Supplemental Digital Content is available in the text.
Key Words: hepatitis C virus, acute hepatitis, RNA fluctuation, clinical presentation, autoantibody
Abstract
Background:
Acute hepatitis C (AHCV) provides a diagnostic challenge with diverse clinical presentations.
Goals:
This study was aimed to examine the clinical and demographic features as well as outcomes in AHCV patients identified from inpatient and outpatient hospital settings.
Study:
Patients with suspected AHCV were recruited from Philadelphia VA Medical Center, Hospital of University of Pennsylvania and Brooklyn VA Medical Center between 2000 and 2010. AHCV was diagnosed by acute serum alanine aminotransferase elevation with anti-hepatitis C virus (HCV) seroconversion, HCV-RNA fluctuations above 1 log, and/or recent high-risk exposure without prior HCV infection, excluding those with human immunodeficiency virus infection. Clinical and therapeutic outcomes were monitored for at least 6 months.
Results:
A total of 40 AHCV patients were enrolled with a median follow-up of 129 weeks. They were mostly men (68%) and whites (73%) with median age of 43 years, diverse risk factors (33% injection drugs, 20% health care–associated, 3% sexual, and 45% unknown), and wide variations in peak alanine aminotransferase (143 to 3435 U/L) and total bilirubin levels (0.4 to 19.3 mg/dL). Viremia resolved spontaneously in 23% and persisted without therapy in 27%, whereas 50% received interferon α-based therapy with 90% cure (18/20). Distinct clinical scenarios included: (1) wide viremic fluctuations >1 log (65%) and intermittent HCV-RNA negativity; (2) autoantibodies (25% antinuclear antibodies, 69% antismooth muscle antibodies) or autoimmune features; (3) delayed spontaneous viral clearance in 2 patients; (4) rapid cirrhosis progression in 2 patients.
Conclusions:
AHCV is a heterogenous disease that requires careful monitoring. The lack of apparent risk factor in high proportion of patients and its diverse presentations warrant diagnostic vigilance.
Chronic hepatitis C virus (HCV) infection is a leading cause of chronic liver disease worldwide. Although the incidence of acute hepatitis C (AHCV) has been declining in recent years, such cases continue to occur in the community in the setting of injection drug use (IDU), health care–associated (HCA) procedures, and/or sexual exposures as well as unknown risk factors.1–7 While most untreated AHC progresses to chronicity, antiviral therapy–based on interferon α (IFNα) has been more effective when initiated during AHCV rather than with established chronic hepatitis C.8–11 Thus, a timely diagnosis and therapy of AHCV can prevent liver disease progression while limiting further spread of HCV infection.
However, diagnosis of AHCV can be challenging due to a wide spectrum of clinical presentation from completely asymptomatic to acute icteric and symptomatic hepatitis as well as fluctuating course of viremia and transaminase elevation.2,3,8,12,13 Diagnostic criteria for AHCV can include documented anti-HCV seroconversion (77%), alanine aminotransferase (ALT) elevation (68%), and HCV-RNA detection (63%).14 It remains unclear as to when anti-HCV seroconversion occurs relative to the timing of clinical presentation and laboratory testing.3,6,13 HCV seroconversion may not even occur with low-level exposures.15 In this regard, >1 log viremic fluctuations and low-titer HCV-RNA (uncommon in established chronic hepatitis C) support the diagnosis of AHCV.12,16
The virological outcome of AHCV is associated with various host and viral factors including age, gender, presence of symptoms and/or jaundice, antiviral T-cell responsiveness, immunogenetic polymorphisms, and viral coinfections.2,3,8,16–21 Typically, patients with self-limited AHCV have sustained viral clearance within the first 12 weeks of disease onset, whereas viremia beyond 6 months generally indicate chronic evolution (CE).5,8,10 If viremia is not spontaneously resolved within 12 weeks, IFNα-based treatment can achieve excellent sustained virological response (SVR) rate above 80% regardless of treatment regimen (monotherapy combined with ribavirin) or duration (typically 12 to 48 wk).1,8,9,11,22 However, up to 30% of the patients are not treated due to comorbidities that may present challenges to IFNα-based therapy.1,22 Chronic hepatitis C therapy is rapidly evolving into an IFN-free and directly acting antiviral regimens, with or without ribavirin, for as short a duration as 8 to 12 weeks and with high response rates.23 Although such regimens have not yet been fully evaluated in AHCV, they can be used in such cases as reported by Fierer et al24 and possibly even prophylactically after HCV exposure although the latter is contentious.
In our center, we have been prospectively enrolling AHCV patients since 2000 to examine the role of cellular immunity in HCV infection.16,17 In this study, we analyzed the clinical and demographic findings in our patient cohort recruited over a decade of IFNα-based therapeutics to highlight their natural history and outcomes.
MATERIALS AND METHODS
Patient Recruitment and Diagnosis
Patients with suspected AHCV were recruited between 2000 and 2010 at the Philadelphia VA Medical Center, Hospital of the University of Pennsylvania and Brooklyn VA Medical Center with written informed consent approved by the respective Institutional Review Boards. All patients underwent detailed qualitative interview and review of medical record by the clinical research coordinator and/or physicians for risk factor history including HCA procedures (eg, transfusion, surgical/medical procedures), piercing, tattooing, IDU, recreational drug use, and sexual exposures within a year of clinical onset as well as other history relevant for liver disease. Laboratory parameters included standard liver function parameters as well as serology for hepatitis A/B and human immunodeficiency virus (HIV). All patients were enrolled within 1 year of clinical onset and monitored for at least 6 months. Serum HCV-RNA, HCV genotype, and anti-HCV status were determined by standard commercial assays through the clinical laboratory (see Supplementary document for details, Supplemental Digital Content 1, http://links.lww.com/JCG/A122).
AHCV was diagnosed by acute serum ALT elevation with documented HCV seroconversion, spontaneous serum HCV-RNA fluctuations over 10-fold, and/or risk history within 1 year of clinical onset without a history of prior HCV infection or other causes of liver disease.12,14,16 HIV-coinfected patients were excluded. Among 50 AHCV patients initially identified, 10 were excluded for HIV coinfection or follow-up duration of <6 months, resulting in 40 AHCV patients with documented seroconversion (70%) and/or viremic fluctuations above 10-fold (65%) (Fig. 1). As the timing of HCV exposure was difficult to define, the clinical onset was operationally defined as the time of first documented ALT elevation. Among those with HCV seroconversion (70%), the median time from a negative HCV Ab test to clinical onset of AHCV was 16 weeks, although some may have been “recent” rather than “acute” hepatitis C.
FIGURE 1.
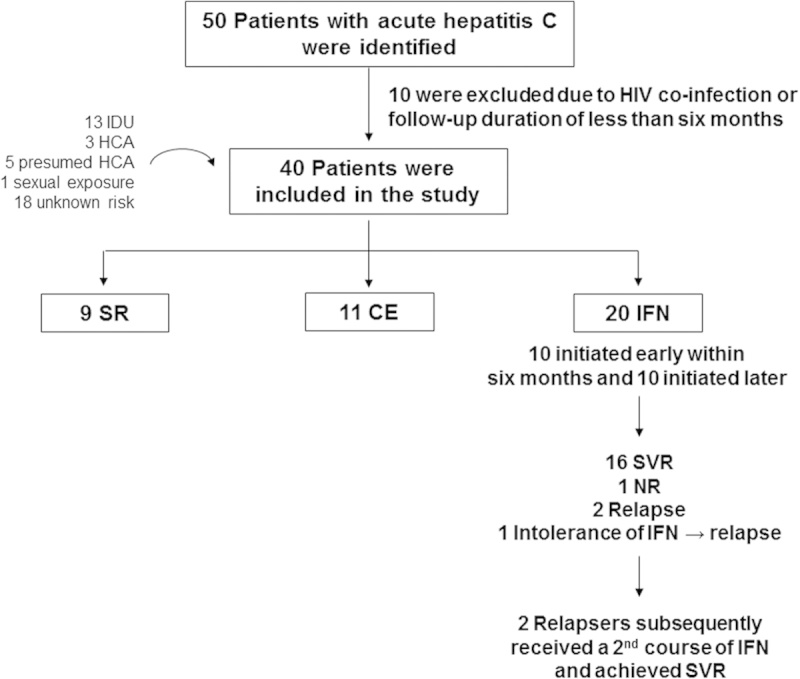
Study flow. CE indicates chronic evolution; HCA, health care–associated; HIV, human immunodeficiency virus; IDU, injection drug users; IFN, interferon; NR, nonresponse; SR, spontaneous resolution; SVR, sustained virological response.
Patient Grouping
Patients were grouped as: (1) spontaneous resolution (SR) with undetectable serum HCV-RNA and ALT normalization on at least 2 consecutive occasions at 3 or more months apart; (2) CE with persistent HCV-RNA for over 6 months; (3) IFN group who received 2 or more doses of IFNα±ribavirin.
HCV Therapy
HCV therapy included nonpegylated to pegylated IFN either alone or with ribavirin, initiated by the primary clinical provider based on clinical indications and contraindications as well as patient’s motivation.25 Treatment was defined as early or late, based on their initiation before or after 6 months (or 26 wk) from clinical onset, respectively. SVR was defined as undetectable HCV-RNA at 24 weeks from treatment cessation.
Statistical Analysis
Categorical variables were analyzed using the Pearson χ2 or the Fisher exact test. Continuous variables were examined using nonparametric Mann-Whitney U or Kruskal-Wallis test, with P-value below 0.05 considered significant.
RESULTS
Patient Characteristics
A total of 40 subjects with AHCV were enrolled between 2000 and 2010 as described in Materials and methods section and monitored over a median duration of 129 weeks. They included 9 patients with SR (23%), 11 patients with CE without therapy (27%), and 20 with IFNα-based therapy (50% IFN) (Tables 1, 2). The patients were 68% males, 73% white, and wide in age range (18 to 75 y). IL28B genotype was defined in 38/40 subjects as 53% CC, 30% CT, and 13% TT (Table 1B). HCV genotype 1 was most prevalent (80%) followed by genotype 3 (13%) (Table 1C). HCV clearance was ultimately achieved in 27/40 (68%) including 9 SR, 16 IFN patients achieving SVR with initial therapy, and 2 IFN patients who initially relapsed but achieved SVR with second therapy.
TABLE 1.
Summary of Demographic, Virological, and Clinical Parameters
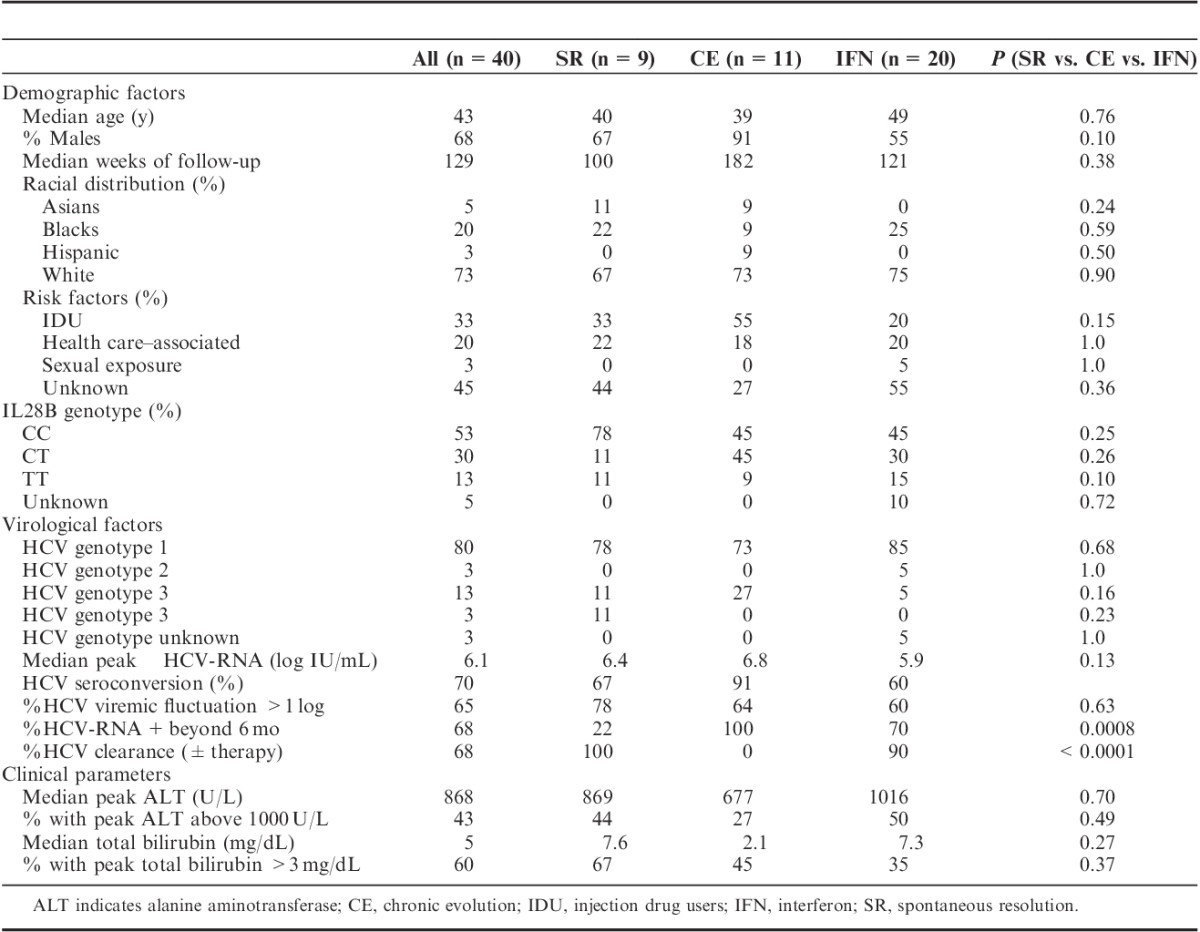
TABLE 2.
Demographic, Clinical, and Virological Parameters in Individual Patients
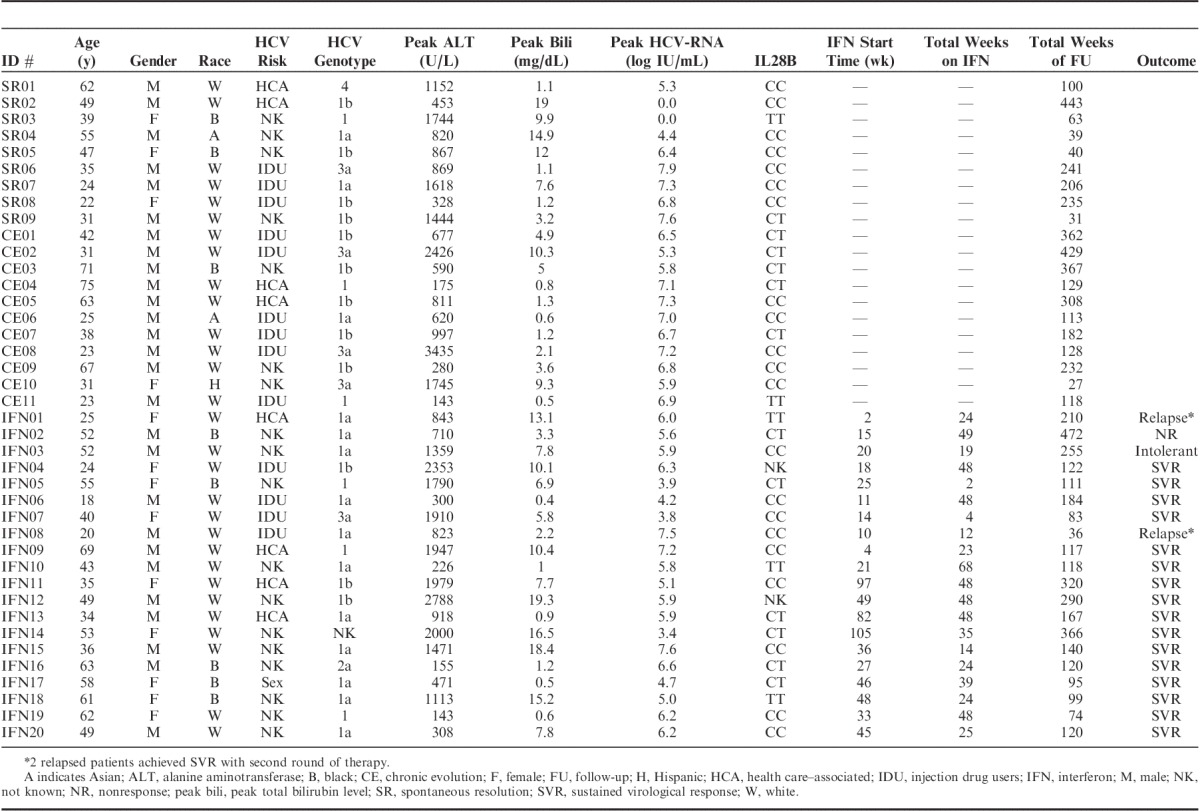
All patients had elevated ALT activity consistent with our inclusion criteria, whereas 24/40 (60%) displayed jaundice with total bilirubin ≥3 mg/dL (Tables 1, 2, Fig. 2A). Severe liver inflammation and/or jaundice occurred in 17/40 (43%) with ALT above 1000 U/L (Table 1D), including 5/40 (13%) with ALT above 2000 U/L and 11/40 (28%) with total bilirubin above 10 mg/dL. As for HCV-RNA, median peak HCV-RNA titer was 6.1 log10 IU/L with titers above 7 log in 11/40 patients (28%). ALT activity was associated with total bilirubin levels (R’s=0.57, P=0.00014) but not HCV-RNA titers (Fig. 2B). The patient groups did not differ in clinical or demographic parameters or IL28B genotype distribution.
FIGURE 2.

Clinical and virological course in acute hepatitis C with spontaneous resolution or chronic evolution. ALT indicates serum alanine aminotransferase; CE, chronic evolution; IFN, interferon; SR, spontaneous resolution.
Diverse Risk Factors for AHCV
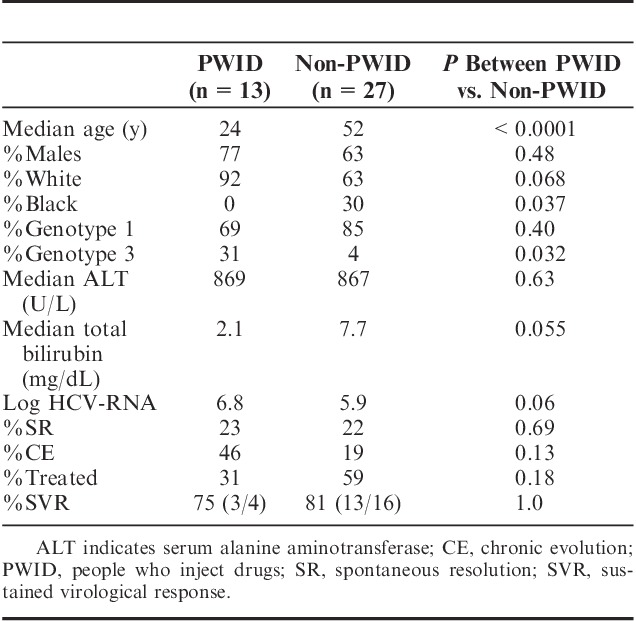
Potential risk factors for HCV transmission included (Table 1A): IDU (33%), HCA (20%), sexual exposures (3%). No risk factors were identified in 19/40 (45%). There were no associated recent transfusions, piercing, or tattoos. As shown in Table 3, persons who inject drugs (PWID) included mostly young white males who started injection drugs within 1 to 2 years. They were enriched for HCV genotype 3 compared with non-PWID (31% vs. 4%, P=0.032) with a tendency for lower total bilirubin level (P=0.055). PWID also showed a tendency for greater CE (46% vs. 18%, P=0.13) and less treatment initiation (31% vs. 59%, P=0.18) in part due to poor follow-up with ongoing drug use and/or incarceration, highlighting a need to systematically motivate, educate, and treat PWID and persons in correctional facilities.26,27 There was no difference in %SVR between PWID and non-PWID (75% vs. 81%, P=1.0), similar to previous reports.28
TABLE 3.
Characteristics of People Who Inject Drugs
Among 8 patients with HCA risk factors, 2 had documented HCV exposures: IFN01 was a nurse with HCV-contaminated needle-stick accident, whereas IFN13 received HCV-contaminated tendon graft. Another patient was on chronic hemodialysis—a known risk factor for HCV infection. In the remaining 5 patients in HCA group, the precise mode of transmission was not defined except for recent medical/surgical procedures (eg, cardiac catheterization, prostate biopsy, upper endoscopy, or abdominal surgery within 6 mo of clinical presentation). HCV risk factor was presumed to be sexual in a 58-year-old female patient IFN17 (3%) who was newly married to an HCV-infected male in the absence of other known risk factors, although specific details of their sexual practice were not obtained. Thus, IDU accounted for only 30% of HCV transmission in our AHCV cohort, whereas the majority had either no apparent risk factor (47%) or only temporal associations with medical/surgical procedures (20%).
Virological Outcome to IFNα-based Antiviral Therapy
Twenty patients received IFNα therapy (standard or pegylated) either alone or combined with ribavirin (Table 2B). Median time from onset to treatment initiation was 25.8 weeks (range, 2 to 105 wk). Median treatment duration was 30 weeks (range, 2 to 68 wk). Antiviral therapy was initiated within 6 months in 10 patients (IFN01 to 10) and beyond 6 months in 10 others (IFN11 to 20). The later therapy was in part due to fluctuating viremia with transiently low to undetectable HCV-RNA in 7 as well as delayed referral, concurrent medical issues, and/or personal preference.
SVR was achieved in 16/20 (80%) with initial therapy, including 2/3 patients with IL28B TT genotype. Paradoxically, all 4 SVR-negative patients received early IFN therapy, resulting in a lower %SVR in early than late IFN group (60% vs. 100%, P=0.087). In particular, IFN02 was refractory to antiviral treatment and maintained viremia above 5 logs throughout 49 weeks of combination IFNα and ribavirin therapy. IFN03 was intolerant of IFN therapy and required treatment cessation at 19 weeks with subsequent virological relapse. Two patients (IFN01, IFN08) with viremic relapses after 24 and 12 weeks, respectively, achieved SVR after a second round of therapy. Of note, IFN01 has IL28B TT genotype, whereas IFN08 had IL28B CC genotype. There were no apparent differences in demographic, clinical, and virological characteristics between early and late IFN groups or between SVR+ and SVR− patients (data not shown), although our sample size was small. Thus, the overall treatment response was excellent in our AHCV cohort regardless of early and late therapy or IL28B genotype.
Distinct Features in Natural History and Outcomes of AHCV
There were several distinct features in our AHCV cohort as shown below.
Viremic Course in Early and Late Phase of AHCV
Spontaneous viremic fluctuations over 10-fold (median 1.5 log fold) were observed in 26/40 (65%) patients, irrespective of IL28B genotype. This included rapid and sustained loss of detectable HCV-RNA titers in most SR patients within the first 6 months and multiple bouts of intermittent HCV-RNA negativity followed by continued viremia in some CE patients (eg, CE2 in Fig. 3B and IFN11 in Supplementary Figure 1S, Supplemental Digital Content 2, http://links.lww.com/JCG/A123). Interestingly, 2 SR patients (SR06, SR08) with stable viremia in the early phase cleared viremia beyond 6 months from onset. As shown in Figure 3C, SR06 was persistently viremic with the latest documented viremia at week 46, before becoming temporarily lost to follow-up for recurrent drug use, incarceration, and pregnancy. She then returned with undetectable HCV-RNA and normal ALT at weeks 191 and 241. Similarly, SR08 was viremic up to 98 weeks, but became repeatedly HCV-RNA-negative at weeks 119, 194, and 235 with normal ALT. Neither patients showed evidence of concurrent HBV infection that can influence HCV outcome29 and were negative for HBsAg, anti-HBc, and anti-HBs. Both patients had IL28B CC genotype and had not received antiviral therapy. Thus, among 20 patients without antiviral therapy, 2 (10%) achieved delayed viral clearance beyond the first 6 months, similar to delayed viral clearance in 3/15 Italian patients during an AHCV outbreak.30
FIGURE 3.
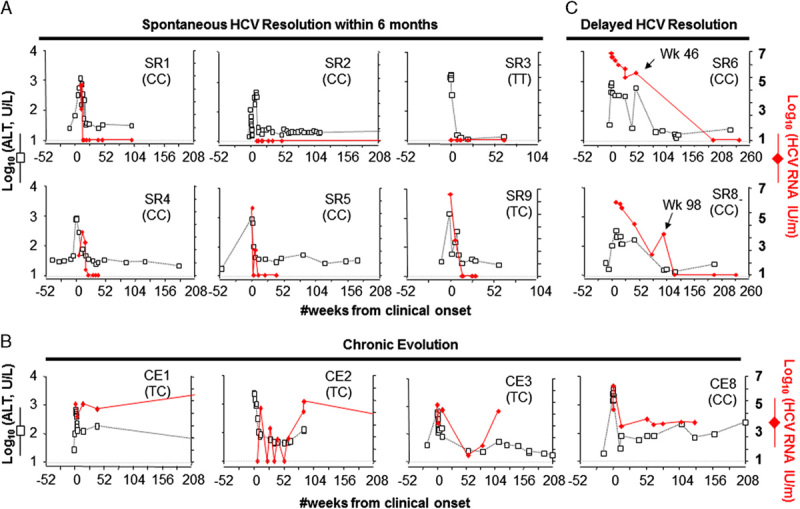
Clinical and virological parameters in acute hepatitis C relative to outcome and IL28B genotype. Letters in parentheses (CC, TT, CT) are IL28B genotypes. ALT indicates serum alanine aminotransferase; SR, spontaneous resolution; CE, chronic evolution.
Autoantibodies and/or Autoimmune Hepatitis (AIH)
On the basis of the associations between HCV infection and autoimmune markers,31,32 serum antinuclear antibodies (ANA) and antismooth muscle antibodies (ASMA) were obtained in 28 and 16 patients, respectively. ANA were detected in 7/28 patients tested (25%) and ASMA were detected in 11/16 patients tested (69%) although mostly at low titers (≤1:80) and without preexisting autoimmune conditions. However, higher ANA titers (1:2560) were detected in IFN11, a 35-year-old white woman without associated autoimmune condition. A second patient (IFN14) was a 53-year-old white woman with liver histology showing interface hepatitis suggestive of AIH but no detectable autoantibodies that developed an ALT flare upon corticosteroid therapy. Fortunately, both patients achieved SVR upon subsequent IFNα-based therapy. Their clinical details are described in Supplementary document and Figure 1S (Supplemental Digital Content 2, http://links.lww.com/JCG/A123).
Rapid Liver Disease Progression
Cirrhosis generally develops gradually over decades of chronic hepatitis C.33 However, rapid fibrosis progression into frank clinical cirrhosis occurred in CE05 and IFN02 without a prior history of liver disease. As shown in Table 2, CE05 was a 63-year-old white male with CE of AHCV who did not undergo antiviral therapy due to concurrent medical issues and chaotic lifestyle due to homelessness. Unfortunately, he developed clinically apparent cirrhosis with thrombocytopenia, splenomegaly, and ascites within 5 years of AHCV presentation. The second patient (IFN02) was a 52-year-old African American male who remained persistently viremic despite multiple rounds of IFNα-based antiviral with platelet count spontaneously declining below 130 k within 7 years from AHCV and F4 fibrosis on liver biopsy within 10 years.
On the basis of these findings, fibrosis progression was estimated in a noninvasive manner using Fib-4 index based on the formula previously defined: age ([y]×AST [U/L])/((platelets [109/L])×(ALT [U/L])(1/2)).34,35 As shown in Figure 4, transient Fib-4 index elevations were observed during initial acute hepatitis due to high AST levels (eg, SR02, SR04, CE03, and CE08). Fib-4 index elevations were seen in both patients with IL28B CC and non-CC genotypes. Prolonged Fib-4 index elevation during the first 2 year was also seen in CE09 whose liver biopsy during acute hepatitis showed moderate to bridging fibrosis, although cirrhosis could not be assessed due to competing medical issues (metastatic lung cancer, prostate cancer, and cardiovascular disease). By contrast, both CE05 and IFN02 showed Fib-4 index levels persistently above 3.25 within the first 3 years of AHCV (Fig. 4B), consistent with their clinical cirrhosis progression. Otherwise, prolonged Fib-4 elevation and clinical cirrhosis was not seen in patients with spontaneous or IFN-related HCV clearance. Thus, rapid fibrosis progression was observed in 2/13 (15%) patients with persistent viremia after AHCV and in 2/40 (5%) of the overall AHCV cohort.
FIGURE 4.
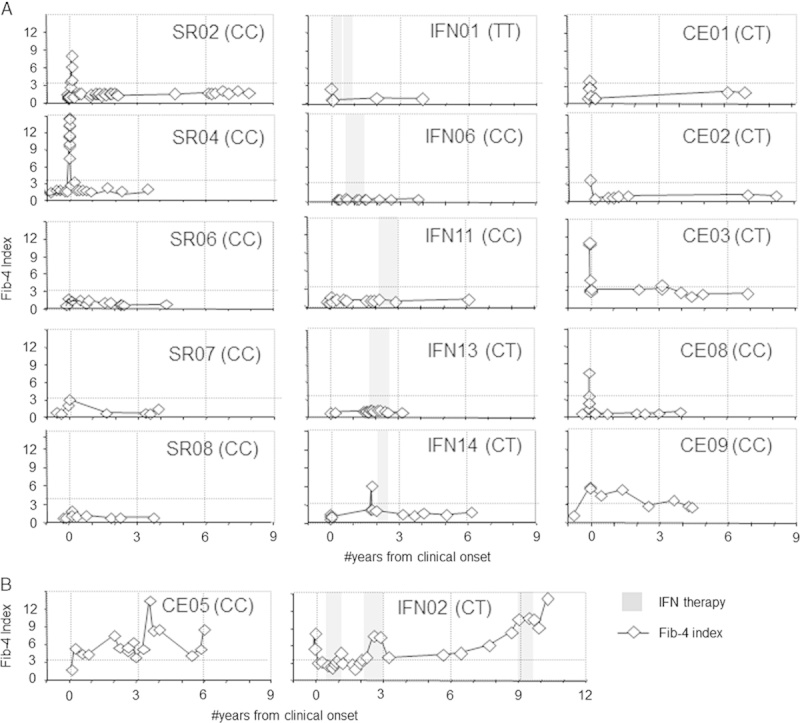
Evolution in Fib-4 index during and after acute hepatitis C. Letters in parentheses (CC, TT, CT) are IL28B genotypes. CE indicates chronic evolution; IFN, interferon; SR, spontaneous resolution.
DISCUSSION
In this study, we examined AHCV patients identified from inpatient and outpatient hospital settings by clinical symptoms, exposure history, and/or clinical laboratory abnormalities. Our cohort is characterized by predominantly white ethnicity, male gender, HCV genotype 1 infection, and wide viremic fluctuations as well as relatively high levels of ALT and total bilirubin. HCV infection spontaneously resolved in 23%, resolved with therapy in 90% of those treated, and persisted regardless of therapy in 33% with several distinct demographic and clinical features. AHCV identified in the context of clinical suspicion may differ in natural history and outcome, with a greater likelihood for SR when compared with clinically silent cases,2,8,19 although SR was observed in only 23% of our cohort.
HCV risk factors in our cohort were diverse with several notable features. First, we were struck by the lack of defined HCV-associated risk factors in 18/40 (45%) patients. Moreover, among 8 subjects with HCA risk factors, exposure was only presumed without a clear source in 5 with recent medical, surgical, and/or dental interventions. This ultimately resulted in 23/40 without a clear route of HCV transmission (58%). Our findings contrast from the MMWR surveillance summary listing PWID as the highest risk factor (48%), followed by multiple sexual partners (42%) and unknown (35%).36 These differences reflect different data collection methodologies and populations examined. For example, we did not collect explicit information on sexual exposures or non-IDU (eg, methamphetamine), as described in HIV-infected men who have sex with men in the New York City epidemic.37 Nevertheless, a lack of identifiable risk factor, as was noted in our AHCV cohort, can result in psychological distress for the patients (and their families) while limiting preventative strategies. Second, our findings suggest that AHCV should be considered in all persons presenting with acute hepatitis, even without apparent risk factors. Indeed, 2 patients in our cohort were over 70 years old and AHCV may not have been considered given their age. Recent hospitalizations with presumably an exposure to HCV infection may have been the mode of acquisition in one of these patients. Of note, risk factors for AHCV may differ between HIV-negative and HIV-positive patients with rising incidence of AHCV observed among HIV-positive men who have sex with men.37–40 Third, CE was more frequent in PWID compared with others (46% vs. 19%, P=0.13). Although the difference did not reach statistical significance, this could reflect reinfection or superinfection previously reported among recently HCV-infected patients with ongoing IDU.41
Overall, SVR was achieved in 80% upon initial therapy and in an additional 2/2 patients with repeat therapy (overall 90% in treated patients) regardless of IL28B genotype. All 4 patients with initial treatment failure received early therapy within 6 months of clinical onset, contrasting with a superior therapeutic response associated with early therapy in some but not all studies.8–10 The rate of SVR was similar among patients who acquired AHCV through injection drugs and other routes (75% vs. 81%, P=1.0) as reported previously.28,42 Of note, 90% SVR in our AHCV cohort is higher than the 50% SVR rates reported for patients with chronic HCV genotype 1 infection.43 Among 2 patients who failed IFN therapy, IFN02 showed primary IFN resistance, whereas IFN08 was intolerant. Limited initial treatment duration may have been a factor in IFN01 and IFN08 who relapsed but achieved SVR with retreatment. However, short-term therapy can be effective for AHCV, as IFN05 and IFN15 achieved SVR with only 2 and 14 weeks of therapy, respectively. Thus, virological response to IFN-based therapy was generally excellent in AHCV regardless of timing and duration of therapy, IDU, or IL28B genotype.
The rate of fibrosis progression in HCV-infected patients can depend on various host, environmental, and viral factors.44 One large systematic review of 111 studies estimated the prevalence of cirrhosis after 20 years of infection to be 14% to 19%.45 In our cohort, 2 patients (CE05, IFN02) rapidly progressed to cirrhosis within 5 to 8 years without HIV coinfection or HBV coinfection46,47 or immunosuppression.48 Although CE05 also suffered from diabetes and obesity, IFN02 had no known factors associated with liver disease progression. Unfortunately, CE05 was not a candidate for IFNα-based therapy, whereas IFN02 was IFN resistant likely related to African American heritage and HCV genotype 1 infection49 as well as IL28B non-CC genotype.
It is important to emphasize the potential benefits of timely AHCV diagnosis. For example, this can provide valuable window of opportunity for risk reduction counseling, disruption of transmission networks, evaluation for other blood-borne or sexually transmitted infections, education about HCV and liver disease, therapeutic options, and monitoring strategies to ultimately limit health risks at the individual and population levels. Our finding of AHCV in young IDUs also highlights a continued need to screen this population and for preventative interventions (eg, access to clean syringes, addiction treatment, and opiate replacement therapy).
Of interest is the delayed spontaneous HCV clearance beyond 46 to 98 weeks of infection in 2 patients with IL28 CC genotype. Such a phenomenon has been reported in 3/15 Italian patients with AHCV,30 although the IL28B genotype of the Italian patients is unknown. One can only speculate that the dynamic host-virus interactions in early HCV infection may be protracted and that such a phenomenon might be related to IL28B polymorphism.
Chronic hepatitis C has been associated with autoimmune conditions50 and AIH with serum autoantibodies detected in up to 18% although not anti-KLM.31,32 Although pathogenetic mechanisms remain unclear, continued antigenic exposure during chronic HCV infection could lead to B-cell stimulation. Autoantibodies were frequent in our AHCV cohort (25% ANA, 69% ASMA), suggesting that their induction does not require long-term HCV persistence. As for their clinical relevance, the presence of autoantibodies in HCV-infected patients may mislead clinicians to diagnose and treat for AIH. Indeed, corticosteroid administration for suspected AIH resulted in worsening transaminases in IFN14, whereas high ANA titers contributed to delayed treatment in IFN11. In both patients, however, IFNα therapy led to resolution of hepatitis without an apparent AIH flare, indicating that their autoimmune features were HCV-mediated and innocent bystanders.
In conclusion, AHCV can present in a heterogenous manner. A heightened awareness regarding AHCV is needed, given the high proportion of patients with no apparent HCV-associated risk factor and its diverse presentations. Although therapeutic outcome of AHCV is excellent with IFNα-based therapy, greater improvement is anticipated with the use of direct-acting antivirals, provided that there is accurate and timely diagnosis of AHCV.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the research subjects who participated in this study and the clinical and laboratory staff that provided assistance.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.jcge.com.
This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs with VA Merit Review IOBX000649 and with the resources and the use of facilities at the Philadelphia VA Medical Center. This study was also supported by NIH Grants R01-AI-47519 and R01-AA-12849; the Philadelphia VA Medical Research; NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases P30DK50306 and its Molecular Biology and Cell Culture Core Facilities; the NIH Public Health Service Research Grant M01-RR00040. D.E.K. was supported by the NIH T32 DK 07066, NIH Loan Repayment Program, AASLD/Schering Advanced Hepatology Fellowship, NIH K12-RR-017625, and the VA Career Development Award and the VA Stars and Stripes Award.
C.B.: study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; L.M.J., M.K., and A.A.: acquisition of data; M.E.V., D.E.K., and F.A.N.: patient recruitment and acquisition of data; K.R.R.: study concept and design, patient recruitment, interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision; K.-M.C.: study concept and design, patient recruitment, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtained funding, administrative support, and study supervision. The content of this article does not necessarily reflect the views of the Department of Veterans Affairs or of the US government.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Dore GJ, Hellard M, Matthews GV, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138:123–135e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol. 2008;103:1283–1297quiz 1298. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Rivera MM, McBurney R, et al. The natural history of acute hepatitis C: clinical presentation, laboratory findings and treatment outcomes. Aliment Pharmacol Ther. 2011;33:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morin T, Pariente A, Lahmek P, et al. Acute hepatitis C: analysis of a 126-case prospective, multicenter cohort. Eur J Gastroenterol Hepatol. 2010;22:157–166. [DOI] [PubMed] [Google Scholar]
- 5.Santantonio T, Medda E, Ferrari C, et al. Risk factors and outcome among a large patient cohort with community-acquired acute hepatitis C in Italy. Clin Infect Dis. 2006;43:1154–1159. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Krantz E, Klarquist J, et al. Acute hepatitis C in a contemporary US cohort: modes of acquisition and factors influencing viral clearance. J Infect Dis. 2007;196:1474–1482. [DOI] [PubMed] [Google Scholar]
- 7.Spada E, Mele A, Mariano A, et al. Risk factors for and incidence of acute hepatitis C after the achievement of blood supply safety in Italy: results from the national surveillance system. J Med Virol. 2013;85:433–440. [DOI] [PubMed] [Google Scholar]
- 8.Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003;125:80–88. [DOI] [PubMed] [Google Scholar]
- 9.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. [DOI] [PubMed] [Google Scholar]
- 10.Kamal SM, Fouly AE, Kamel RR, et al. Peginterferon alfa-2b therapy in acute hepatitis C: impact of onset of therapy on sustained virologic response. Gastroenterology. 2006;130:632–638. [DOI] [PubMed] [Google Scholar]
- 11.Kamal SM, Moustafa KN, Chen J, et al. Duration of peginterferon therapy in acute hepatitis C: a randomized trial. Hepatology. 2006;43:923–931. [DOI] [PubMed] [Google Scholar]
- 12.McGovern BH, Birch CE, Bowen MJ, et al. Improving the diagnosis of acute hepatitis C virus infection with expanded viral load criteria. Clin Infect Dis. 2009;49:1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang CT, Tobler LH, Haesche C, et al. Fluctuation of HCV viral load before seroconversion in a healthy volunteer blood donor. Transfusion. 2003;43:541–544. [DOI] [PubMed] [Google Scholar]
- 14.Hajarizadeh B, Grebely J, Dore GJ. Case definitions for acute hepatitis C virus infection: a systematic review. J Hepatol. 2012;57:1349–1360. [DOI] [PubMed] [Google Scholar]
- 15.Heller T, Werner JM, Rahman F, et al. Occupational exposure to hepatitis C virus: early T-cell responses in the absence of seroconversion in a longitudinal cohort study. J Infect Dis. 2013;208:1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DE, Sugimoto K, Newton K, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan DE, Ikeda F, Li Y, et al. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol. 2008;48:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller T, Rehermann B. Acute hepatitis C: a multifaceted disease. Semin Liver Dis. 2005;25:7–17. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Fisher BE, Thomas DL, et al. Spontaneous clearance of primary acute hepatitis C virus infection correlated with high initial viral RNA level and rapid HVR1 evolution. Hepatology. 2012;55:1684–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tillmann HL, Thompson AJ, Patel K, et al. A polymorphism near IL28B is associated with spontaneous clearance of acute hepatitis C virus and jaundice. Gastroenterology. 2010;139:1586–1592e1. [DOI] [PubMed] [Google Scholar]
- 22.Wiegand J, Buggisch P, Boecher W, et al. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43:250–256. [DOI] [PubMed] [Google Scholar]
- 23.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. [DOI] [PubMed] [Google Scholar]
- 24.Fierer DS, Dieterich DT, Mullen MP, et al. Telaprevir in the treatment of acute HCV infection in HIV-infected men: SVR 12 results. Hepatology. 2013;55:227A. [Google Scholar]
- 25.Yee HS, Chang MF, Pocha C, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107:669–689quiz 690. [DOI] [PubMed] [Google Scholar]
- 26.McGovern BH, Wurcel A, Kim AY, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–1670. [DOI] [PubMed] [Google Scholar]
- 27.Robaeys G, Grebely J, Mauss S, et al. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis. 2013;57suppl 2S129–S137. [DOI] [PubMed] [Google Scholar]
- 28.Grebely J, Raffa JD, Meagher C, et al. Directly observed therapy for the treatment of hepatitis C virus infection in current and former injection drug users. J Gastroenterol Hepatol. 2007;22:1519–1525. [DOI] [PubMed] [Google Scholar]
- 29.Liaw YF, Yeh CT, Tsai SL. Impact of acute hepatitis B virus superinfection on chronic hepatitis C virus infection. Am J Gastroenterol. 2000;95:2978–2980. [DOI] [PubMed] [Google Scholar]
- 30.Larghi A, Zuin M, Crosignani A, et al. Outcome of an outbreak of acute hepatitis C among healthy volunteers participating in pharmacokinetics studies. Hepatology. 2002;36:993–1000. [DOI] [PubMed] [Google Scholar]
- 31.Williams MJ, Lawson A, Neal KR, et al. Autoantibodies in chronic hepatitis C virus infection and their association with disease profile. J Viral Hepat. 2009;16:325–331. [DOI] [PubMed] [Google Scholar]
- 32.Reddy KR, Krawitt EL, Homberg JC, et al. Absence of anti-LKM-1 antibody in hepatitis C viral infection in the United States of America. J Viral Hepat. 1995;2:175–179. [DOI] [PubMed] [Google Scholar]
- 33.Ghany MG, Kleiner DE, Alter H, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. [DOI] [PubMed] [Google Scholar]
- 34.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. [DOI] [PubMed] [Google Scholar]
- 35.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 36.Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis—United States, 2007. MMWR Surveill Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- 37.Fierer DS, Factor SH, Uriel AJ, et al. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60:945–950. [PubMed] [Google Scholar]
- 38.Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis-C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaphe S, Bozinoff N, Kyle R, et al. Incidence of acute hepatitis C virus infection among men who have sex with men with and without HIV infection: a systematic review. Sex Transm Infect. 2012;88:558–564. [DOI] [PubMed] [Google Scholar]
- 40.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grebely J, Pham ST, Matthews GV, et al. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57suppl 2S80–S89. [DOI] [PubMed] [Google Scholar]
- 43.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. [DOI] [PubMed] [Google Scholar]
- 44.Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008;134:1699–1714. [DOI] [PubMed] [Google Scholar]
- 45.Thein HH, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. [DOI] [PubMed] [Google Scholar]
- 46.Fierer DS, Mullen MP, Dieterich DT, et al. Early-onset liver fibrosis due to primary hepatitis C virus infection is higher over time in HIV-infected men. Clin Infect Dis. 2012;55:887–888author reply 888–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel M, Page E, Boesecke C, et al. Liver fibrosis progression after acute hepatitis C virus infection in HIV-positive individuals. Clin Infect Dis. 2012;54:556–559. [DOI] [PubMed] [Google Scholar]
- 48.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver Transpl. 2008;14suppl 2S36–S44. [DOI] [PubMed] [Google Scholar]
- 49.Brau N, Bini EJ, Currie S, et al. Black patients with chronic hepatitis C have a lower sustained viral response rate than non-Blacks with genotype 1, but the same with genotypes 2/3, and this is not explained by more frequent dose reductions of interferon and ribavirin*. J Viral Hepat. 2006;13:242–249. [DOI] [PubMed] [Google Scholar]
- 50.Ferri S, Muratori L, Lenzi M, et al. HCV and autoimmunity. Curr Pharm Des. 2008;14:1678–1685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


