can lead to cancer cell death by induction of ER stress.
If the proliferation of cancer cells occupying hypoxic tumor domains is limited by a lack of unsaturated lipid, then understanding how cancer cells overcome this limitation would likely open up new therapeutic strategies. A recently published study [30] found that hypoxic cells exhibit increased uptake of unsaturated lipid from their environment, thus bypassing the requirement for fatty acid desaturation. Cancer cell lines with activating mutations in Ras also elevate fatty acid uptake, possibly making them more independent of their O2 supply. Using Ras-driven human cancer cells and immortalized baby mouse kidney (iBMK) epithelial cells, it was found that oncogenic Ras renders cells less sensitive to SCD1 inhibition compared to other oncogenes, presumably through elevating lipid import. LC-MS analysis of lipid species in spent media identified lysophospholipids as the predominant lipid form taken up by these cells. Details of mechanisms for uptake of unsaturated lipid are currently unknown and are an area of active research.
These recent lines of investigation paint an interesting picture of cancer cells’ metabolic dependence on their microenvironment (see Figure 1). A normal cell's capacity to regulate its growth rate in response to nutrient availability and retain a pool of unsaturated lipid allows it to maintain homeostasis and avoid ER stress. In contrast, a cancer cell's dysregulated growth rate requires high levels of protein synthesis, creating a metabolic requirement for unsaturated lipid, much like that of Tsc2−/− MEFs. As a tumor grows and outstrips its O2 supply, its source of unsaturated lipid shifts from endogenous desaturation to uptake from the microenvironment. If exogenous unsaturated lipids also become limited, cells experience ER stress and activation of the terminal UPR, ultimately causing cell death. Evidence suggests that Ras-driven cells are more able to import lipid from media than Akt-driven cells, demonstrating that different oncogenic events can alter a tumor cell's ability to survive under harsh microenvironmental conditions [30].
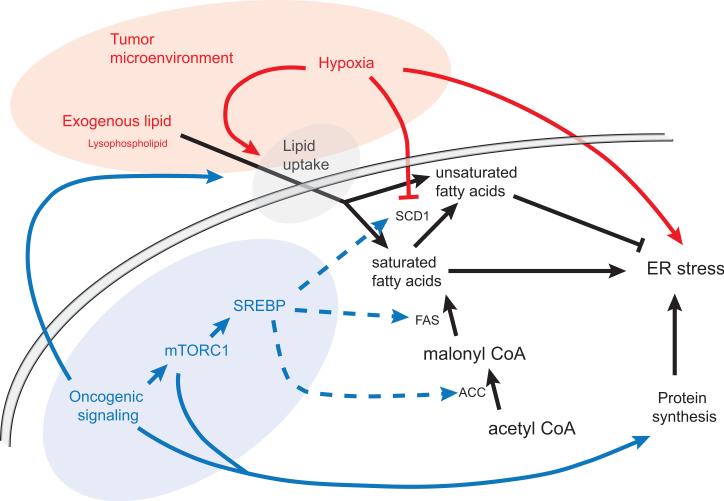
Figure 1.
Unsaturated lipids can be acquired by desaturation of de novo synthesized fatty acids or by uptake of exogenous lipid. Saturated fatty acids have been shown to promote ER stress while unsaturated fatty acids counteract the effect of saturated lipid. The tumor microenvironment can contribute to ER stress by inhibiting optimal protein folding and SCD1 desaturase activity. Hypoxia can also increase lipid uptake, which counteracts the effects of SCD1 inhibition. Oncogenic signaling increases fatty acid synthesis and protein synthesis. Elevated rates of protein synthesis have been shown to increase ER stress. Oncogenic changes, such as activating Ras mutations, also promote lipid uptake.
SCD1: stearoyl-Coenzyme A desaturase 1
FAS: fatty acid synthase
ACC: acetyl-CoA carboxylase
Effects of the ratio of saturated to unsaturated phospholipids on ER stress
Changes in cellular lipid composition, such as the accumulation of free cholesterol in the ER [31] and exogenous addition of saturated fatty acids [32, 33], have previously been shown to induce ER stress and trigger the UPR. In particular, the effect of changes in the saturated:unsaturated lipid ratio on cell function has been investigated extensively due to the role of lipotoxic saturated fatty acids in diabetes and non-alcoholic fatty liver disease [34-36].
Preventing ER stress appears to require balancing saturated and unsaturated lipid species, since the lipotoxic effects of saturated lipids can often be reversed through the addition of unsaturated lipids. Shifting the cellular lipid profile towards saturated lipids by administration of saturated lipid or loss of desaturase activity causes ER stress and cell death [37-39]. In vivo models of hepatic steatosis exhibit a similar relationship between saturated lipid uptake, apoptosis [40] and ER stress [41]. ER stress caused by lipid saturation can be ameliorated or rescued by addition of exogenous, unsaturated lipid or by overexpressing SCD1. ER stress following administration of saturated lipids is known to lead to insulin resistance [42] and can also be overcome by addition of unsaturated fatty acids [39, 43, 44]. Even dietary changes in levels of unsaturated lipid consumption have been shown to improve insulin sensitivity in humans [45-47]. Studies on the effects of lipid saturation on insulin resistance therefore support the view that ER homeostasis requires the correct balance of unsaturated to saturated lipids.
Although a link between the ratio of saturated to unsaturated lipid and ER stress has been shown, identifying the lipid species and cellular compartments that are critical for this effect is more difficult. Recent work supports the theory that lipid-induced ER stress is caused by disruption of ER structure by over-incorporation of saturated phospholipids into the ER membrane [37, 39]. Administering oleic acid may reverse this process by shifting the ratio back towards unsaturated phospholipids. The composition of phospholipid in the ER membrane affects membrane fluidity and the assembly of membrane subdomains [48]. Studies on LPCAT3, an enzyme capable of altering the composition of the ER membrane, support the view that changes in phospholipid saturation can trigger ER stress. The saturation of the phospholipid pool can be influenced by several factors, such as the degree of saturation of the cellular fatty acid pool. Additionally, the Lands cycle is capable of remodeling membrane composition through fatty acyl deacylation and reacylation. LPCAT3 contributes an increased unsaturated phospholipid fraction within membranes by preferentially incorporating arachidonic and linoleic acid into phospholipids. Recent work has shown that loss of LPCAT3 leads to increased phospholipid saturation and elevated ER stress. LPCAT3 inhibition in HeLa cells sensitizes them to UPR activation and cell death under these circumstances [38]. The transcription factor LXR regulates both LPCAT3 and SCD1 and plays an important role in maintaining membrane fluidity and homeostasis through a number of downstream processes [48].
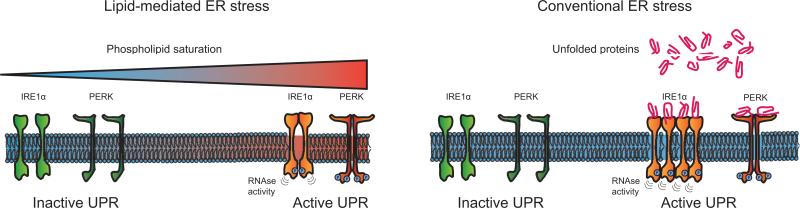
In summary, there is growing evidence that an unsaturated lipid pool is necessary for the maintenance of cell viability, especially in cells with high rates of protein synthesis, because of the essential role of these lipids in maintaining ER homeostasis by counteracting the effects of saturated lipids (see Figure 2). That ER stress occurs because of a shift in phospholipid saturation specifically is supported by the effects of LPCAT3 loss; however, an additional role for unsaturated fatty acids in preventing ER stress cannot be excluded.
Figure 2.
Increases in phospholipid saturation have been shown to activate the IRE1α and PERK branches of the UPR. The mechanisms of activation by lipid saturation are distinct from those triggered by unfolded protein accumulation. Conventional ER stress triggers IRE1α and PERK through either direct binding of unfolded proteins to the luminal domains of the sensors or through their interaction with the BiP chaperone protein [9]. While UPR activation by increased unfolded protein load occurs via the luminal domains of IREα and PERK, these domains are not required for activation by elevated lipid saturation. In addition, IRE1α activation by saturated lipids does not lead to the characteristic formation of IRE1α “foci” indicating that lipid-induced IRE1α activation does not lead to oligomerization.
Sensing ER membrane lipid saturation: A role for IRE1α and PERK
ER stress can be a consequence of high levels of protein synthesis in malignant cells and also exacerbated by a hypoxic tumor microenvironment. Hypoxia has long been known to cause ER stress, possibly due to the elevated levels of reactive oxygen species in hypoxic cells and the role of O2 as the terminal electron acceptor for disulphide bond formation [49]. During conventional ER stress, three primary UPR sensors detect the accumulation of unfolded proteins via direct binding to their ER-luminal domains or through binding of the chaperone protein Grp78.
Furthermore, changes in phospholipid composition of ER membranes have been shown to trigger the UPR. Indeed, electron microscopy of Tsc2−/− MEFs demonstrates a physical distension of the ER during unsaturated lipid deprivation [25], which is often accompanied by engaging the IRE1α and PERK branches of the UPR. Mechanisms of IRE1α and PERK activation through changes in membrane lipid composition are currently being elucidated [48] and appear to be distinct from UPR activation through misfolded proteins.
Several lines of investigation indicate that lipid-induced and conventional ER stress trigger the UPR through separate mechanisms. Fluorescent labeling of IRE1α during ER stress also reveals clear differences in IRE1α activation. Under conventional ER stress, fluorescently labeled IRE1α proteins aggregate in distinct foci in the ER, but these are not visible under lipid-induced ER stress [50]. Furthermore, it was found that the luminal domains of IRE1α and PERK, essential for the detection of misfolded proteins, are mostly dispensable for activation by saturated lipids [51, 52]. These data indicate that IRE1α and PERK are capable of directly sensing biophysical changes in the membrane following changes in saturation. How this is achieved is unclear, although several theories have been postulated. For example, changes in membrane saturation could affect the proteins’ propensity to homodimerize and autophosphorylate or change the tilting angle of transmembrane protein domains, thus leading to changes in activity. Alternatively, some of the known interacting partners of IRE1α may play a role in sensing membrane properties [52].
Persistent ER stress triggers a “terminal” UPR that leads to cell death via several pathways. The question of how ER stress downstream of unsaturated lipid deprivation causes cell death has not been resolved and the answer is likely to be condition-dependent. For example, in non-alcoholic liver disease models, JNK was found to be essential for cell death, but CHOP is not required for saturated fatty acid-dependent apoptosis [34]. In contrast, JNK appears to be dispensable in mTORC1-driven MEFs, where apoptosis was caused by IRE1α activation [25]. Conversely, HeLa cell death from SCD1 loss is suppressed by PERK knockdown and unaffected by IRE1α loss [38]. Increased lipid saturation through SCD1 and SREBP inhibition has also been shown to elevate reactive oxygen species (ROS) levels [29], which can also promote cell death. The source of ROS under lipid-induced stress is unclear: unsaturated lipid deprivation may cause mitochondrial dysfunction, which can lead to ROS generation and ER stress. Alternatively ER stress can lead to oxidative damage directly through release of ROS from the oxidizing environment of the ER lumen [53]. How the mechanisms of a terminal UPR differ and how saturated fatty acid-dependent apoptosis is induced in distinct cell types during variable conditions still needs to be more thoroughly investigated. While there is reason to believe that changes in lipid saturation affect ER stress through changes in ER phospholipid saturation, the involvement of other organelles, such as mitochondria, should also be explored.
Supplying cancer cells with exogenous lipid: Microenvironmental and dietary factors
As evidence accumulates that cancer cells, especially those driven by oncogenic Ras, benefit from exogenously supplied lipids, it remains to be determined how circulating lipids affect tumor growth. Understanding the extent to which cancer cells gain access to unsaturated lipid species from their microenvironment could have therapeutic implications. Adipocytes are a major component of tumor microenvironments [54] and adipocyte-secreted factors have been shown to enhance cell motility and promote expression of genes associated with a more aggressive phenotype, although the exact nature of these secreted factors is under dispute [54, 55]. Adipocyte-derived lipids appear to impact cancer progression in ovarian cancer metastases to the omentum [56]. Metastasis to this site occurs at least partly because adipocytes secrete cytokines that attract cancer cells. Through unknown mechanisms, the malignant cells then cause adipocytes to increase lipolysis and secrete fatty acids that are taken up by the cancer cells to be used for energy production. This work showed that omental adipocytes transfer lipids to ovarian cancer cells and thus promote tumor growth. Moreover, the fatty acid binding protein FABP4 was found to play a critical role in the transfer of lipids and in metastasis in a murine ovarian cancer model.
These data suggest that cancer cells are capable of inducing stromal cells to modify their metabolism and secrete lipid species that are subsequently imported to promote tumor growth. Such a model is reminiscent of cachexia, in which patients with advanced stages of cancer exhibit adipose tissue atrophy. Adipocytes undergo increased lipolysis in cachexic patients and release more fatty acids, which may be triggered by tumor-derived factors [57-61]. The importance of exogenous lipid availability to tumor growth suggests that broader questions of diet and obesity could influence cancer cell metabolism by altering the availability of lipid species to cancer cells. Obesity has long been known to increase the risk of some cancer types, with up to 5% of cancer incidence being attributable to being overweight [62-64]. Whether this increase in cancer risk can be partially explained through lipid availability remains to be seen, as obesity not only affects circulating lipid levels, but is also associated with hormonal changes, cytokine release and inflammation [54].
Concluding remarks
Reflecting the lipogenic nature of many cancers as well as the need for unsaturated lipid in cancer cell proliferation, SCD1 expression levels are frequently elevated in cancer [65, 66]. Several studies on the effects of SCD1 inhibition on xenograft tumor growth have found a modest growth decrease in ccRCC (clear cell renal cell carcinoma) cells [67, 68], gastric carcinoma cells [69] and colon cancer cells [70]. In vitro, SCD1 inhibition leads to induction of ER stress and apoptosis in multiple cell lines [67, 69], which is an expected consequence of increased lipid pool saturation. As predicted, given that unsaturated lipid deprivation has its most pronounced effect on cells that exhibit dysregulated growth [25], SCD1 inhibition has been found to affect cancer cells, but not normal cells in several cases [67, 71]. That SCD1 inhibition fails to have larger effects on tumor growth is not surprising given cancer cells’ ability to take up unsaturated lipid from their microenvironment [30].
While it is established that tumor cells fulfill their ravenous demand for glucose by increasing the expression of surface glucose transporters, recent work has identified macropinocytosis as another route by which nutrient uptake is enhanced in cancer cells. During this process, engulfed extracellular material is internalized in macropinosome vesicles and degraded by the cell's lysosmal compartment. It was recently demonstrated that oncogenes such as Ras and Src increase macropinocytotic rates and that uptake of soluble extracellular protein by cancer cells fulfills a metabolic need for amino acids [72]. Inhibition of macropinocytosis reduces the growth of Ras-driven human pancreatic cancer cell xenograft tumors, demonstrating that this uptake route is an important nutrient source for some cancers [72]. Ras-driven cells have been described as possessing a “scavenging” phenotype and their increased import of lysophospholipids has been shown to render them less sensitive to SCD1 inhibition [30]. It remains to be determined whether Ras-driven cells’ increased rate of macropinocytosis also explains their increased lipid uptake or whether this route is reserved for protein transport.
The studies discussed here suggest the possibility that unsaturated lipid deprivation could be used as a therapeutic intervention to target cancer cells, since dysregulated growth sensitizes cells to terminal UPR pathways. On its own, SCD1 inhibition is unlikely to be effective, as exogenous lipid uptake renders cells resistant to SCD1 inhibition. Delineating the dynamics of lipid uptake will likely open novel therapeutic options for exploiting cancer cells’ dependence on exogenous unsaturated lipid. This approach could be used to target hypoxic tumor domains or used in combination with available SCD1 inhibitors to cause cell death from ER stress due to unsaturated lipid deprivation. A better understanding of the mechanisms by which ER stress and UPR activation are elicited and how they trigger cell death during unsaturated lipid deprivation could also provide important new strategies to sensitize cancer cells to this type of stress.
Box 1: The unfolded protein response (UPR).
The unfolded protein response is a highly conserved signaling program that monitors ER stress and resolves it by decreasing mRNA translation, triggering an expansion of ER membrane, increasing protein degradation and enhancing protein folding capacity. These responses are mediated by three separate pathways and initiated by separate sensors (ATF6, PERK and IRE1α) that reside in the ER membrane and detect accumulation of misfolded and under-glycosylated proteins. ER stress activates ATF6 by causing this transmembrane protein to be transported to the Golgi apparatus in vesicles where it is cleaved by two proteases. The remaining cytosolic fragment enters the nucleus following cleavage and alters gene expression. PERK engagement by ER stress occurs by oligomerization and autophosphorylation. Once active, it phosphorylates eIF2α and inhibits mRNA translation, thus reducing ER protein load. PERK stimulation also leads to the preferential translation of specific proteins, including the transcription factor ATF4, a critical mediator of PERK activity. The bifunctional transmembrane protein IRE1α acts as the third branch of the UPR. Like PERK, detection of unfolded protein leads to autophosphorylation and oligomeryzation. IRE1α exerts much of its effects via RNAse activity (which is capable of degrading mRNAs to reduce peptide synthesis [1]), splicing specific mRNAs [2], and degrading miRNAs [3, 4]. A key component of the IRE1α controlled response is the transcription factor XBP1. Active IRE1α removes an exon from Xbp1 mRNA leading to the accumulation of a more stable XBP1s protein under ER stress. XBP1s enhances the expression of proteins that help improve ER capacity [9]. IRE1α also interacts with cytosolic proteins, such as ASK1 and TRAF2 to modulate p38MAPK and JNK activation and also impacts the ERK and NF-kB pathways. While mild ER stress typically elicits a cytoprotective UPR response, persistent ER stress can induce the UPR to initiate programmed cell death [10]. PERK activation triggers cell death by stimulating the expression of the pro-apoptotic C/EBP-homologous protein (CHOP) [11]. IRE1α can also trigger cell death by JNK activation via TRAF2 recruitment and ASK1 phosphorylation. Once activated, JNK induces apoptosis via phosphorylation of various Bcl-2 protein family members and modulation of their pro-apoptotic and anti-apoptotic activity in favor of cell death [18].In addition to its kinase activity, IRE1α nuclease activity can trigger cell death. The process of regulated IRE1-dependent decay of mRNAs (RIDD), leads to the degradation of mRNA and the reduction of ER-protein burden. RIDD initially helps resolve ER-stress, and can promote cell death under irremediable conditions. IRE1α activity can also induce apoptosis relieving inhibition of pro-apoptotic factors by degradation of key miRNAs [3, 4]. Cleavage of miRNAs occurs at sites that are distinct from DICER sites as well as sites of IRE1α cleavage in Xbp1 mRNA. IRE1α thus plays an integral role in committing the cell to an apoptotic program under persistent and irremediable ER-stress.
Highlights.
Cancer cells inhabit a challenging microenvironment
Malignant cells do not adjust their rate of proliferation in response to nutrient availability
Rapid growth in the context of limited nutrients and oxygen results in metabolic challenges
These challenges create metabolic vulnerabilities for cancer cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, et al. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 3.Upton JP, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerner AG, et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell metabolism. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill FR, et al. The tumor microenvironment at a glance. Journal of cell science. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 6.Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiological reviews. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaupel P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Experimental oncology. 2010;32:125–127. [PubMed] [Google Scholar]
- 8.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer research. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 9.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 10.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nature reviews. Molecular cell biology. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 11.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature cell biology. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cairns RA, et al. Regulation of cancer cell metabolism. Nature reviews. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 13.Zaidi N, et al. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52:585–589. doi: 10.1016/j.plipres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 15.Price DT, et al. Comparison of [18 F]fluorocholine and [18 F]fluorodeoxyglucose for positron emission tomography of androgen dependent and androgen independent prostate cancer. The Journal of urology. 2002;168:273–280. [PubMed] [Google Scholar]
- 16.Liu Y, et al. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30:369–374. [PubMed] [Google Scholar]
- 17.Zha S, et al. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. The Prostate. 2005;63:316–323. doi: 10.1002/pros.20177. [DOI] [PubMed] [Google Scholar]
- 18.Sovolyova N, et al. Stressed to death - mechanisms of ER stress-induced cell death. Biological chemistry. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 19.Kuemmerle NB, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Molecular cancer therapeutics. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Currie E, et al. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duvel K, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. Journal of cell science. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozcan U, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Molecular cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young RM, et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes & development. 2013;27:1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths B, et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer & metabolism. 2013;1:3. doi: 10.1186/2049-3002-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo D, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Science signaling. 2009;2:ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams KJ, et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer research. 2013;73:2850–2862. doi: 10.1158/0008-5472.CAN-13-0382-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamphorst JJ, et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature cell biology. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 32.Cunha DA, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. Journal of cell science. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu S, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leamy AK, et al. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Progress in lipid research. 2013;52:165–174. doi: 10.1016/j.plipres.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nature reviews. Molecular cell biology. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 36.Giacca A, et al. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. American journal of physiology. Endocrinology and metabolism. 2011;300:E255–262. doi: 10.1152/ajpendo.00416.2010. [DOI] [PubMed] [Google Scholar]
- 37.Borradaile NM, et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. Journal of lipid research. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Ariyama H, et al. Decrease in membrane phospholipid unsaturation induces unfolded protein response. The Journal of biological chemistry. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng G, et al. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology. 2011;152:2206–2218. doi: 10.1210/en.2010-1369. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, et al. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. The Journal of nutrition. 2008;138:1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, et al. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, et al. ER stress potentiates insulin resistance through PERK-mediated FOXO phosphorylation. Genes & development. 2013;27:441–449. doi: 10.1101/gad.201731.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coll T, et al. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. The Journal of biological chemistry. 2008;283:11107–11116. doi: 10.1074/jbc.M708700200. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura S, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. The Journal of biological chemistry. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan M, et al. Diabetes and the Mediterranean diet: a beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM : monthly journal of the Association of Physicians. 2000;93:85–91. doi: 10.1093/qjmed/93.2.85. [DOI] [PubMed] [Google Scholar]
- 46.Serra-Majem L, et al. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutrition reviews. 2006;64:S27–47. doi: 10.1111/j.1753-4887.2006.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 47.Vessby B, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44:312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 48.Rong X, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell metabolism. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu BP, Weissman JS. The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Molecular cell. 2002;10:983–994. doi: 10.1016/s1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- 50.Kitai Y, et al. Membrane lipid saturation activates IRE1alpha without inducing clustering. Genes to cells : devoted to molecular & cellular mechanisms. 2013;18:798–809. doi: 10.1111/gtc.12074. [DOI] [PubMed] [Google Scholar]
- 51.Promlek T, et al. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Molecular biology of the cell. 2011;22:3520–3532. doi: 10.1091/mbc.E11-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volmer R, et al. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 54.Nieman KM, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochimica et biophysica acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Experimental and molecular pathology. 2012;92:312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donohoe CL, et al. Cancer cachexia: mechanisms and clinical implications. Gastroenterology research and practice. 2011;2011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islam-Ali B, et al. Modulation of adipocyte G-protein expression in cancer cachexia by a lipid mobilizing factor (LMF). British journal of cancer. 2001;85:758–763. doi: 10.1054/bjoc.2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agustsson T, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer research. 2007;67:5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- 60.Arner P. Medicine. Lipases in cachexia. Science. 2011;333:163–164. doi: 10.1126/science.1209418. [DOI] [PubMed] [Google Scholar]
- 61.Das SK, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 62.Renehan AG, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 63.Reeves GK, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedenreich CM, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 65.Yahagi N, et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005;41:1316–1322. doi: 10.1016/j.ejca.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 66.Li J, et al. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. International journal of cancer. Journal international du cancer. 1994;57:348–352. doi: 10.1002/ijc.2910570310. [DOI] [PubMed] [Google Scholar]
- 67.von Roemeling CA, et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:2368–2380. doi: 10.1158/1078-0432.CCR-12-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung JY, Kim WY. Stearoyl co-A desaturase 1 as a ccRCC therapeutic target: death by stress. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:3111–3113. doi: 10.1158/1078-0432.CCR-13-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roongta UV, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Molecular cancer research : MCR. 2011;9:1551–1561. doi: 10.1158/1541-7786.MCR-11-0126. [DOI] [PubMed] [Google Scholar]
- 70.Mason P, et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PloS one. 2012;7:e33823. doi: 10.1371/journal.pone.0033823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hess D, et al. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PloS one. 2010;5:e11394. doi: 10.1371/journal.pone.0011394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]