Abstract
Human pancreatic β cells have exceptionally high zinc content. In β cells the highest zinc concentration is in insulin secretory granules, from which it is co-secreted with the hormone. Uptake of zinc into secretory granules is mainly mediated by zinc transporter 8 (ZnT8), the product of the SLC30A8 gene. The minor alleles of several single nucleotide polymorphisms (SNPs) in SLC30A8 are associated with decreased risk of type 2 diabetes (T2D), but the precise mechanisms underlying the protective effects remain uncertain. In this article we review current knowledge of the role of ZnT8 in maintaining zinc homeostasis in β cells, its role in glucose metabolism based on knockout mouse studies, and current theories regarding the link between ZnT8 function and T2D.
Keywords: Islet, SLC30A8, Slc30a8
Regulation of intracellular bioavailable zinc
Zinc is essential for human health, with clinical deficiency having potentially fatal consequences, through affects on multiple organs including elements of the epidermal, gastrointestinal, central nervous, immune, skeletal, and reproductive systems (reviewed in [1, 2]). Moreover, disturbances in zinc homeostasis that may contribute to, or exacerbate, pathology have been observed in many chronic conditions including Alzheimer’s disease, cardiovascular disease, cancer, autoimmunity, and diabetes (reviewed by [3, 4]). Zinc is required by all cell types, playing critical catalytic, structural and regulatory roles, by binding to a zinc proteome estimated in humans to contain ~3000 members [5]. However, although intoxication is relatively rare compared to other heavy metal ions, intracellular free zinc concentrations must nonetheless be tightly controlled due to the central role that zinc/cysteine interactions play in redox homeostasis [6], and the consequent capacity for both low and high levels of bio-available Zn2+ to induce oxidative stress and related metabolic disturbances that can trigger cell death [2, 7]. The cytoplasmic zinc concentration is controlled by the actions of three classes of proteins, namely metallothioneins (that control availability), and zinc transporters of the solute linked carrier 30 (SLC30) and SLC39 gene families that together regulate intracellular distribution, mediating efflux from, and influx into, the cytoplasm respectively (recently reviewed in [3, 8–10]).
Compared to other cell types pancreatic β cells have exceptionally high zinc content [11]. Within β cells the highest levels of zinc are located in insulin secretory granules (ISGs), which may contain up to 70% of the total β cell zinc, and where the total concentration is ~10–20 mM [12]. ZnT8, the product of the SLC30A8 gene (UniGene Hs.532270) is responsible for the very high level of zinc accumulation in ISGs [13, 14]. The major intra-granular ligand for zinc is insulin, which is stored in a crystalline lattice of insoluble hexamers in which 6 insulin molecules are complexed with 2 Zn2+ ions and 1 Ca2+ ion (reviewed by [15, 16]). The high capacity binding provided by nascent (pro)insulin hexamers likely acts as a “sink” to drive uptake, evidenced by the fact that guinea pigs, that lack the insulin B10 His that coordinates Zn2+, accumulate only low levels of zinc in their islets [17], although whether guinea pigs actually express a ZnT8 isoform is currently unclear. Additional ISG Zn2+ ligands include inorganic ions such as phosphate, and other proteins that are co-secreted together with insulin [12]. Of particular note is islet amyloid polypeptide (IAPP) [18, 19], which is the major constituent of the amyloid plaques that are present in the pancreas of the majority of individuals with T2D [20] and have been implicated in β-cell apoptosis [21] and islet inflammation [22]. Intriguingly, ISG Zn2+ may play a key role in preventing amyloidogenesis, acting both to increase the lag time of fiber formation and decrease the rate of addition of monomers to existing fibrils [23, 24]. Moreover, IAPP can also interact with monomeric and crystalline insulin [25], which may also influence amyloidogenesis [18]. At rest, β cell cytoplasmic free Zn2+ is estimated to be approximately 400–450 pM [26]. Zinc in ISGs only slowly exchanges with Zn2+ in the cytoplasm [27], thus the increases in cytoplasmic Zn2+ concentrations that occur during GSIS [26, 28] are unlikely to involve internal release of the ion from ISGs. However, since upon exocytosis the elevated pH (~5.5 in granules, ~7.4 in blood) destabilizes insulin crystals releasing the monomeric hormone and free Zn2+ and Ca2+, re-uptake of co-secreted zinc could contribute to the increases observed. Co-secreted Zn2+ might potentially act in a paracrine manner to regulate glucagon secretion from α-cells [29, 30], although this remains a subject of controversy [31], but may also act in an autocrine fashion to potentiate GSIS [32].
ZnT8 expression
In humans high-level expression of SLC30A8 is mainly restricted to the endocrine pancreas. It is absent from pancreatic exocrine tissue [33], but is expressed in some extra-pancreatic sites, most notably retinal pigment epithelium [34] and several layers of the retina [35], where its loss may contribute to the pathology of ischemic retinopathy. SLC30A8 mRNA has also been detected in human adipocytes [36] and lymphocytes [37], although evidence of ZnT8 protein expression is still lacking. Within human islets, SLC30A8 mRNA and ZnT8 protein levels are highly enriched in β cells, but both are also present in α cells, albeit at significantly lower levels [33, 38–40]. This is also true in rodents [41], but not in pigs, where ZnT8 appears to be restricted exclusively to β cells [42]. In rodents, expression of Slc30a8 has also been detected in extra-pancreatic endocrine glands including pituitary, adrenal, and thyroid [43, 44].
At steady state in human β cells, ZnT8 shows a high degree of co-localization with insulin, consistent with its principal role of facilitating uptake of Zn2+ into ISGs. However, the overlap appears incomplete [45], suggesting that ZnT8 may also traffic to other intra-cellular compartments. At present the sorting signal(s) responsible for targeting ZnT8 to ISGs have not been defined, and the possibility that the alternatively spliced isoforms (see Box 1) have distinct intra-cellular itineraries, like ZnT2 [46] and ZnT5 [47], cannot be excluded.
BOX 1. Zinc transporter 8 (ZnT8) regulation.
There are 10 members of the SLC30 gene family in humans, and at least 8 of these (SLC30A1, 2, 4, 5, 6, 7, 8, and 9) are expressed in human β cells [38, 40]. Of these, the most abundant transcript encodes ZnT8, the product of the SLC30A8 gene located on chromosome 8 at position 8q24.11.
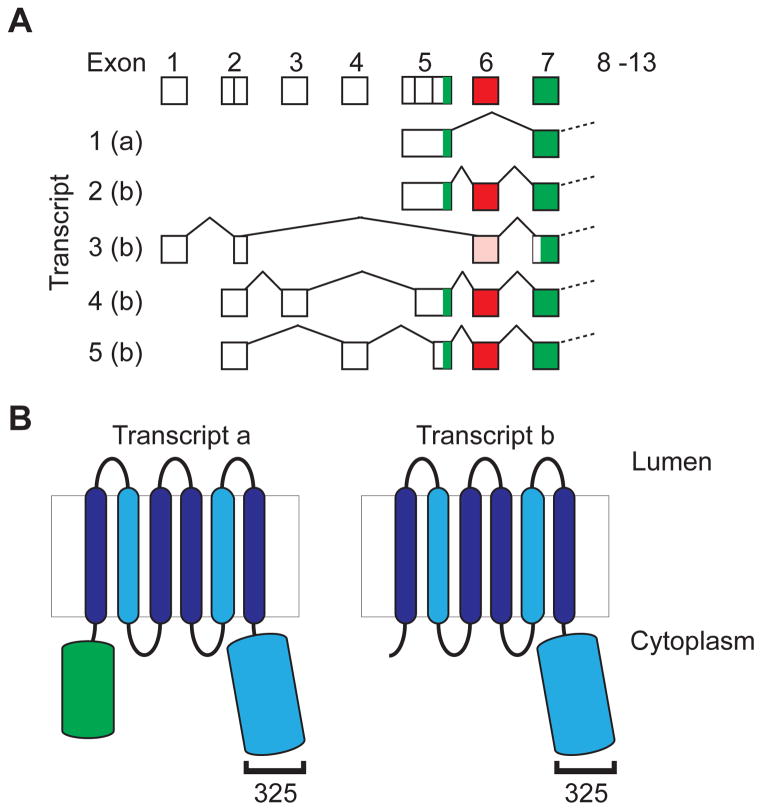
The SLC30A8 gene contains 13 exons (exons 2 and 5 having multiple 5′ splice donor sites; Fig IA) and spans ~226kB. It is processed to generate 5 primary transcripts, at least two of which are co-expressed in β cells [38 ] and can potentially encode 2 protein isoforms. The longest open reading frame (ORF), derived from transcript 1, encodes a 369 aa integral membrane protein translated from a start site in exon 5 (Fig IA). Exon 6, present in transcripts 2–5, contains 2 in frame stop codons, which may prevent efficient expression of transcripts 2, 4 and 5, that retain the start codon in exon 5. Transcript 3 lacks this exon, and could generate a truncated product of 320 aa derived from an alternative start site in exon 7. This isoform would lack most of the N-terminal cytoplasmic domain (Fig IB), a region that is also absent in the majority of non-mammalian orthologs [48]. However, direct evidence for expression of the truncated protein is lacking, and potential functional differences remain largely un-explored.
Relatively little is known about the transcription factors regulating SLC30A8 expression, in part because the proximal SLC30A8 promoter is only active in stable and not transient transfections [33]. Pound and colleagues identified a strong transcriptional enhancer in the second intron of the mouse Scl30a8 gene [49] that is highly conserved (>80%) in humans [33]. This enhancer is active in islet-derived β cells but not α cells [33, 49]. Analysis of the enhancer by scanning mutagenesis indicates that it binds multiple transcription factors, one of which was identified through gel retardation and ChIP analyses as Pdx-1 [49].
Several groups have studied factors that might regulate SLC30A8 expression. For example, zinc depletion, glucose and cytokine treatments have all been reported to decrease Slc30a8 gene expression in mouse and rat islet-derived cell lines [50, 51], although cytokine treatment had little apparent effect on SLC30A8 transcript levels in cultured human islets [38]. Such regulation might potentially contribute to disease since acute suppression of ZnT8 impairs GSIS in rodent β-cells [52] and in vivo may represent an early event in diabetes [53].
Figure I for BOX 1. Schematic representation of human ZnT8 transcripts and proteins.
A. Exons comprising the 5′ UTR and initiator methionine of the 5 NCBI RefSeq transcripts are shown. Coding exons are shown in green and the 30bp exon 6 that contains 2 in-frame stop codons in red, or pink in the case of transcript 3 that lacks the start site in exon 5 and could give rise to a truncated product from a downstream in frame ATG in exon 7. Exons 2 and 5 contain multiple splice acceptor sites. All transcripts contain exons 8–13. The ZnT8 isoform potentially encoded by each transcript is shown. It should be noted that most studies of the expression of SLC30A8 mRNA in non-β cell tissues use primer pairs that will not distinguish the various transcripts. B. The domain structure of the two isoforms are shown. The light blue shapes indicate domains implicated in Zn2+ binding, and the bracket the approximate location of the polymorphic residue 325.
ZnT8 and T2D in humans
Perhaps the most striking aspect of ZnT8 biology is the observation that polymorphisms in the SLC30A8 gene are associated with altered risk of T2D. Exon 13, which encodes the final 52 aa of both isoforms (Box 1) contains 6 reported SNPs, two of which cause amino acid changes at position 325 (276 in isoform b), from an Arg to a Trp (rs13266634) or Gln (rs16889462) respectively. Genome Wide Association Study (GWAS) data showed that the major allele of rs13266634 was associated with a modest increased risk of T2D in some [61–64], but not all [65, 66], populations. However, Flannick and colleagues recently described 12 rare loss-of-function ZnT8 mutants that collectively are associated with a 65% decrease in T2D risk [67]. This suggests that the GWAS data should instead more properly be interpreted in terms of the minor allele of rs13266634 being associated with modest protection from T2D. It should also be noted that the probes used to detect rs13266634 cannot distinguish between the Arg and Gln variants since both have a C at that position, although in contrast to rs13266634, which has a relatively high minor allele frequency in many populations, the Gln encoding rs16889462 SNP is relatively rare. Thus although in most of the subjects included in the GWAS studies the C allele of rs13266634 likely encoded an Arg, it cannot be concluded that this was always the case.
The other four SNPs on exon 13 are located in the 3′ UTR, and two (rs3802177 and rs11558471) that are in strong linkage disequilibrium with rs13266634 have also been linked to T2D risk [17, 18]. The rs13266634 Trp325-encoding T-allele is also associated with a decrease in the plasma proinsulin to insulin ratio during oral glucose tolerance tests in a population of individuals at risk for T2D [68]. However there are conflicting reports as to whether rs13266634, rs3802177 and/or rs11558471 are linked to altered fasting blood glucose, fasting insulin, insulin sensitivity, or glucose tolerance and insulin secretion [69–73] [68, 74, 75].
A key issue therefore, is the mechanism that underpins the T2D association of rs13266634. The initial and simplest hypothesis was that the coding change directly influences ZnT8’s ability to transport zinc. Evidence that this could be the case was reported by Nicolson and co-workers who used fluorescent dyes to monitor cytosolic (FluoZin-3) and vacuolar (Zinquin) accumulation of zinc in murine MIN6 cells transiently expressing the Arg or Trp variants of human ZnT8 [13]. They observed significantly greater fluorescence in cells expressing the Trp variant, and concluded that this variant was therefore a more active transporter. Similarly Kim and colleagues reported increased uptake of 65Zn into vesicles from INS-1 cells over-expressing the Trp compared to the Arg variant [60]. However, how this change is deleterious to β cell function is unclear since Cauchi and colleagues [73] reported that the Trp encoding variant is not associated with improved basal or glucose-stimulated insulin secretion (GSIS) from human islets. Moreover, the observation by Flannick and colleagues [67] that haploinsufficiency is associated with marked decreased in T2D risk appears to conflict with the conclusion that expression of a slightly less active form of ZnT8 causes a modest increased risk of T2D, and suggests that a more complex mechanism is responsible for the observed rs13266634 T2D association. Intriguingly, Nica and colleagues found evidence of allele specific expression of the rs11558471 SNP [40] that might be consistent with differential expression of the 2 alleles. However, Cauchi and colleagues found no evidence that rs13266634 (and by implication those SNPs in linkage disequilibrium with it) influenced SLC30A8 mRNA levels [73], arguing against changes in mRNA expression or stability as the primary factor underlying T2D association with rs13266634.
In light of the effects of haploinsufficiency [67], other potential explanations that deserve further exploration are that the protective Trp variant turns over at a faster rate than the Arg isoform (thereby mimicking the effect of haploinsufficiency), or that it traffics differently within the cell. Evidence for the latter possibility comes from the study of Nicolson and colleagues [13], since the most dramatic difference between the Arg and Trp variants was seen with respect to cytoplasmic accumulation of zinc. This suggests that ZnT8, like ZnT5 variant B [76], can operate bi-directionally, and mediate uptake across the plasma membrane [13]. Consequently the protective effect of the Trp variant might stem from greater activity at the plasma membrane than the Arg variant. How this might provide protection is unclear. Of note, GSIS is associated with increased production of reactive oxygen and nitrogen species [77]. Given the central role of zinc in mitigating oxidative stress [6] a unified theory that might reconcile the apparently conflicting observations is that both the Trp and loss of function variants might act by transiently providing a higher cytoplasmic zinc concentration in the β cell during GSIS. In the former case this would be through greater activity at the plasma membrane, and in the latter through a slower rate of depletion through uptake into granules.
ZnT8 and β cell function in mice
The role of ZnT8 in mice in vivo has been investigated by six studies that examined the effect of either global [13, 78–80] or β cell specific [81, 82] deletion of the Slc30a8 gene (Table 1). There were several points on which most or all of these studies concurred. Most studies reported no changes in islet insulin content [13, 78–80, 82] or on islet size, numbers and cell composition [13, 78, 81, 82] in the ZnT8 knockout (KO) mice (Table 1). Global or β cell-specific deletion of the gene resulted in marked changes in the appearance of insulin secretory granules [13, 80–82] in most cases, however, one study observed no changes [79] (Table 1). A clear decrease in islet zinc content was also observed (Table 1), though subtle differences were apparent. Specifically Nicolson et al. [13] detected a decrease in zinc content when using zinquin and dithizone but not FluoZin-3 whereas Pound et al. [78, 79] detected a marked decrease through the use of FluoZin-3. Also, using zinquin, Wijesekara et al. [81] detected a much more modest decrease in islet zinc in β-cell specific Slc30a8 KO than global KO [13] mice.
Table 1.
Data from Slc30a8 KO mouse studies using male mice.
| Deletion Location |
Deletion Domain1 |
Genetic B/G2 |
Body Mass3 |
Islet Insulin Content |
Islet Zinc Content |
Islet # Islet Size α/β # |
Islet EM |
GSIS In Situ |
ITT/ EH Clamp4 |
Fasting Glucos e |
Fasting Insulin |
IPGTT 4–6 Weeks |
IPGTT 12–22 Weeks |
OGTT | GSIS In Vivo |
REF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global | Exon 3 2 Transmembrane | Mixed | No change | No change | Down | No change | N.R.5 | Down | N.R. | No change | Down | N.R. | No change | N.R. | N.R. | [14] |
| Global | Exon 3 2 Transmembrane | C57BL /6J | No change | No change | Down | N.R. | No change | No change | No change | No change | No change | IGT8 | No change | No change | N.R. | [79] |
| Global | Exon 1 Initiation codon | Mixed | No change | No change | Down | No change | Altered | No change | No change | No change | N.R. | IGT | IGT | N.R. | N.R. | [13] |
| Global | Exon 1 Initiation codon | Mixed | No change | N.R. | Down | No change | Altered | Up6 | No change | Up7 | N.R. | IGT | No change | N.R. | Down | [13] |
| Global | Exon 1 Initiation codon | Mixed | No change | No change | Down | N.R. | Altered | No change | No change | No change | N.R. | No change | No change | N.R. | N.R. | [80] |
| Beta Cell | Exon 1 Initiation codon | Mixed | No change | N.R. | Down | No change | Altered | Down | N.R. | No change | No change | N.R. | N.R. | IGT | No change | [81] |
| Beta Cell | Exon 5 Zinc binding | C57BL /6J | No change | No change | Down | No change | Altered | Up | No change | No change | No change | IGT | IGT | N.R. | Down | [82] |
The mouse Slc30a8 gene has 8 exons (Chimienti et al. Diabetes 53:2330–2337,2004).
Designated mixed unless speed congenic backcrossing or at least 10 backcrosses onto a defined genetic background (B/G).
Body weight on a chow diet.
EH, euglycemic-hyperinsulinemic clamp
N.R., not reported.
GSIS was increased in islets from the Toronto colony mice at 12 weeks but unchanged in the London colony.
Fasting glucose was elevated at 6 weeks but normal by 12 weeks.
IGT, impaired glucose tolerance
Impact of ZnT8 on glucagon secretion
As discussed above, the Zn2+ that is co-secreted with insulin could potentially act in a paracrine manner to regulate glucagon secretion from α-cells [29, 30], although this remains a subject of controversy [31]. Neither global [13] nor β cell-specific [82] deletion of Slc30a8 appears to affect glucagon secretion from isolated islets. In addition, plasma glucagon levels were unaltered in α cell-specific Slc30a8 KO mice [81], β cell-specific KO mice [82] and global KO mice [13, 78–80]. The function of ZnT8 in α cells is therefore uncertain. However, it is important to note that none of these studies examined the most important stimulator of glucagon secretion in vivo, namely hypoglycemia [83].
Impact of ZnT8 on GSIS and proinsulin processing
One of the most striking aspects of the KO mouse studies are the discordant results with respect to the effect of Slc30a8 deletion on GSIS from isolated islets (Table 1). Two studies report no effect of Slc30a8 deletion on GSIS [79, 80], two report impaired GSIS [78, 81] and two report enhanced GSIS [13, 82] (Table 1). Tamaki et al. [82] have suggested that an increase in GSIS in Slc30a8 KO mouse islets could be explained by the previously reported inhibitory effect of zinc on insulin secretion. One potential technical explanation as to why four of the six studies did not detect an increase in GSIS might involve differences in the volume of media used in GSIS assays since this would alter the degree of dilution of co-secreted zinc. However, whether such differences existed is not apparent from the methods sections of these papers.
In addition to playing a structural role in the storage of insulin, Zn2+ is an essential co-factor for many components of the proinsulin processing machinery [84]. Thus, ZnT8 may play a role both in sorting/retaining proinsulin into the regulated secretory pathway, as well as in regulating the activity of enzymes such as prohormone convertase (PC)1/3, PC2, or carboxypeptidase E (CPE) and modulating intragranular pH or Ca2+ concentration. Zn2+ is an essential co-factor for CPE [85], and loss of CPE activity reduces the overall rate of conversion of proinsulin to insulin [86] probably through product inhibition of PC1/3 and 2 by conversion intermediates. Thus, several of the mouse KO studies examined the effect of Slc30a8 deletion on proinsulin levels and processing. Inconsistent results were obtained, with two studies reporting no effect on proinsulin processing [13, 80] whereas two other studies reported a small increase in plasma proinsulin [81, 82]. In contrast, Pound and colleagues [79] reported an unexpected decrease in plasma proinsulin levels, although the effect was influenced by genetic background, leading to the conclusion that in mice, the absence of Slc30a8 has only a minor effect on proinsulin processing, despite gross changes in ISG morphology [13, 81].
Role of ZnT8 on in vivo physiology
All six Slc30a8 KO mouse studies investigated the influence of ZnT8 on in vivo physiology, particularly with respect to glucose metabolism. As with the studies on β cell function, there were several points on which most or all of these studies concurred. All six studies found that Slc30a8 deletion does not affect body weight when mice are fed a standard chow diet (Table 1), and, where analyzed, using either insulin tolerance tests or euglycemic-hyperinsulinemic clamps, no changes in insulin sensitivity were observed [13, 79, 80, 82]. Five of the studies reported no change in fasting blood glucose (FBG) [78–82] whereas one study reported an elevation in FBG, but only in young mice [13]. Similarly, three of the studies reported no change in fasting insulin [79, 81, 82] whereas one study reported a marked decrease [78]. Most studies also reported impaired glucose tolerance during intraperitoneal glucose tolerance tests (IPGTTs) in young mice [13, 79, 80, 82], but only two studies observed impairment in older mice [13, 78–80, 82] (Table 1). A slight impairment in glucose tolerance at a single time point was observed during oral glucose tolerance tests (OGTTs) in young mice [81] but no impairment was apparent in older mice [79] (Table 1).
Impact of high fat diet (HFD)
Several of the KO studies also examined the effect of HFD, expecting that the additional insulin secretory demand imposed by HFD induced insulin resistance might unmask a clear phenotype in KO mice [87]. As with the studies on chow fed mice, the outcome of these HFD studies were variable. Pound et al. [79] and Tamaki et al. [82] reported no difference in glucose tolerance relative to wild type (WT) mice following HFD in global and β cell-specific Slc30a8 KO mice, respectively, though Pound et al. [79] reported that the global KO mice gained slightly less weight and had lower fasting insulin levels. Lemaire et al. [80] also reported little effect in global Slc30a8 KO mice on glucose tolerance or body weight after 10 weeks on HFD relative to WT mice, though interestingly, after prolonged feeding (20 weeks) diabetes developed in 2 out of 4 KO mice versus 0 out of 8 WT mice. Finally, Hardy et al. [88] reported that global Slc30a8 KO mice gained more weight than WT mice on HFD and had higher fasting insulin levels, consistent with preliminary studies described by Nicolson and colleagues [13] on the same mouse colony. Strikingly, Hardy et al. [88] demonstrated that these effects of HFD on body weight were not replicated in β cell-specific Slc30a8KO mice, suggesting that the effect of HFD on body weight they observed in global KO mice required deletion of Slc30a8 in other cell types. Interestingly in humans SLC30A8 is preferentially associated with T2DM in lean and not obese individuals [89].
Tamaki and colleagues [82] recently reported that the absence of ZnT8 affects insulin clearance by the liver. Using an elegant comparison of pancreas perfusion versus dual pancreas and liver perfusion, they demonstrated enhanced GSIS from the pancreas of KO mice using pancreas perfusion that was lost following dual pancreas and liver perfusion. Tamaki et al. [82] suggested that this enhanced insulin clearance was likely not due to the absence of ZnT8 per se but instead due to a reduction in zinc secretion from KO beta cells resulting in enhanced clathrin-dependent insulin endocytosis. Indeed, Lemaire et al. [80] showed that zinc release is markedly reduced from Slc30a8 KO islets. Thus Tamaki and colleagues [82] hypothesized that the absence of ZnT8 has dual effects, promoting GSIS and hepatic insulin clearance, both of which are mediated via the absence of zinc. Since the GSIS observed using dual pancreas and liver perfusion was the same between WT and KO mice, these effects at first glance appear to be offsetting [82]. However, Tamaki et al. [82] also reported a marked decrease in insulin secretion in vivo during IPGTTs. While this decrease is consistent with the observed impairment in glucose tolerance, it implies that the initial absence of zinc during insulin secretion must enhance not only the first pass clearance of the hypersecreted insulin though the liver but also subsequent passages. In support of their model Tamaki et al. have shown that the rs13266634 SNP is associated with altered insulin clearance in humans, as assessed by c-peptide/insulin ratios [82]. However, only two other mouse studies examined insulin secretion in vivo during GGTs with one seeing a similar decrease in insulin secretion [13] and the other reporting no change [81]. Nevertheless, a change in insulin clearance would explain the low fasting plasma insulin observed in one study [78], though it would not explain why a similar decrease was not observed in others (Table 1).
The influence of genetic background and non-beta cell Slc30a8 expression
The variable results observed between the six KO mouse studies suggest that other factors must be influencing the effect of Slc30a8 deletion. Environmental differences might be important [90]; indeed, two groups [91, 92] have recently described an interaction between SCL30A8 SNPs and plasma trans-β-carotene and zinc, respectively, that modulate the influence of these SNPs on T2D risk. However, the most likely explanation for the variable results is the variable genetic background and hence the variable influence of compensatory modifier genes (Table 1). The influence of genetic background was initially apparent from the studies of Nicolson et al. [13] who described data derived from independent colonies of mixed genetic background global Slc30a8 KO mice established in London and Toronto. For example, glucose tolerance in males was normal in the Toronto colony at 12 weeks of age but impaired in the London colony, whereas GSIS was increased in isolated islets from KO mice in the Toronto colony at 12 weeks of age but no change was observed in isolated islets from KO mice in the London colony [13]. Similarly, when Pound et al. examined the effect of Slc30a8 deletion on a mixed [78] and pure C57BL/6J [79] genetic background, multiple differences were observed. Most notably, the reduction in fasting insulin in male mice and the reduction in isolated islet GSIS observed on a mixed genetic background were lost on the pure C57BL/6J background. Interestingly, the rs13266634 SLC30A8 variant is associated with T2D risk in Asians and Europeans, but not in Africans [66], suggesting that modifier genes may also influence the effect of SLC30A8 variations in humans.
The observation that mice with a global deletion of Slc30a8 do not have the same phenotype as mice with β cell-specific deletion [79, 82] (Table 1), even when the two colonies are on a pure C57BL/6J genetic background, suggests that Slc30a8 expression in other tissues also influences the phenotype of global KO mice. Indeed, although predominantly expressed in β cells in mice, ZnT8 is also found at lower levels in the testis, submaxillary glands, the cubical epithelium that lines the thyroid follicles and the adrenal cortex [43].
The potential influence of developmental compensation
The presumptive deleterious effects of a complete loss of ZnT8 function may not be apparent in global KO mice due to unknown compensatory gene expression changes during development. Consistent with this hypothesis, Fu an colleagues [52] demonstrated diminished uptake of exogenous zinc, reduced insulin content, and decreased GSIS following an acute ~90% reduction of ZnT8 using an shRNA-mediated approach in the INS-1 pancreatic β cell line. These acute suppression studies might reflect the true biological importance of ZnT8, but are unlikely to be relevant to providing mechanistic explanations for why lower expression of ZnT8 would be associated with reduced T2D risk.
The obvious candidates for compensatory factors are the other Slc30a family members. RT-PCR analyses showed low expression of several Slc30a isoforms in both mouse [13] and human islets [79], at least at the RNA level, but no evidence for a compensatory increase in the expression of these or Slc39a members in islets from mice with either global [13, 80] or β cell-specific [81] deletion. However, knockdown and KO mouse studies suggest that ZnT3 [50, 93] and ZnT7 [94] may play a functional role in regulating GSIS independent of ZnT8, at least in mice. Although clearly unable to mediate high-level zinc accumulation in ISGs, these data suggest that factors affecting the activity of other zinc transporters may be able to partially compensate for the loss of ZnT8, and could explain some of the variation in results obtained between the various animal models.
The impact of gender
It is apparent that multiple gender differences exist with respect to the effects of Slc30a8 deletion [95], though gender cannot explain the variable data shown in Table 1, which are all derived from studies on male mice. For example, Pound and colleagues [79] reported a decrease in fasting insulin levels in female but not male KO mice on the C57BL/6J genetic background. Similarly, Nicolson et al. [13] reported that female KO mice were glucose intolerant in their Toronto colony at 12 weeks of age whereas males were normal. In contrast, male mice were glucose intolerant in their London colony at 12 weeks of age whereas females were normal. Most strikingly, Lemaire et al. [80] reported no effect of Slc30a8 deletion on glucose tolerance in male mice at any age whereas female mice showed impaired glucose tolerance at 6 weeks of age, improved glucose tolerance at 12 weeks of age and no change at 25 weeks of age.
Concluding remarks and future perspectives
Overall, the six KO mouse studies show that in rodents the effects of Slc30a8 deletion are fairly modest (Table 1), especially given the uniformly dramatic decrease in free zinc levels in KO islets. Indeed, they imply that very low levels of zinc are sufficient for proper insulin secretion and that Zn2+ accumulation in ISGs is probably not as important for insulin secretion as previously thought. Consistent with this conclusion, guinea-pig insulin lacks a histidine residue in the B10 position of the molecule, which normally binds zinc in hexamer formation, and the zinc content of guinea pig islets is very low compared to mice and rats [96]. The extent to which GSIS and ISG biogenesis place a burden on the capacity of SLC30 and SLC39 family members to maintain zinc homeostasis is unknown, although it is evident that islet zinc content and insulin content do not change in parallel [27]. Given that the ISG compartment can turn over every 24h, this is clearly a highly dynamic process, and both net influx and the efflux of zinc from β cells are high relative to other cell types [97]. This may create vulnerability, such that an imbalance in ZnT8 function may lead to increased β cell stress that over time accelerates failure and progression to glucose intolerance and overt T2D.
The KO mouse studies were initiated with the anticipation that a complete loss of ZnT8 would be deleterious and would likely lead to the development of T2D. This assumption was based, at least in part, on an extrapolation of the initial GWAS studies in humans [61–64]. However, in the light of the recent study by Flannick and colleagues [67] it appears clear that the previous focus on the major, rather than the minor rs13266634 allele, may have been misleading. Indeed some of the KO data, such as the global Slc30a8KO mouse study by Pound et al. [33], which showed decreased GSIS, decreased fasting insulin and normal glucose tolerance, suggest improved rather than impaired glucose homeostasis, and are thus more consistent with the conclusion that decreased ZnT8 expression is protective against T2D.
At least four key questions remain to be answered. First, how is ZnT8 haploinsufficiency protective against T2D? Second, is ZnT8 inhibition a viable therapeutic strategy to prevent T2D? Third, since ZnT8 has been evolutionarily conserved, is there a biologically critical function of ZnT8 that is hidden by compensatory developmental mechanisms in KO mice? Fourth, how can the rare loss-of-function variants and the common rs13266634 encoded Trp variant, which has increased rather than decreased transporter activity [13, 60], share a common phenotype (reduced risk of T2D)? Do they have distinct mechanisms of action? The hypothesis that elevated ZnT8 activity is deleterious rather than protective has not been explored, and studies with transgenic mice will likely be required to test this possibility. Similarly, since murine IAPP, unlike the human protein, is not amyloidogenic, crosses of the slc30a8 KO and hIAPP transgenic mice will be required to determine the role, if any, of ZnT8 in amyloidogenesis. Intriguingly T2D is also associated with elevated plasma proIAPP/IAPP ratios [98], although a linkage with the ZnT8 rs13266634 SNP remains to be investigated.
It is also possible that the events that link ZnT8 to T2D occur outside the β cell. Indeed, two reports have shown that the rs13266634 SNP is linked to non-β cell changes. Thus, even though SLC30A8 is not expressed in liver or muscle, Tamaki and colleagues have shown that the SNP is associated with altered insulin clearance, as assessed by C-peptide/insulin ratios, consistent with their KO mouse data [82]. Similarly, Sprouse et al. [99] reported that the SNP is associated with altered muscle function, perhaps suggesting that ZnT8 influences communication between β cells and other tissues. The fact that these fundamental questions remain to be addressed indicates that much still remains to be learned about ZnT8.
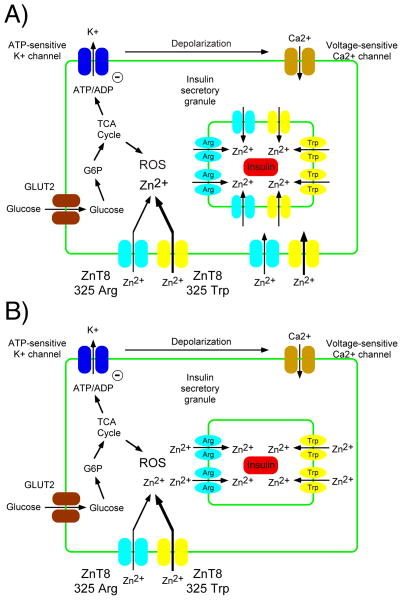
Figure 1. Hypothetical unified model to reconcile rare and common SLC30A8 genetic data.
The best characterized pathway for GSIS is shown, though other pathways clearly contribute [100]. We speculate that the minor rs13266634 allele encoding the 325 Trp ZnT8 variant and rare mutations leading to haploinsufficiency are protective against T2D due by prolonging the transient increase in cytoplasmic zinc levels that occur during GSIS thereby reducing the deleterious effects of ROS. In Panel A we speculate that the minor rs13266634 allele encoding the 325 Trp ZnT8 variant enhances cytoplasmic zinc levels mainly through increased re-uptake of co-secreted Zn2+ at the plasma membrane. In Panel B we speculate that rare mutations leading to haploinsufficiency enhance cytoplasmic Zn2+ levels mainly through a reduced rate of uptake into ISGs and that SLC39 family members can compensate for the slight decrease in ZnT8 mediated uptake at the plasma membrane.
BOX 2. ZnT8 structure and function.
ZnT8 is a member of the ancestral Cation Diffusion Facilitator (CDF) family of heavy metal transporters [48]. Based on protein sequence similarities reflecting metal ion specificities, most CDF members can be assigned to one of 3 groups: Zn (Group I), Fe/Zn (Group II), or Mn (Group III). Apart from ZnT9, the SLC30 family all show the molecular signature of group I [48]. ZnT8 shows the highest sequence similarity with ZnT2, ZnT3, and ZnT4 that form a distinct sub-group [48, 54], consistent with their orthologous roles in the secretory pathway where they mediate Zn2+ uptake into conventional and secretory lysosomes (ZnT2), synaptic vesicles (ZnT3), endosomes and constitutive secretory vesicles (ZnT4) and regulated secretory granules (ZnT8) respectively [55]. Like most CDF family members, ZnT8 is predicted to have 6 trans-membrane helices with both N and C termini localized to the cytoplasm ([45]; Box 1, Fig IB), and to function as a homo-dimer [13, 43]. Key residues regulating metal ion specificity are located in transmembrane domains II and V [56].
The 3-dimensional structure of ZnT8 is unknown, although two groups have developed models [13, 57] based upon the distantly related E. coli group II transporter FieF (YiiP) [58]. They are broadly in agreement, and support the proposed topology, but differ in their interpretation of the potential effect of substitutions at position 325, a key ZnT8 residue linked to T2D. Nicolson and colleagues predict that an Arg to Trp substitution will likely impact key Zn2+ binding sites [13], whereas Weijers concluded that the residue at position 325 is shielded, and hence unable to influence ZnT8’s metal ion sensing capacity [57]. Although informative, these models must be interpreted with care [59], as they use low-resolution templates to model regions of low sequence conservation (i.e. the region surrounding residue 325). An accurate assessment of the structural implications of substitutions at position 325 must await crystallization of human ZnT8.
Studies of ZnT8’s ability to transport zinc have so far been indirect, being based on measurements in over-expressing or knockdown cells [13, 45, 52, 56, 60]. They confirm that ZnT8 is a bona fide zinc transporter with specificity for Zn2+ but not Cd2+ [56], but cannot accurately measure kinetic parameters or relative efficiencies due to localization of ZnT8 to multiple subcellular compartments with distinct ionic environments, and the potentially confounding presence of other family members. Accurate measurement of transporter function must await reconstitution of purified ZnT8 into proteoliposomes.
Highlights.
ZnT8, the product of the SLC30A8 gene, mediates zinc uptake into insulin secretory granules within pancreatic islet beta cells.
Polymorphisms in SLC30A8 are associated with decreased risk for T2D in humans.
Slc30a8 knockout mice show a marked reduction in beta cell zinc content but a mild metabolic phenotype.
Potential mechanisms linking polymorphisms in SLC30A8 with changed risk for T2D include reduced insulin secretion, increased insulin clearance, or greater sensitivity to reactive oxygen species.
Acknowledgments
This manuscript is dedicated to the memory of John C. Hutton, PhD, mentor, collaborator, and colleague of the authors, who first stimulated our interest in ZnT8, and contributed many of the ideas expressed herein. We also thank Dr. David Powell for insightful comments on knockout mouse physiology. Work in H.W.D’s laboratory is supported by NIH R01-DK052068, Juvenile Diabetes Research Foundation research grants 17-2012-120 and 17-2012-609, and the Children’s Diabetes Foundation of Denver. Work in R.O’B’s laboratory is supported by NIH R01-DK092589 and American Diabetes Association grant 7-13-BS-119.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roohani N, et al. Zinc and its importance for human health: An integrative review. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 2.Plum LM, et al. The essential toxin: impact of zinc on human health. International journal of environmental research and public health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers SA, et al. Zinc transporters, mechanisms of action and therapeutic utility: implications for type 2 diabetes mellitus. Journal of nutrition and metabolism. 2012;2012:173712. doi: 10.1155/2012/173712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocchegiani E, et al. Zinc signalling and subcellular distribution: emerging targets in type 2 diabetes. Trends Mol Med. 2008;14:419–428. doi: 10.1016/j.molmed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oteiza PI. Zinc and the modulation of redox homeostasis. Free radical biology & medicine. 2012;53:1748–1759. doi: 10.1016/j.freeradbiomed.2012.08.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends in pharmacological sciences. 2011;32:480–486. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambe T. Molecular architecture and function of ZnT transporters. Current topics in membranes. 2012;69:199–220. doi: 10.1016/B978-0-12-394390-3.00008-2. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki E. ZnT8 and type 1 diabetes. Endocr J. 2012;59:531–537. doi: 10.1507/endocrj.ej12-0069. [DOI] [PubMed] [Google Scholar]
- 10.Vasak M, Meloni G. Chemistry and biology of mammalian metallothioneins. Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry. 2011;16:1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- 11.Zalewski PD, et al. Video image analysis of labile zinc in viable pancreatic islet cells using a specific fluorescent probe for zinc. J Histochem Cytochem. 1994;42:877–884. doi: 10.1177/42.7.8014471. [DOI] [PubMed] [Google Scholar]
- 12.Hutton JC, et al. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983;210:297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolson TJ, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–2083. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pound LD, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodson G, Steiner D. The role of assembly in insulin’s biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. doi: 10.1016/s0959-440x(98)80037-7. [DOI] [PubMed] [Google Scholar]
- 16.Dunn MF. Zinc-ligand interactions modulate assembly and stability of the insulin hexamer -- a review. Biometals. 2005;18:295–303. doi: 10.1007/s10534-005-3685-y. [DOI] [PubMed] [Google Scholar]
- 17.Voight BF, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westermark P, et al. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 20.Kahn SE, et al. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 21.Haataja L, et al. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29:303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masters SL, O’Neill LA. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends Mol Med. 2011;17:276–282. doi: 10.1016/j.molmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Brender JR, et al. Role of zinc in human islet amyloid polypeptide aggregation. J Am Chem Soc. 2010;132:8973–8983. doi: 10.1021/ja1007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salamekh S, et al. A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc. J Mol Biol. 2011;410:294–306. doi: 10.1016/j.jmb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knight JD, et al. Interaction of membrane-bound islet amyloid polypeptide with soluble and crystalline insulin. Protein science : a publication of the Protein Society. 2008;17:1850–1856. doi: 10.1110/ps.036350.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellomo EA, et al. Glucose regulates free cytosolic Zn(2) concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet beta-cells. J Biol Chem. 2011;286:25778–25789. doi: 10.1074/jbc.M111.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figlewicz DP, et al. 65Zinc and endogenous zinc content and distribution in islets in relationship to insulin content. Endocrinology. 1984;115:877–881. doi: 10.1210/endo-115-3-877. [DOI] [PubMed] [Google Scholar]
- 28.Slepchenko KG, Li YV. Rising intracellular zinc by membrane depolarization and glucose in insulin-secreting clonal HIT-T15 beta cells. Exp Diabetes Res. 2012;2012:190309. doi: 10.1155/2012/190309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin I, et al. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–1815. doi: 10.2337/diabetes.54.6.1808. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, et al. Zinc, not insulin, regulates the rat alpha-cell response to hypoglycemia in vivo. Diabetes. 2007;56:1107–1112. doi: 10.2337/db06-1454. [DOI] [PubMed] [Google Scholar]
- 31.Hardy AB, et al. Regulation of glucagon secretion by zinc: lessons from the beta cell-specific Znt8 knockout mouse model. Diabetes Obes Metab. 2011;13(Suppl 1):112–117. doi: 10.1111/j.1463-1326.2011.01451.x. [DOI] [PubMed] [Google Scholar]
- 32.Richards-Williams C, et al. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet beta-cells to potentiate insulin secretion. Purinergic signalling. 2008;4:393–405. doi: 10.1007/s11302-008-9126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pound LD, et al. Characterization of the human SLC30A8 promoter and intronic enhancer. J Mol Endocrinol. 2011;47:251–259. doi: 10.1530/JME-11-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung KW, et al. Expression of ZnT and ZIP zinc transporters in the human RPE and their regulation by neurotrophic factors. Invest Ophthalmol Vis Sci. 2008;49:1221–1231. doi: 10.1167/iovs.07-0781. [DOI] [PubMed] [Google Scholar]
- 35.Deniro M, Al-Mohanna FA. Zinc transporter 8 (ZnT8) expression is reduced by ischemic insults: a potential therapeutic target to prevent ischemic retinopathy. PLoS One. 2012;7:e50360. doi: 10.1371/journal.pone.0050360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smidt K, et al. Zinc-transporter genes in human visceral and subcutaneous adipocytes: lean versus obese. Molecular and cellular endocrinology. 2007;264:68–73. doi: 10.1016/j.mce.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Overbeck S, et al. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368–380. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 38.Eizirik DL, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohanasundaram D, et al. Ultrastructural analysis, zinc transporters, glucose transporters and hormones expression in New world primate (Callithrix jacchus) and human pancreatic islets. Gen Comp Endocrinol. 2011;174:71–79. doi: 10.1016/j.ygcen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Nica AC, et al. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome research. 2013;23:1554–1562. doi: 10.1101/gr.150706.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyulkhandanyan AV, et al. Investigation of transport mechanisms and regulation of intracellular Zn2+ in pancreatic alpha-cells. J Biol Chem. 2008;283:10184–10197. doi: 10.1074/jbc.M707005200. [DOI] [PubMed] [Google Scholar]
- 42.Schweiger M, et al. The zinc transporter ZnT8 (slc30A8) is expressed exclusively in beta cells in porcine islets. Histochemistry and cell biology. 2013 doi: 10.1007/s00418-013-1137-2. [DOI] [PubMed] [Google Scholar]
- 43.Murgia C, et al. Diabetes-linked zinc transporter ZnT8 is a homodimeric protein expressed by distinct rodent endocrine cell types in the pancreas and other glands. Nutr Metab Cardiovasc Dis. 2009;19:431–439. doi: 10.1016/j.numecd.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhong ML, et al. Widespread expression of zinc transporter ZnT (SLC30) family members in mouse endocrine cells. Histochemistry and cell biology. 2012;138:605–616. doi: 10.1007/s00418-012-0979-3. [DOI] [PubMed] [Google Scholar]
- 45.Chimienti F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–4206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 46.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson KA, et al. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J Biol Chem. 2007;282:10423–10431. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- 48.Montanini B, et al. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pound LD, et al. The pancreatic islet beta-cell-enriched transcription factor Pdx-1 regulates Slc30a8 gene transcription through an intronic enhancer. Biochem J. 2011;433:95–105. doi: 10.1042/BJ20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smidt K, et al. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One. 2009;4:e5684. doi: 10.1371/journal.pone.0005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egefjord L, et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: a potential role for zinc transporters in beta-cell apoptosis? BMC Endocr Disord. 2009;9:7. doi: 10.1186/1472-6823-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu Y, et al. Down-regulation of ZnT8 expression in INS-1 rat pancreatic beta cells reduces insulin content and glucose-inducible insulin secretion. PLoS One. 2009;4:e5679. doi: 10.1371/journal.pone.0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamaki M, et al. Downregulation of ZnT8 expression in pancreatic beta-cells of diabetic mice. Islets. 2009;1:124–128. doi: 10.4161/isl.1.2.9433. [DOI] [PubMed] [Google Scholar]
- 54.Kambe T, et al. Sequence similarity and functional relationship among eukaryotic ZIP and CDF transporters. Genomics Proteomics Bioinformatics. 2006;4:1–9. doi: 10.1016/S1672-0229(06)60010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kambe T. An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Bioscience, biotechnology, and biochemistry. 2011;75:1036–1043. doi: 10.1271/bbb.110056. [DOI] [PubMed] [Google Scholar]
- 56.Hoch E, et al. Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci U S A. 2012;109:7202–7207. doi: 10.1073/pnas.1200362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weijers RN. Three-dimensional structure of beta-cell-specific zinc transporter, ZnT-8, predicted from the type 2 diabetes-associated gene variant SLC30A8 R325W. Diabetol Metab Syndr. 2010;2:33. doi: 10.1186/1758-5996-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- 59.Dunbrack RL., Jr Sequence comparison and protein structure prediction. Curr Opin Struct Biol. 2006;16:374–384. doi: 10.1016/j.sbi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Kim I, et al. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. Pharmacogenomics J. 2011;11:191–198. doi: 10.1038/tpj.2010.22. [DOI] [PubMed] [Google Scholar]
- 61.Saxena R, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 62.Scott LJ, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 64.Zeggini E, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mtiraoui N, et al. Contribution of common variants of ENPP1, IGF2BP2, KCNJ11, MLXIPL, PPARgamma, SLC30A8 and TCF7L2 to the risk of type 2 diabetes in Lebanese and Tunisian Arabs. Diabetes & metabolism. 2012;38:444–449. doi: 10.1016/j.diabet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Xu K, et al. Association between rs13266634 C/T polymorphisms of solute carrier family 30 member 8 (SLC30A8) and type 2 diabetes, impaired glucose tolerance, type 1 diabetes--a meta-analysis. Diabetes Res Clin Pract. 2011;91:195–202. doi: 10.1016/j.diabres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Flannick J, et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014 doi: 10.1038/ng.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirchhoff K, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 69.Staiger H, et al. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS One. 2007;2:e832. doi: 10.1371/journal.pone.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruchat SM, et al. Association between insulin secretion, insulin sensitivity and type 2 diabetes susceptibility variants identified in genome-wide association studies. Acta Diabetol. 2009;46:217–226. doi: 10.1007/s00592-008-0080-5. [DOI] [PubMed] [Google Scholar]
- 71.Groenewoud MJ, et al. Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia. 2008;51:1659–1663. doi: 10.1007/s00125-008-1083-z. [DOI] [PubMed] [Google Scholar]
- 72.Boesgaard TW, et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients--the EUGENE2 study. Diabetologia. 2008;51:816–820. doi: 10.1007/s00125-008-0955-6. [DOI] [PubMed] [Google Scholar]
- 73.Cauchi S, et al. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Strawbridge RJ, et al. Genome-wide association identifies nine common variants associated with fasting proinsulin levels and provides new insights into the pathophysiology of type 2 diabetes. Diabetes. 2011;60:2624–2634. doi: 10.2337/db11-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimas AS, et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes. 2013 doi: 10.2337/db13-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valentine RA, et al. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J Biol Chem. 2007;282:14389–14393. doi: 10.1074/jbc.M701752200. [DOI] [PubMed] [Google Scholar]
- 77.Newsholme P, et al. Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J Endocrinol. 2012;214:11–20. doi: 10.1530/JOE-12-0072. [DOI] [PubMed] [Google Scholar]
- 78.Pound LD, et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J. 2009;421:371–376. doi: 10.1042/BJ20090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pound LD, et al. The physiological effects of deleting the mouse SLC30A8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One. 2012;7:e40972. doi: 10.1371/journal.pone.0040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lemaire K, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wijesekara N, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamaki M, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. The Journal of clinical investigation. 2013 doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gylfe E, Gilon P. Glucose regulation of glucagon secretion. Diabetes Res Clin Pract. 2014;103:1–10. doi: 10.1016/j.diabres.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Hutton J, et al. Proprotein processing and pancreatic islet function. Adv Exp Med Biol. 2004;552:39–65. [PubMed] [Google Scholar]
- 85.Davidson HW, Hutton JC. The insulin-secretory-granule carboxypeptidase H. Purification and demonstration of involvement in proinsulin processing. Biochem J. 1987;245:575–582. doi: 10.1042/bj2450575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naggert JK, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 87.Young GS, Kirkland JB. Rat models of caloric intake and activity: relationships to animal physiology and human health. Appl Physiol Nutr Metab. 2007;32:161–176. doi: 10.1139/h06-082. [DOI] [PubMed] [Google Scholar]
- 88.Hardy AB, et al. Effects of high-fat diet feeding on Znt8-null mice: differences between beta-cell and global knockout of Znt8. Am J Physiol Endocrinol Metab. 2012;302:E1084–1096. doi: 10.1152/ajpendo.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cauchi S, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet. 2008;9:45. doi: 10.1186/1471-2350-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovacs AD, Pearce DA. Location- and sex-specific differences in weight and motor coordination in two commonly used mouse strains. Scientific reports. 2013;3:2116. doi: 10.1038/srep02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel CJ, et al. Systematic identification of interaction effects between genome- and environment-wide associations in type 2 diabetes mellitus. Human genetics. 2013;132:495–508. doi: 10.1007/s00439-012-1258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kanoni S, et al. Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis. Diabetes. 2011;60:2407–2416. doi: 10.2337/db11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petersen AB, et al. siRNA-mediated knock-down of ZnT3 and ZnT8 affects production and secretion of insulin and apoptosis in INS-1E cells. APMIS. 2011;119:93–102. doi: 10.1111/j.1600-0463.2010.02698.x. [DOI] [PubMed] [Google Scholar]
- 94.Huang L, et al. Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J Biol Chem. 2012;287:33883–33896. doi: 10.1074/jbc.M111.309666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.da Silva Xavier G, et al. Animal models of GWAS-identified type 2 diabetes genes. Journal of diabetes research. 2013;2013:906590. doi: 10.1155/2013/906590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Havu N, et al. Zinc and manganese contents of micro-dissected pancreatic islets of some rodents. A microchemical study in adult and newborn guinea pigs, rats, Chinese hamsters and spiny mice. Acta Endocrinol (Copenh) 1977;86:570–577. [PubMed] [Google Scholar]
- 97.Formby B, et al. Relationship between insulin release and 65zinc efflux from rat pancreatic islets maintained in tissue culture. Diabetes. 1984;33:229–234. doi: 10.2337/diab.33.3.229. [DOI] [PubMed] [Google Scholar]
- 98.Zheng X, et al. Serum levels of proamylin and amylin in normal subjects and patients with impaired glucose regulation and type 2 diabetes mellitus. Acta Diabetol. 2010;47:265–270. doi: 10.1007/s00592-010-0201-9. [DOI] [PubMed] [Google Scholar]
- 99.Sprouse C, et al. SLC30A8 Non-synonymous Variant Is Associated with Recovery Following Exercise and Skeletal Muscle Size and Strength. Diabetes. 2013 doi: 10.2337/db13-1150. [DOI] [PubMed] [Google Scholar]
- 100.Jensen MV, et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]