Abstract
Studies of brain connectivity have focused on two modes of networks: structural networks describing neuroanatomy and the intrinsic and evoked dependencies of functional networks at rest and during tasks. Each mode constrains and shapes the other across multiple time scales, and each also shows age-related changes. Here we argue that understanding how brains change across development requires understanding the interplay between behavior and brain networks: changing bodies and activities modify the statistics of inputs to the brain; these changing inputs mold brain networks; these networks, in turn, promote further change in behavior and input.
Keywords: development, brain networks, connectivity, embodiment, dynamic system
Human cognition and behavior emerge from dynamic neural activity that unfolds within distributed structural and functional brain networks [Box 1; 1–2]. There has been an expansion of studies examining age-related changes in these networks [3–4] that set the stage for the critical next step: understanding the processes through which brain networks at one age turns into brain networks at a later age. Both theory and data strongly implicate changing brain connectivity as both cause and consequence of developmental changes in behavior [5–8]; accordingly, an understanding of development requires viewing brain networks as part of larger systems of dynamically interwoven processes that extend from the brain through the body into the world [9–11].
Box 1: Structural and functional networks.
Structural networks refer to the set of anatomical connections linking distinct cortical and subcortical brain regions, such as the arcuate fasciculus that links temporal regions to the inferior frontal gyrus. Functional networks refer to the set of connections among brain regions that are derived from statistical dependencies among their temporal patterns of neural activity observed during tasks and during rest. For example, during reading, when left inferior occipitotemporal regions are active, temporally correlated evoked activity is also observed in left posterior superior temporal cortex and in left inferior frontal gyrus [63]. These regions thus form part of a reading functional network; parts of this reading network also participate in functional networks for spoken language [63, 68]. In addition to such task-evoked functional networks, intrinsic (or resting-state) functional networks are derived from spontaneous neural activity when no specific task is being performed.
Brain networks are dynamic. Structural networks are relatively stable but can change gradually over longer timescales of days or weeks, due to changes in myelination and other axonal properties [28]. Functional networks capture statistical dependencies and can be measured over various time intervals. Measured over short intervals from milliseconds to seconds, functional networks undergo continual change, reflecting spontaneous and task-evoked fluctuations of neural activity [25, 89]. Over longer time intervals of several minutes, functional networks exhibit robustly stable features across and within individuals even at rest [90] that are thought to reflect the brain’s intrinsic functional architecture [2,12,13]. Nonetheless, these stable features of functional networks can also change over longer timescales, in response to changes in sensory input or behavior [24–27, 29, 30, 73]. A main point of this paper is that structural and functional networks interact on multiple time scales, mutually shaping and constraining one another within the brain on short time scales, while both generating and being modulated by patterns of behavior and learning on long time scales.
Here, we propose a network-based account of developmental process in terms of the nested dependencies and interdependent time scales of change within structural and functional brain networks (Fig. 1). This interplay between functional and structural networks provides the basis for a developmental perspective that explicitly views brain networks as extending from the brain into the sensorimotor environment: brain-body-behavior networks actively select and create information that in turn modifies the brain’s own internal structure and dynamics. These ideas have consequences for understanding behavioral development, neurodevelopmental disorders, atypical developmental trajectories, and education.
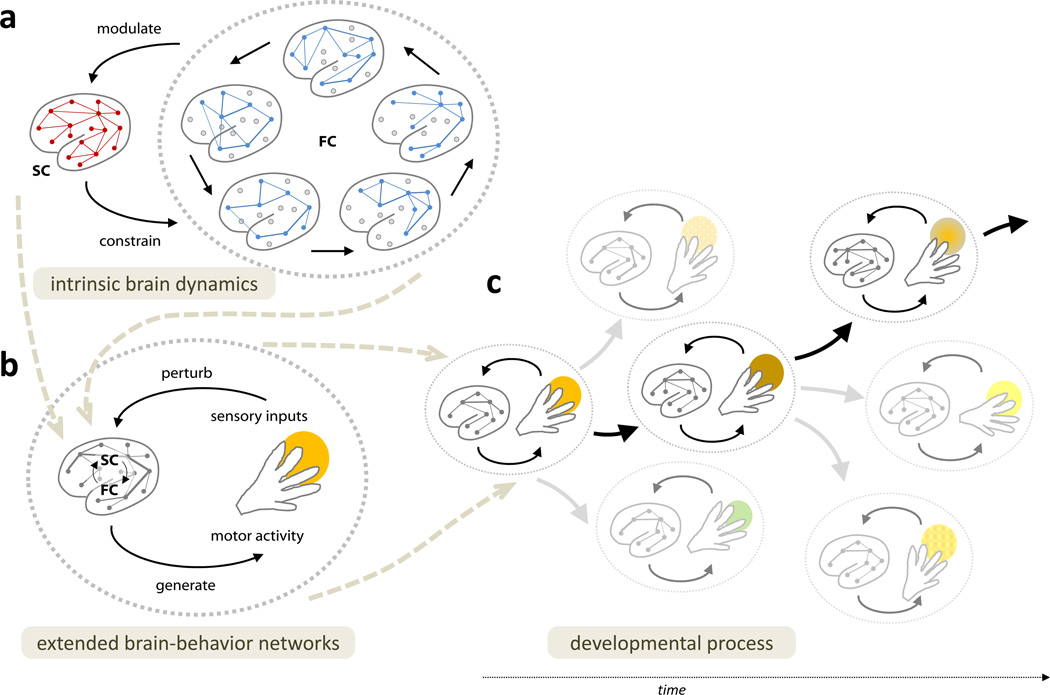
Figure 1.
Extended brain-body-behavior networks mutually shape and constrain one another across time scales, with developmental process emerging from these multi-scale interactions. Figure 1A: Within the brain, intrinsic functional networks (FC; blue nodes and edges) fluctuate and evolve over fast time scales. FC networks are constrained by structural connectivity (SC; red nodes and edges) which they in turn modulate over longer time scales. Figure 1B: Behavior extends brain networks into the world by selecting inputs that perturb the interplay between structural and functional networks within the brain. These stimulus-evoked perturbations cascade into intrinsic brain dynamics, producing changes in functional and structural networks over short and long timescales, changes that modulate subsequent behavior. Figure 1C: These extended-brain-behavior networks undergo profound changes over development, with changes in the dynamics of the body and behavior (e.g. sitting, crawling, walking, or reading) creating different regularities in the input to the brain – and in turn modulating functional and structural networks of the brain, which in turn modify later behavioral patterns. Overall, across multiple time scales, brain networks (A) are shaped by interactions within extended brain-body-behavior networks (B), producing unique developmental trajectories (C) and thus contributing to the individual differences observed in adult brain networks.
Links between structural and functional networks and behavior
Although there are sp ecialized brain regions that have been associated with specific cognitive competencies, research over the last 20 years has shown that different brain regions cooperate with one another to yield systematic patterns of co-activation in different cognitive tasks [12]. Detailed analyses of these patterns of functional connectivity have also been recorded during task-free “resting-state” brain activity and these analyses reveal statistical dependencies in neural activity across regions that are highly similar to those that are activated when individuals are engaged in specific tasks [12,13]. Thus functional networks have enduring connectivity patterns even when not specifically engaged. Other studies have shown that these functional networks are also constrained by patterns of structural connectivity [14]. Individual differences in both structural and functional brain networks have been observed and these are associated with differences in cognitive and behavioral performance [15–17]. Further, moment-to-moment fluctuations in intrinsic functional connectivity predict moment-to-moment variations in performance, including ambiguous perceptual decisions and detection of stimuli at threshold [18,19]. All these advances point to the centrality of understanding functional and structural connectivity patterns in understanding human cognition.
These advances in understanding the dynamic properties of brain networks clarify several conceptual issues. First, the role of connectivity goes beyond channeling specific information between functionally specialized brain regions. Instead, connectivity generates complex system-wide dynamics that enable local regions to participate across a broad range of cognitive and behavioral tasks (Box 1). Second, the role of external inputs goes beyond the triggering or activating of specific subroutines of neural p rocessing that are encapsulated in local regions – rather, inputs act as perturbations of ongoing activity whose widespread effects depend on how these inputs become integrated with the system’s current dynamic state [20,21]. Third, the cumulative history of perturbations as recorded in changing patterns of connectivity – in-the-moment and over progressively longer timescales – defines the system’s changing capacity to both respond to input and to generate increasingly rich internal dynamics.
None of this can be fully understood by studying the brain in isolation. Brain networks do not arise autonomously, but instead they emerge in a constant dialogue between intrinsic and evoked dynamics, local and global neural processing, and, perhaps most importantly, constant interaction between brain, body and environment [2, 9–11, 22]. While studies of brain connectivity have been extraordinarily successful in disclosing patterns of interrelationships among functionally segregated and specialized regions of the brain, a fuller understanding of how brain networks relate to cognition across an individual’s lifespan requires extending these networks out into the world.
Behavior modulates structural and functional connectivity
Brain networks drive real-time behavior; behavior in turn evokes neural activity that can change patterns of connectivity – for instance, when we hold a cup or read a book, different and potentially overlapping sets of neural regions become functionally connected. These changes in connectivity occur across multiple time scales, extending beyond the moment of co-activation to more enduring functional and structural changes. Evoked neural activity from performing even relatively brief tasks such as looking at images causes perturbations to intrinsic activity that last from minutes to hours [19, 23–25] and are functionally relevant, predicting later memory for the seen images [24]. Longer tasks produce longer perturbations [23]. For example, 30 minutes of neurofeedback training yield intrinsic activity changes that persist for a day [26] and many sessions of intensive reasoning training yield measurable effects on intrinsic activity that persist for months [27]. These “reverberations” of evoked activity may also modulate structural topology via longer-lasting synaptic plasticity [23]. Extensive practice in tasks such as juggling produces changes in the structure of cerebral white matter, likely by affecting activity-dependent myelination [28] over slow time scales of weeks and longer [16], with task-induced modulations of functional and structural connectivity occurring in tandem [29].
Thus, across fast and slow time scales, behaviorally evoked activity shapes network structure and function, which in turn results in changes in behavior – strongly suggesting that an individual brain’s network topology and dynamics at one time point reflects a cumulative history of past behavior [e.g. 30; see also 19]. Brain networks are thus adaptive networks in the sense that they combine two types of dynamics that co-evolve–the dynamics of the topology of the network and the dynamics of the activity on the network [31]. Network topology constrains fast on-the-network dynamics; fast dynamics in turn shape network topology over slower time scales [31; see also 32].
Behavior extends brain networks into the environment
Behavior exerts its powerful causal influence on the brain’s evolving network topology and dynamics by selecting inputs and by dynamically coupling different components of the neural system; in so doing, behavior modulates functional connectivity. For example, where one looks determines what one sees, shaping the statistics of visual inputs as well as the resulting patterns of functional connectivity across the brain [33]. Moreover, by contributing to action and behavior, neural activity in one part of the brain can drive neural activity elsewhere – not by going through the brain but by going through the sensorimotor environment [2]. Active input selection via eye movements also modulates neural responses throughout visual cortex [34]. Head and hand movements also structure the input statistics; for example, as we hold, rotate and use objects, we actively generate dynamic visual information that supports efficient visual object recognition [35]. Because hand movements so often select what we look at, objects near hands have visual priority [36]. All these bodily actions not only shape the unimodal input statistics, they also generate and modulate correlations among sensorimotor systems, creating higher-order multimodal regularities exploitable by the brain and critical for perceptual and reward learning as shown in robotic models [2,10, 22, 37].
In sum, sampling of the external world through action creates structure in the input, which in turn perturbs ongoing brain activity, modulating future behavior and input statistics, and changing both structural and functional connectivity patterns. But this active sampling of the world is itself driven by neural activity, as motor neurons modulated by intrinsic activity [18, 38] and network topology [15] guide the movements of eyes and body. Thus, in a circular process that was once one of the foundations of cybernetics [39], the brain’s outputs influence its inputs, and these inputs in turn shape subsequent outputs [2] -- binding brain networks to the organism’s environment over short time scales, and cumulatively over developmental time [5, 19, 40].
Extended brain-body-behavior networks change across development
All aspects of this circular process change with development– the brain, its outputs, and its inputs. The development of structural and functional brain networks is protracted, with age-related changes throughout the first decades arising from extended postnatal pruning and myelination along with the synaptic tuning and remodeling that persist over the lifespan [41]. Consistent with computational models [32; see also 31, 42], some empirical evidence supports ontogenetic coevolution of structural and functional brain networks, which exhibit coordinated age-related change [43] and become more coupled with age [44]. One example, in ferret, shows increasing correspondence between visual input statistics, evoked visual activity and spontaneous activity, such that spontaneous activity increasingly matches activity evoked from visual inputs [40].
In early human development, the body’s morphology and behavior change concurrently which results in continual but developmentally ordered changes in the input statistics. Figure 2 illustrates the dramatic changes in the motor abilities of humans over the first 18 months of life. A large literature documents dependencies between these specific motor achievements and changes in perceptual and other developments in typically [45,46] and atypically developing children [47]. For example, pre-crawlers, crawlers, and walkers have different experiences with objects, different visual spatial experiences, different social experiences, and different language experiences that are tied to posture and can be influenced by experimentally changing the infant’s posture [45, 48–51]. Input statistics change profoundly when infants become able to sit steadily, such that their hands are free to manipulate and functionally use objects. The visual information self-generated by object manipulation in stably sitting infants has been shown to support changes in object memory, object discrimination and view-invariant object recognition [52–54]. Further, when toddlers handle an object, they do so with short arms that bring the object near the eyes so that it dominates the visual field, creating optimal moments for object name learning [55,56]. Changes in infant babbling [57], locomotor status [49], and hand actions [58] all have been shown to shape caregivers’ verbal responses and thus influence the regularities available in the language input.
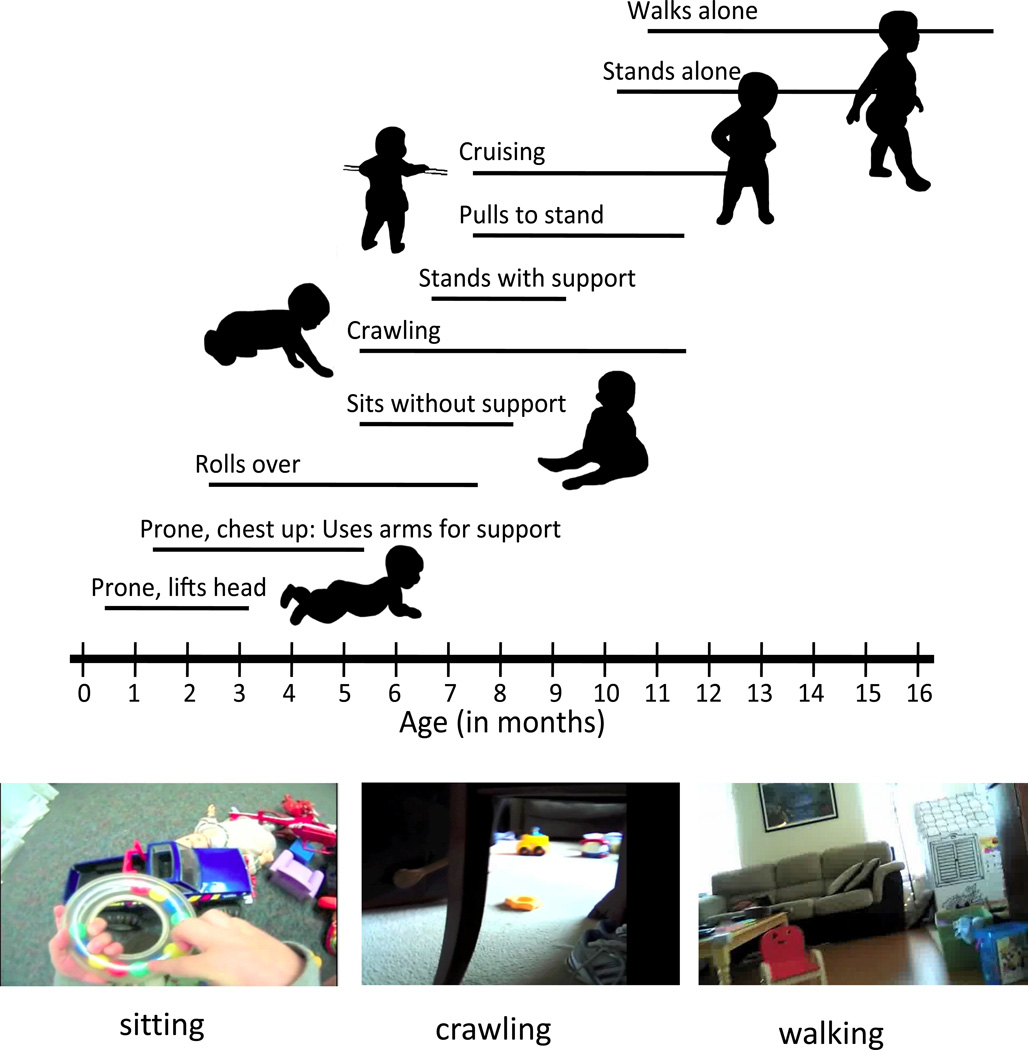
Figure 2.
Sensory-motor skills and postures change dramatically in the first year and a half of life, with each new sensory-motor achievement leading to new sensory experiences. The top portion of the figure shows the sequence of postural and locomotor skills over the first 16 months. The horizontal lines indicate the normative range of emergence of each posture. The figure is an adaptation of an original figure drawn by Nancy Bayley in 1969 (The Psychological Corporation), and from Denver II Screening Manual (Frankenburg, et al., 1992, Denver, CO: Denver Developmental Materials, Inc) and presented in many variations in textbooks and empirical papers. The bottom images were captured from head cameras worn by a sitting infant holding a toy, by a crawling infant, and by a walking infant, and they illustrate the different views and perspective provided by changing sensory-motor skills.
At present there is little direct evidence linking these changes in motor development and multisensory input to changes in brain networks [but see 8]. However, studies of older children learning to read, write, and compute provide direct evidence of brain networks being modulated by changes in behavior and input statistics [7, 59–61]. Literacy acquired during childhood and adulthood is associated with largely similar patterns in structural [62] and functional [63] brain networks, underscoring the importance of behavior in creating those changes. Extra reading practice in children needing remediation is also associated with modulation of structural networks [64; see also 65] and intrinsic connectivity [66]. The state of these structural and functional networks in turn supports future behavior, with structural topology [67] and intrinsic connectivity [17,66] predicting reading competence.
Developmental changes in experiences and in the active sampling of information will restructure the input statistics and over time yield changes in brain network topology and dynamics, changes that in turn support and influence behavior and new experiences. The sources of brain changes relevant to some development can be indirect and overlapping, with handwriting practice influencing reading networks [7], and reading practice influencing auditory language networks [68]. Importantly, many of these behavioral changes are common and linked with age, and thus seem likely to contribute to the age-related changes now being observed in brain network structure and function.
In sum, the changing dynamics of the child’s body and behavior modulate the statistics of sensory inputs as well as functional connectivity within the brain, which contribute to developmental changes in functional and structural networks that support behavioral performance across disparate domains.
Development emerges from change in brain-body-behavior networks
The essential question of development is to understand the processes by which new form and pattern emerge from existing form and pattern [10, 42, 69], and so the next frontier is to understand age-related changes in brain networks in terms of the processes that take a brain network from an earlier state to a more mature one. Because of the interplay of structural and functional networks with behavior and sensory inputs, understanding brain network development will require extending these brain networks out into the world: developmental process emerges from the interplay between different modes and different time scales of these extended brain-body-behavior networks.
New form (structural topology) and pattern (intrinsic and evoked activity) emerges in young brain networks as the dynamics of the child’s body and behavior change and restructure the input to the brain. Perturbations from these behavioral dynamics combine with ongoing neural dynamics [19–21]. Thus behavioral and neural dynamics interact to produce the ongoing network dynamics that form the brain’s functional repertoire [13, 70] and shape brain network topology over time [31]. Importantly, modulations of network topology and function associated with one particular behavior are not limited to that specific behavior [6–7, 68]. As these modulated network components are reassembled in subsequent tasks, the changes produced in one task context feed into the next task context. This developmental cascade supports new dynamics and complexity in behavior [e.g. 71] and both reflects and produces the deep interdependencies between apparently disparate developmental achievements [46].
One example concerns the conse quences of providing infants with weeks of precocious reaching experience via Velcro mittens that enable them to grasp and explore objects much earlier than control infants; this early experience leads to increases in later visual attention to objects, and oral exploration of objects [72]. Although speculative, it is quite possible that the new coordination between vision and action produced by the Velcro mittens, and the increased and early reaching that follows, increases functional coupling between neural systems for vision, spatial orienting of attention, and manual action, and strengthens associated structural connectivity. Such behaviorally-driven changes in functional and structural connectivity would also influence ongoing activity at rest. Responses to sensory input depend in part on ongoing neural activity at the time of input [20, 73, 74], raising the possibility that the increased looking at objects and responding to them as targets for exploration is supported by changes in ongoing activity produced by mitten training in this paradigm – and, outside the laboratory, by many hours of reaching practice [e.g. 75]. Increases in object exploration, in turn, lead to numerous subsequent developmental achievements [52–54, 46].
Development thus emerges from such interactions within extended brain-body-behavior networks, going beyond the mere unrolling of information pre-existing in the organism or the absorption of environmental information [10, 42, 69, 76–78]. Instead, brain and behavioral development is a process through which the information that moves the system forward is created probabilistically in interactions that cross time scales and span the brain, the body, and behavior.
Implications for future research
Understanding how brain networks develop requires zooming out and considering how the system as a whole develops. On this view, a snapshot of the system at a given time cannot be divorced from its history or ‘lifeline’ [79; see also 10, 80]: a specific behavior or brain network property may depend on some earlier activity that in turn depended on the network status at that time. This perspective requires taking a broader view of sources of variation and change in brain networks. Instead of ascribing age-related changes to maturational processes that unfold autonomously, it is necessary to consider neural and behavioral activity as key modulators of the physiological growth processes that produce the observed changes in brain networks and in turn behavior. Because action and input selection influence brain networks [e.g. 81], it is unlikely that individual differences in brain network properties are solely the result of heritable variations, but instead must be understood in light of the history of network interactions unfolding over time, building upon one another.
These ideas have direct implications for atypical development (Box 2) and education. Emerging evidence suggests that targeted measures of brain networks in children enhance and even surpass [71, 82, 83] behavioral measures in predicting future learning. But only with a mechanistic understanding of the sequences of events – including behaviors and inputs – that lead to the emergence of the network properties associated with best learning outcomes can these findings be translated into educational programs that can improve outcomes in all children.
Box 2: Implications for atypical development.
Much research is currently investigating the use of structural and functional brain network metrics as biomarkers of developmental disorders, for diagnostic purposes and for providing mechanistic insights on heterogeneous behavioral phenotypes [2,91]. Consideration of the developmental trajectory leading to the appearance of any such network metrics -- including the child’s physical behavior and surroundings -- is critical for informing mechanism and intervention alike [see also, 92]. As Karmiloff-Smith has noted [92], children with developmental disorders often inhabit very different environments from typically developing children, leading to different input regularities available to be selected.
In the case of autism, differences between typically developing children and children at high risk of autism in the self-generated selection of inputs via gaze patterns and orienting to name and to other social stimuli emerge early [93]. Indeed, several researchers have implicated the child’s selection of atypical inputs as a key contributor to the developmental cascade leading to the emergence of the atypical behavior characteristic of autism and atypical development of brain networks alike [93–95].
Prospective long itudinal study of siblings at high risk of autism is crucial not just for charting the trajectories associated with eventual diagnoses, but also for understanding the pathways followed by high risk children who do not develop autism, including differences in protective factors such as executive function [96] or differences in input regularities due to the child’s own behavior or environment. Pediatric neuroimaging has revealed common but atypical brain activation in diagnosed and non-diagnosed siblings along with “compensatory” brain activity in non-diagnosed siblings that differs from both typically developing and diagnosed children and may support their more typical behavior [97]. Future work combining longitudinal study of early developmental trajectories with neuroimaging to understand what differences in the child’s behavior and environment can promote the emergence of such compensatory brain activity despite familial and neural risk factors will thus critically inform the development of interventions.
Examining early developmental trajectories may also provide critical mechanistic insights into the characteristic heterogeneity in symptoms and neural markers [94, 98]. Recent work has identified several distinct developmental trajectories to an autism diagnosis in siblings at high risk [99], characterized by differences in timing and in behavior, differences that are likely to differentially influence the development of brain networks. Important progress will be made by linking these differences in very early pathways – including self-generated behavior – to the emergence of the later heterogeneity in brain and behavior [see also 98].
Given the nonlinearities, unexpected dependencies, and multiple routes that characterize development [42, 46], integrative empirical and synthetic (Box 3) study of the pathways leading to particular network properties will yield important insights beyond the study of behavior alone. These contributions include understanding the neural mechanisms underlying the many dependencies between developmental achievements [45,46, 72], and the multiple routes to these achievements within both typically developing individuals and those with morphological differences [84]. Although the role of behavior and inputs has been emphasized here, other behavioral states such as sleep will also be critical to a more complete understanding of developing brain networks. Advancing research implicates sleep in the consolidation of experience and points to a generally heightened neuroplasticity that may contribute to long-term changes in functional and structural connectivity [106]. Finally, this work will inform the boundaries of activity-related functional and structural network modulation across different neural systems [e.g. 85] and different individuals with different developmental histories. These are all critical contributions for informing the timing and content of rehabilitation and interventions.
Box 3: Insights from developmental robotics.
The synthetic study of mutual interactions between brain networks and embodied behavior over time is an important complement to the empirical study of developing biological organisms. In the field of developmental robotics [100–102], models of mechanisms of developmental change can be instantiated in robots that behave and learn, by combining neurobiologically plausible neural networks with physical bodies that move and sense. This approach allows testing models of development, studying brain-behavior interactions and other emergent developmental change at multiple temporal and physical scales, and importantly permits manipulations that are not ethically possible in human studies.
Several such models have studied how the structuring of inputs through physical behavior influences the development of neural circuits supporting particular functions and have generally found sensorimotor coupling between the robot and the environment to be critical to the formation of circuitry supporting adaptive behavior [103,104]. In one model, self-generated movements were critical to the gradual development of pattern-based object recognition, which was supported by changes in connectivity in higher-order visual circuits [103]; in a related system, physical behavior was essential for visual binding and object discrimination and modulated the underlying neural circuits [104]. Both random self-generated movements or ‘motor babbling’ [105] and limb twitching [106] have been shown to lead to the self-organization of sensorimotor circuits; ‘motor babbling’ has also been linked longitudinally to the emergence of reaching and object manipulation [107]. The work of Oudeyer and colleagues [102] has shown how active exploratory sampling coordinates behavior and enables the discovery of novel tasks including communicatory babbling. Finally, the robotic models of Jun Tani and colleagues demonstrated how the compositionality reflected in higher-order behaviors including language can emerge in systems with multi-scale temporal dynamics that are grounded in sensorimotor processing [108].
Extensions of information theory provide a means of quantifying the mutual interactions between neural circuits and sensing and motor behavior by mapping directions of information flow, reflecting patterns of causality [2,37]. This approach has demonstrated that sensorimotor coupling due to behavior creates information not present in the stimulus alone, in a way that is dependent on sensor morphology and changes based on contingencies in the environment. This method can be extended to study more protracted developmental changes at multiple neural and temporal scales. The extension of such formal models to empirical studies of behaving -- and developing – organisms is an important goal for the future.
Future research investigating the role of extended brain-body-behavior networks in the emergence of age-related change in networks within the brain will rely on longitudinal designs combining neuroimaging and behavioral methods. How the child’s selection of visual inputs reconfigures functional brain networks in-the-moment and at rest can be measured using near-infrared spectroscopy (NIRS) even in infants and toddlers [86]. Understanding how these changes in functional coupling during tasks feed into changes in brain networks over longer timescales can be achieved by combining longitudinal NIRS with longitudinal structural and functional magnetic resonance imaging during sleep [87]. Training studies that provide age-linked experiences precociously [7,72] will be particularly promising for distinguishing more behaviorally-driven brain network changes from more growth-associated changes. Integrating measures of change in behaviors including the child’s selection of input within their environment [e.g. 88] will inform individual variation within brain network development.
Taking seriously the idea that individual history shapes and constrains development presents practical and conceptual research challenges. But studies that explicitly examine how extended network interactions at one time point influence network interactions at subsequent time points will be indispensable for gaining a more complete understanding of the development of the system as a whole.
Conclusions
Development emerges from interactions within extended brain-body-behavior networks, across multiple overlapping timescales (Fig. 1). The dynamics of body and behavior change profoundly over development. These changes result in continuous and discontinuous change in both the input regularities created by behavior and the resulting coupling of neural systems. These in-the-moment perturbations of ongoing activity and modulations of coupling ultimately shape not only task-related functional networks but also functional networks at rest. Such functional network changes can persist, becoming part of the intrinsic functional architecture of the system and modulating structural networks over longer timescales. Such changes in functional and structural networks in turn support changes in behavior – resulting in changes in input and functional coupling, and so on.
These cycles of change in behavior and brain networks are not self-contained. They overlap in developmental time, in neural space, and in behavioral domains. This overlap creates and reflects deep inter-dependencies, and pushes the developing system forward. In other words, achievements in one task context (e.g. self-locomotion) tune brain networks in a way that supports behavioral achievements in another context (e.g. the A-not-B task [10,45]). Thus, understanding how age-related changes in brain networks emerge requires considering not only the brain, but also the rest of the extended brain-body-behavior network.
By re-situating the developing brain within the developing organism, an extended network perspective permits a more mechanistic while less deterministic understanding of developmental process. On this view, shared biological, physical, and cultural constraints result in brains and behaviors that are modally similar. Human variation emerges as each individual organism travels along a unique path [9], pushed forward by interactions within their own unique brain-body-behavior networks.
Highlights.
Functional and structural brain networks and behavior are all mutually interdependent.
Change in networks within the brain must be understood within the context of extended brain-body-behavior networks.
Development features marked changes in the dynamics of body and behavior, and resulting input to the brain – changes must be both cause and consequence of the age-related changes observed in brain networks.
The interplay within extended-brain-body-behavior networks, spanning timescales, creates development.
Acknowledgments
LB was supported by the National Science Foundation Graduate Research Fellowship. OS was supported by the J.S. McDonnell Foundation. LBS was supported in part by in part by National Institute of Child Health and Human Development Grants R01HD28675 and R21HD068475. The authors thank Gregory Kohn and Dan Kennedy for valuable feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn. Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Sporns O. Networks of the Brain. MIT press; 2011. [Google Scholar]
- 3.Power JD, et al. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17:627–640. doi: 10.1016/j.tics.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Edelman GM. Neural Darwinism: The theory of neuronal group selection. Basic Books; 1987. [DOI] [PubMed] [Google Scholar]
- 6.Sporns O, Edelman GM. Solving Bernstein's problem: A proposal for the development of coordinated movement by selection. Child Dev. 1993;64:960–981. [PubMed] [Google Scholar]
- 7.James KH. Sensori-motor experience leads to changes in visual processing in the developing brain. Dev. Sci. 2010;13:279–288. doi: 10.1111/j.1467-7687.2009.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lloyd-Fox S, et al. Cortical activation to action perception is associated with action production abilities in young infants. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varela FJ, et al. The embodied mind: Cognitive science and human experience. MIT Press; 1991. [Google Scholar]
- 10.Thelen E, Smith LB. Dynamic systems approach to the development of cognition and action. MIT press; 1994. [Google Scholar]
- 11.Chiel HJ, Beer RD. The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci. 1997;20:553–557. doi: 10.1016/s0166-2236(97)01149-1. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, et al. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichle ME. Two views of brain function. Trends Cogn. Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Honey CJ, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 16.Zatorre RJ, et al. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15:528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama MS, et al. Resting-State Functional Connectivity Indexes Reading Competence in Children and Adults. J. Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MD, et al. Intrinsic Fluctuations within Cortical Systems Account for Intertrial Variability in Human Behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Sadaghiani S, Kleinschmidt A. Functional interactions between intrinsic brain activity and behaviour. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.04.100. [DOI] [PubMed] [Google Scholar]
- 20.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J. Neurophysiol. 2008;100:1160–1168. doi: 10.1152/jn.90592.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Destexhe A. Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Curr. Opinion Neurobiol. 2011;21:717–725. doi: 10.1016/j.conb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Pfeifer R, Bongard J. How t he body shapes the way we think: a new view of intelligence. MIT press; 2007. [Google Scholar]
- 23.Han F, et al. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tambini A, et al. Enhanced Brain Correlations during Rest Are Related to Memory for Recent Experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betti V, et al. Natural Scenes Viewing Alters the Dynamics of Functional Connectivity in the Human Brain. Neuron. 2013;79:782–797. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmelech T, et al. The Day-After Effect: Long Term, Hebbian-Like Restructuring of Resting-State fMRI Patterns Induced by a Single Epoch of Cortical Activation. J. Neurosci. 2013;33:9488–9497. doi: 10.1523/JNEUROSCI.5911-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackey AP, et al. Intensive Reasoning Training Alters Patterns of Brain Connectivity at Rest. J. Neurosci. 2013;33:4796–4803. doi: 10.1523/JNEUROSCI.4141-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampaio-Baptista C, et al. Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. J. Neurosci. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taubert M, et al. Long-term effects of motor training on resting-state networks and underlying brain structure. Neuroimage. 2011;57:1492–1498. doi: 10.1016/j.neuroimage.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 30.Luo C, et al. Musical training induces functional plasticity in perceptual and motor networks: insights from resting-state FMRI. PloS One. 2012;7:e36568. doi: 10.1371/journal.pone.0036568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross T, Blasius B. Adaptive coevolutionary networks: a review. J. R. Soc. Interface. 2008;5:259–271. doi: 10.1098/rsif.2007.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinov M, et al. Symbiotic relationship between brain structure and dynamics. BMC Neurosci. 2009;10 doi: 10.1186/1471-2202-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder CE, et al. Dynamics of active sensing and perceptual selection. Curr. Opin. Neurobiol. 2010;20:172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engel AK, et al. Where's the action? The pragmatic turn in cognitive science. Trends Cogn. Sci. 2013;17:202–209. doi: 10.1016/j.tics.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Harman KL, et al. Active manual control of object views facilitates visual recognition. Curr. Biol. 1999;9:1315–1318. doi: 10.1016/s0960-9822(00)80053-6. [DOI] [PubMed] [Google Scholar]
- 36.Gozli DG, et al. Hand position alters vision by biasing processing through different visual pathways. Cognition. 2012;124:244–250. doi: 10.1016/j.cognition.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer R, et al. On the information theoretic implications of embodiment–principles and methods. In: Lungarella M, et al., editors. 50 years of artificial intelligence. Springer: 2007. pp. 76–86. [Google Scholar]
- 38.Ramot M, et al. Coupling between spontaneous (resting state) fMRI fluctuations and human oculo-motor activity. NeuroImage. 2011;58:213–225. doi: 10.1016/j.neuroimage.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Ashby WR. Design for a brain. John Wiley; 1960. [Google Scholar]
- 40.Berkes P, et al. Spontaneous Cortical Activity Reveals Hallmarks of an Optimal Internal Model of the Environment. Science. 2011;331:83–87. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagmann P, et al. MR connectomics: a conceptual framework for studying the developing brain. Front. Syst. Neurosci. 2012;6:1–17. doi: 10.3389/fnsys.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb G, et al. The significance of biology for human development: A developmental psychobiological systems view. In: Damon W, Lerner RM, editors. Handbook of child psychology. 5th edn. Wiley; 1998. pp. 210–257. [Google Scholar]
- 43.Raznahan A, et al. Patterns of C oordinated Anatomical Change in Human Cortical Development: A Longitudinal Neuroimaging Study of Maturational Coupling. Neuron. 2011;72:873–884. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagmann P, Sporns O, et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertenthal BI, Campos JJ. A systems approach to the organizing effects of self-produced locomotion during infancy. Adv. Infancy Res. 1990;6:1–60. [Google Scholar]
- 46.Smith LB. It’s all connected: Pathways in visual object recognition and early noun learning. Am. Psychol. 2013;68:618–629. doi: 10.1037/a0034185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhat AN, et al. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011;91:1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- 48.Adolph KE, et al. Locomotor experience and use of social information are posture specific. Dev. Psychol. 2008;44:1705–1714. doi: 10.1037/a0013852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karasik LB, et al. Crawling and walking infants elicit different verbal responses from mothers. Dev. Sci. 2013 doi: 10.1111/desc.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kretch KS, et al. Crawling and walking infants see the world differently. Child Dev. 2013 doi: 10.1111/cdev.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soska KC, Adolph KE. Postural Position Constrains Multimodal Object Exploration in Infants. Infancy. 2014;19:138–161. doi: 10.1111/infa.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruff HA. Effect of context on infants' responses to novel objects. Dev. Psychol. 1981;17:87–89. [Google Scholar]
- 53.Soska KC, et al. Systems in development: motor skill acquisition facilitates three-dimensional object completion. Dev. Psychol. 2010;46:129–138. doi: 10.1037/a0014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James KH, et al. Young children's self-generated object views and object recognition. J. Cogn. Dev. 2013 doi: 10.1080/15248372.2012.749481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu C, Smith LB. Embodied attention and word learning by toddlers. Cognition. 2012;125:244–262. doi: 10.1016/j.cognition.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira A, et al. A Bottom-up View of Toddler Word Learning. Psychon. Bull. Rev. 2014;21:178–185. doi: 10.3758/s13423-013-0466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein MH, West MJ. Consistent responses of human mothers to prelinguistic infants: the effect of prelinguistic repertoire size. J. Comp. Psychol. 1999;113:52–58. doi: 10.1037/0735-7036.113.1.52. [DOI] [PubMed] [Google Scholar]
- 58.Olson J, Masur EF. Infants' gestures influence mothers' provision of object, action and internal state labels. J. Child Lang. 2011;38:1028–1054. doi: 10.1017/S0305000910000565. [DOI] [PubMed] [Google Scholar]
- 59.James KH, Engelhardt L. The effects of handwriting experience on functional brain development in pre-literate children. Trends Neurosci. Educ. 2012;1:32–42. doi: 10.1016/j.tine.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Y, et al. Enhanced white matter tracts integrity in children with abacus training. Hum. Brain Mapp. 2011;32:10–21. doi: 10.1002/hbm.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, et al. The neural pathway underlying a numerical working memory task in abacus-trained children and associated functional connectivity in the resting brain. Brain Res. 2013;1539:24–33. doi: 10.1016/j.brainres.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 62.De Schotten MT, et al. Learning to Read Improves the Structure of the Arcuate Fasciculus. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs383. [DOI] [PubMed] [Google Scholar]
- 63.Dehaene S, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330:1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 64.Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gebauer D, et al. Differences in integrity of white matter and changes with training in spelling impaired children: a diffusion tensor imaging study. Brain Struct. Funct. 2011;217:747–760. doi: 10.1007/s00429-011-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koyama MS, et al. Cortical Signatures of Dyslexia and Remediation: An Intrinsic Functional Connectivity Approach. PloS One. 2013;8:e55454. doi: 10.1371/journal.pone.0055454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wandell BA, et al. Learning to See Words. Annu. Rev. Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monzalvo K, Dehaene-Lambertz G. How reading acquisition changes children’s spoken language network. Brain Lang. 2013;127:356–365. doi: 10.1016/j.bandl.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Lehrman DS. A critique of Konrad Lorenz’s theory of instinctive behavior. Q. Rev. Biol. 1953;28:337–363. doi: 10.1086/399858. [DOI] [PubMed] [Google Scholar]
- 70.Pizoli CE, et al. Resting-state activity in development and maintenance of normal brain function. Proc. Natl. Acad. Sci. U.S.A. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saygin ZM, et al. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33:13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Needham A, et al. A pick-me-up for infants’ exploratory skills: Early simulated experiences reaching for objects using ‘sticky mittens’ enhances young infants’ object exploration skills. Infant Behav. Dev. 2002;25:279–295. [Google Scholar]
- 73.Lewis CM, et al. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. U.S.A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corbetta M, et al. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adolph KE, et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psych. Sci. 2012;23:1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gottlieb G. Experiential canalization of behavioral development: Theory. Dev. Psychol. 1991;27:4–13. [Google Scholar]
- 77.Bateson PPG, Gluckman P. Plasticity, robustness, development and evolution. Cambridge University Press; 2011. [Google Scholar]
- 78.Oyama S. The ontogeny of information: Developmental systems and evolution. Cambridge University Press; 1985. [Google Scholar]
- 79.Rose S. Lifelines: Biology, freedom, determinism. Allen Lane; 1997. [DOI] [PubMed] [Google Scholar]
- 80.Li SC. Brain in macro experiential context: biocultural co-construction of lifespan neurocognitive development. Progr. Brain Res. 2009;178:17–29. doi: 10.1016/S0079-6123(09)17802-0. [DOI] [PubMed] [Google Scholar]
- 81.James TW, James KH. Expert individuation of objects increases activation in the fusiform face area of children. NeuroImage. 2013;67:182–192. doi: 10.1016/j.neuroimage.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 82.Supekar K, et al. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc. Natl. Acad. Sci. U.S.A. 2013;110:8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoeft F, et al. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. U.S.A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stoeckel MC, et al. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2395–2400. doi: 10.1073/pnas.0803733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stevens C, Neville H. Specificity of experiential effects in neurocognitive development. In: Gazzaniga M, editor. The Cognitive Neurosciences V. MIT Press; 2013. [Google Scholar]
- 86.Aslin RN. Questioning the questions that have been asked about the infant brain using near-infrared spectroscopy. Cog. Neuropsych. 2012;29:7–33. doi: 10.1080/02643294.2012.654773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Redcay E, et al. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Franchak JM, Adolph KE. Visually guided navigation: Head-mounted eye-tracking of natural locomotion in children and adults. Vis. Res. 2010;50:2766–2774. doi: 10.1016/j.visres.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baker AP, et al. Fast transient networks in spontaneous human brain activity. eLife. 2014;3 doi: 10.7554/eLife.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biswal BB, et al. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Karmiloff-Smith A. Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Developmental Psychology. 2009;45(1):56. doi: 10.1037/a0014506. [DOI] [PubMed] [Google Scholar]
- 93.Jones EJ, et al. Developmental pathways to autism: A review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 2013 doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones W, Klin A. Het erogeneity and homogeneity across the autism spectrum: the role of development. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- 95.Mundy P, et al. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Res. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson MH. Executive function and developmental disorders: the flip side of the coin. Trends Cogn. Sci. 2012;16:454–457. doi: 10.1016/j.tics.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 97.Kaiser MD, et al. Neural signatures of autism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Uddin LQ, et al. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013;7:458. doi: 10.3389/fnhum.2013.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Landa RJ, et al. Latent class analysis of early developmental trajectory in baby siblings of children with autism. J. Child Psychol. Psychiatry. 2012;53:986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lungarella M, et al. Developmental robotics: a survey. Conn. Sci. 2003;15:151–190. [Google Scholar]
- 101.Fitzpatrick P, et al. Shared challenges in object perception for robots and infants. Infant Child Dev. 2008;17:7–24. [Google Scholar]
- 102.Gottlieb J, et al. Information-seeking, curiosity, and attention: computational and neural mechanisms. Trends Cogn. Sci. 2013;17(11):585–593. doi: 10.1016/j.tics.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Almassy N, et al. Behavioral constraints in the development of neuronal properties: a cortical model embedded in a real-world device. Cereb. Cortex. 1998;8:346–361. doi: 10.1093/cercor/8.4.346. [DOI] [PubMed] [Google Scholar]
- 104.Seth AK, et al. Visual binding through reentrant connectivity and dynamic synchronization in a brain-based device. Cereb. Cortex. 2004;14:1185–1199. doi: 10.1093/cercor/bhh079. [DOI] [PubMed] [Google Scholar]
- 105.Olsson LA, et al. From unknown sensors and actuators to actions grounded in sensorimotor perceptions. Conn. Sci. 2006;18:121–144. [Google Scholar]
- 106.Blumberg MS, et al. Twitching in sensorimotor development from sleeping rats to robots. Curr. Biol. 2013;23:R532–R537. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Law J, et al. A psychology based approach for longitudinal development in cognitive robotics. Front. Neurorobot. 2014;8 doi: 10.3389/fnbot.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugita Y, Tani J. Learning semantic combinatoriality from the interaction between linguistic and behavioral processes. Adapt. Behav. 2005;13:33–52. [Google Scholar]