Abstract
Epithelial-mesenchymal transition (EMT) is an essential step for tumor progression, although the mechanisms driving EMT are still not fully understood. In an effort to investigate these mechanisms, we observed that heregulin-mediated activation of HER2, or HER2 overexpression, resulted in EMT, which is accompanied with increased expression of a known EMT regulator Slug, but not TWIST or Snail. We then investigated how HER2 induced Slug expression and found, for the first time, that there are four consensus HSF Sequence-binding Elements (HSEs), the binding sites for heat shock factor-1 (HSF-1), located in the Slug promoter. HSF-1 bound to and transactivated the Slug promoter independent of heat shock, leading to Slug expression in breast cancer cells. Mutation of the putative HSEs ablated Slug transcriptional activation induced by heregulin or HSF-1 overexpression. Knockdown of HSF-1 expression by siRNA reduced Slug expression and heregulin-induced EMT. The positive association between HSF-1 and Slug was confirmed by immunohistochemical staining of a cohort of 100 invasive breast carcinoma specimens. While investigating how HER2 activated HSF-1 independent of heat shock, we observed that HER2 activation resulted in concurrent phosphorylation of Akt and HSF-1. We then observed, also for the first time, that Akt directly interacted with HSF-1 and phosphorylated HSF-1 at S326. Inhibition of Akt using siRNA, dominant-negative Akt mutant, or small molecule inhibitors prevented heregulin-induced HSF-1 activation and Slug expression. Conversely, constitutively active Akt induced HSF-1 phosphorylation and Slug expression. HSF-1 knockdown reduced the ability of Akt to induce Slug expression, indicating an essential that HSF-1 plays in Akt-induced Slug upregulation. Together, our study uncovered the existence of a novel Akt-HSF-1 signaling axis that leads to Slug upregulation and EMT, and potentially contributes to progression of HER2-positive breast cancer.
Keywords: Slug, EMT, Akt, HSF-1, HER2, gene regulation, phosphorylation, cancer
Introduction
Epithelial-mesenchymal transition, EMT, is a cellular process whereby epithelial cells are reprogrammed to mesenchymal cells. Both EMT and the reverse process MET (mesenchymal-epithelial transition) are critically important in multiple stages of development in vertebrates and invertebrates.1 Both EMT and MET are also important processes in tumor progression and metastasis whereby EMT facilitates the migration of epithelial tumor cells from the primary site to distant locations, while MET allows for extravasation and subsequent colonization at the secondary sites.2 Direct evidence for these models has been established using animal models of different cancer types, including breast cancer.3, 4
The transcription factor Slug (SNAI2) promotes EMT by binding to the E-cadherin promoter and repressing E-cadherin expression in epithelial cells5, which is accompanied by changes in cell morphology indicating EMT.6 Slug is considered a marker for malignancy.7 Another member of the SNAI family, Snail (SNAI1), also binds to the E-cadherin promoter and represses E-cadherin expression in epithelial cells, leading to EMT.8, 9 Additional EMT transcription factors (e.g. TWIST1, ZEB1 and ZEB2) can also repress E-cadherin promoter, causing dissolution of cell junctions, loss of cell polarity, and enhanced cell migration.10, 11
HER2 is a member of the ERBB family of receptor tyrosine kinases.12 HER2 is expressed in 15–20% of breast cancers and HER2-positivity is associated with poor clinical prognosis.13–15 Overexpression of HER2 results in over-activation of several pathways in cells, including PI3K-Akt and Ras-MAPK among others. HER2 utilizes these pathways to support tumor growth by promoting cell proliferation, cell survival, tumor angiogenesis, and metastasis.16 Overexpression of HER2 has been shown to associate with E-cadherin downregulation.17, 18 There is also clinical evidence indicating that patients with HER2-positive metastatic breast cancer have circulating tumor cells that have undergone EMT.19 However, the mechanisms by which HER2 promotes EMT have not been fully elucidated and are likely complex.
To provide new mechanistic insights into the relationship between HER2 and EMT, we undertook the current study using breast cancer as the study model, and our study provided evidence showing that activation of HER2 induces EMT by upregulating Slug expression in human breast cancer cells we had examined. A search of the human Slug gene promoter revealed the existence of several putative binding sites for the transcription factor, heat shock factor-1 (HSF-1). HSF-1 is classically activated by heat stress leading to induction of heat shock proteins (HSPs), which are molecular chaperones that permit repair and refolding to damaged proteins. HSF-1 is constitutively expressed in most tissues but activation is regulated by post-translational modification, specifically phosphorylation at S326 upon heat stress.20 Active HSF-1 trimerizes allowing recognition of HSF Sequence-binding Elements (HSEs) and upregulation of target genes.21 Activation of HSF-1 is enhanced in several cancer types, which is associated with a poor prognosis.22–24 Subsequently, we found for the first time that HSF-1 directly binds to the Slug promoter to induce Slug expression independent of heat shock, and that HER2-activated Akt directly phosphorylates HSF-1 at S326 and activates HSF-1 transcriptional activity. Through these observations, we uncovered a novel HER2-Akt-HSF-1 signaling axis that induces Slug expression and promotes EMT in breast cancer cells, thereby shedding new light on the molecular basis by which HER2-overexpressing breast cancer cells undergo EMT and potentially subsequent metastasis.
Results
Heregulin induces EMT and Slug expression in HER2-amplified breast cancer cells
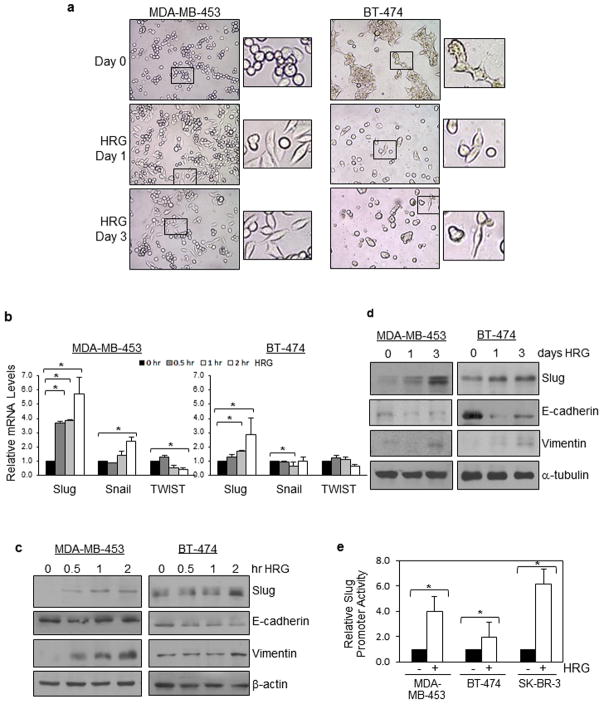
We first investigated whether heregulin induced EMT of two HER2-amplified breast cancer cell lines, MDA-MB-453 and BT-474. Both cell lines displayed typical epithelial morphology after serum starvation overnight (day 0) and underwent changes to the mesenchymal-like morphology after heregulin (HRG) treatment (Figure 1a). To determine which of the EMT regulators may be involved in the observed EMT, we determined levels of Slug, Snail and TWIST with heregulin stimulation for 0–120 min, and the results (Figure 1b) showed that the Slug transcripts were significantly induced by heregulin in both cell lines. This observation was further confirmed at the protein levels using western blotting (WB; Figure 1c). Consistent with increased expression of Slug, a transcriptional repressor of E-cadherin5, E-cadherin expression level was reduced by heregulin. In agreement with observed EMT-like morphological changes in Figure 1A, the mesenchymal marker Vimentin was elevated after heregulin treatment (Figure 1c). We further show that levels of Slug and Vimentin were elevated while E-cadherin expression was suppressed after prolonged heregulin treatment (Figure 1d).
Figure 1. Heregulin induces EMT and Slug expression in HER2-amplified breast cancer cells.
(a) Heregulin (HRG) induced EMT of HER2-amplified breast cancer cells. MDA-MB-453 and BT-474 were serum-starved overnight (day 0) and then treated with heregulin (100 ng/ml) for 1 or 3 days. Cultured cells were imaged using a phase-contract microscope. Representative images are shown.
(b) Slug transcripts were induced by heregulin in HER2-amplified breast cancer cells. MDA-MB-453 and BT-474 were starved from serum overnight, treated with heregulin (100 ng/ml) for 0–120 min, and harvested for total RNA extraction and RT-qPCR. Levels of EMT regulators, Slug, Snail and TWIST were determined. * indicates p-values < 0.05.
(c) (d) Slug protein expression was induced by heregulin in HER2-amplified breast cancer cells. MDA-MB-453 and BT-474 were starved from serum overnight, treated with heregulin (100 ng/ml) for 0–120 min (c) or for 1–3 days (d), and harvested for protein extraction and WB. Levels of Slug, E-cadherin (epithelial marker), and Vimentin (mesenchymal marker) were analyzed.
(e) Slug promoter was significantly activated by heregulin in breast cancer cells with HER2 amplification. MDA-MB-453, BT-474 and SK-BR-3 cells were transfected with a firefly luciferase reporter under the control of the human Slug promoter, serum-starved for 16 hrs and treated with heregulin (100 ng/ml) for 2 hrs. Treated cells were lysed and subjected to luciferase assay. All cells were co-transfected with the Renilla luciferase expression vector, pRL-CMV, to control for transfection efficiency. The results were derived from at least three experiments. The student t-test was conducted to compute p-values. * indicates p-values < 0.05.
Using a luciferase reporter under the control of the human Slug promoter, we found the Slug promoter was significantly activated by heregulin in all three breast cancer cell lines with HER2 amplification (Figure 1e). Together, these observations indicate that heregulin induces EMT and Slug expression in HER2-amplified breast cancer cells.
HER2 overexpression enhances Slug expression, leading to EMT in breast cancer cells
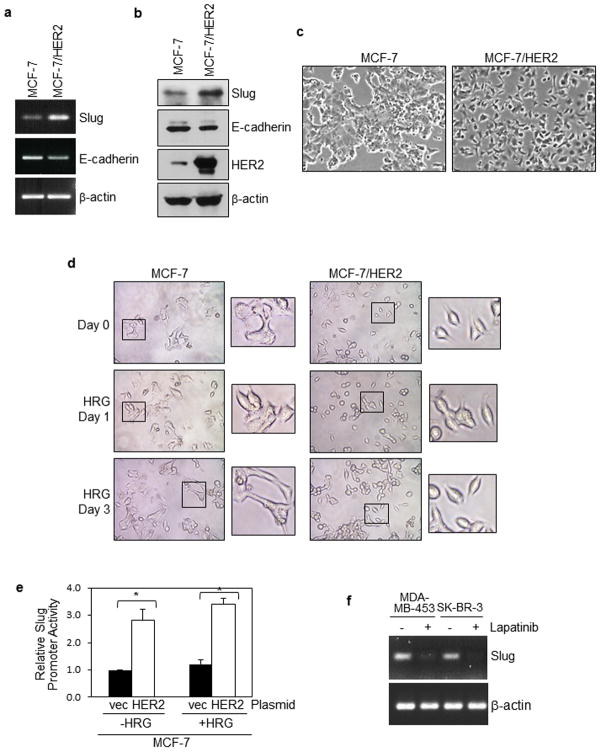
We next asked whether HER2 overexpression induced EMT using MCF-7 (with normal HER2 expression level) and MCF-7/HER2 (MCF-7 cells stably expressing ectopic HER2). As shown by RT-PCR in Figure 2a, forced expression of HER2 upregulated Slug expression which led to reduced E-cadherin expression. WB in Figure 2B confirmed the RT-PCR data and HER2 overexpression in MCF-7/HER2 cells. We further show that HER2 overexpression converted the epithelial appearance of MCF-7 cells into the mesenchymal morphology (Figure 2c). In the presence of heregulin, MCF-7 cells (with normal HER2 expression) also underwent EMT-like morphological changes (Figure 2d). This observation is consistent with the results in Figure 2E showing that heregulin induced slug promoter activity in MCF-7 cells. Figure 2e also shows that HER2 transient transfection induced Slug promoter activation, which is in agreement with the results with MCF-7/HER2 stable transfectants cells. Conversely, we observed that Lapatinib, a small molecule HER2/EGFR inhibitor reduced Slug expression in HER2-amplified MDA-MB-453 and SK-BR-3 cells (Figure 2f). Taken together, these results demonstrate that HER2 overexpression enhances Slug expression, leading to EMT in breast cancer cells.
Figure 2. HER2 overexpression enhances Slug expression, leading to EMT in breast cancer cells.
(a)(b) Ectopic HER2 expression induced Slug expression in breast cancer cells. MCF-7 (with normal HER2 expression level) and MCF-7/HER2 (MCF-7 cells stably expressing ectopic HER2) were analyzed for Slug and E-cadherin expression using RT-PCR (a) and WB (b). Enhanced HER2 expression in MCF-7/HER2 cells is indicated by WB.
(c)(d) Ectopic HER2 expression induced EMT-like morphology changes in breast cancer cells. In panel c, both cell lines were cultured in normal growth condition with fetal calf serum. In panel d, cells were serum-starved and treated with heregulin (100 ng/ml) for 0–3 days. Representative images are shown.
(e) Ectopic HER2 expression induced Slug promoter activity in breast cancer cells. MCF-7 cells (with normal HER2 levels) were transiently transfected with HER2 and the Slug luciferase reporter, serum-starved, and then stimulated with heregulin (100 ng/ml) for 2 hrs. All cells were co-transfected with the Renilla luciferase expression vector, pRL-CMV, to control for transfection efficiency. The results were derived from at least three experiments, and analyzed by the student t-test to compute p-values. * indicates p-values < 0.05.
(f) Lapatinib, a small molecule HER2/EGFR inhibitor reduced Slug expression in HER2-amplified MDA-MB-453 and SK-BR-3 cells. Cells were pre-treated with lapatinib (5 uM) for 24 hrs and subjected to total RNA extraction and RT-PCR for Slug transcript levels.
HSF-1 binds to and transactivates the Slug gene promoter, leading to Slug expression in breast cancer cells
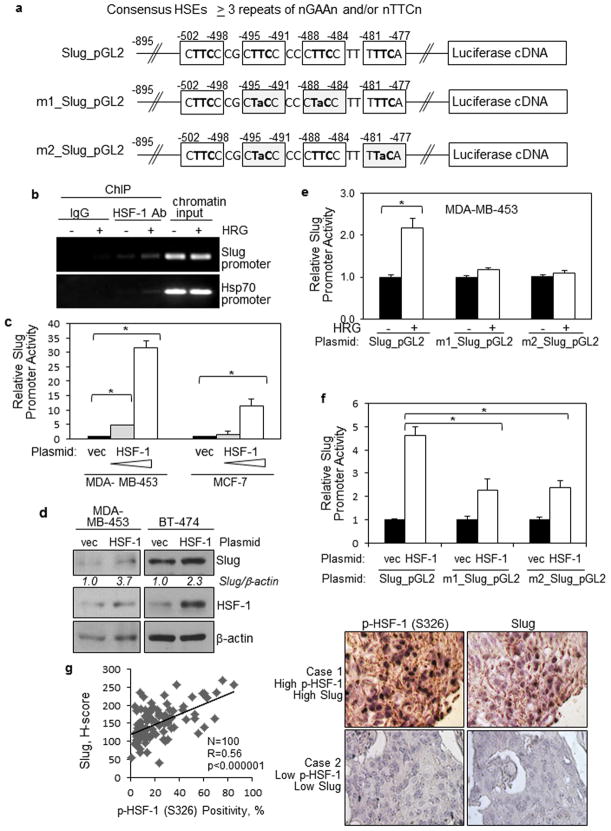
To investigate the mechanisms by which Slug expression is upregulated by HER2/heregulin, we searched the Slug promoter using TFSearch, a web-based search engine for transcription factor-binding sites. Our search revealed that there are four putative HSF-1-binding sites, HSF Sequence-binding Elements (HSEs), within the Slug promoter (Figure 3a). Of note, HSF-1 proteins form trimers and recognize three repeats of HSEs, nGAAn or nTTCn (Figure 3a).21 Using the ChIP assay, we found that HSF-1 bound to the Slug promoter and the binding was enhanced by heregulin (Figure 3b). As a positive control, we observed HSF-1 also bound to the Hsp70 promoter, a known HSF-1 target gene. In the ChIP assay, IgG was used as negative controls for immunoprecipitation while chromatin inputs were used as loading controls for PCR. Using the luciferase reporter assay and WB, we found that HSF-1 expression significantly induced Slug promoter activity (Figure 3c) and expression (Figure 3d), respectively.
Figure 3. HSF-1 binds to and transactivates the Slug gene promoter, leading to Slug expression in breast cancer cells.
(a) Identification of four putative HSEs within the human Slug gene promoter. TFSearch, a web-based search engine for transcription factor-binding sites, was used to search for HSEs within the Slug promoter. Consensus HSEs are shown on the top. Structures of the wild-type Slug promoter reporter and two mutant reporters are shown. Each of the two mutant promoters contains mutations at two of the four putative sites, in order to destroy the three repeats required for binding to HSF-1 trimers. Clear boxes mark the putative HSEs. Lower case letters indicate mutated bases.
(b) HSF-1 binds to the Slug promoter and the binding was enhanced by heregulin. Serum-starved BT-474 cells treated with and without heregulin (100 ng/ml) were used in the ChIP assay. HSF-1 antibody (Ab) was used to immunoprecipitate HSF-1 while IgG served as the negative controls. Chromatin input was used to control for loading. PCR was conducted to detect HSF-1 binding to Slug promoter and a known HSF-1 target gene, Hsp70.
(c) Ectopic HSF-1 expression significantly induced Slug promoter activity. Cells were transfected with the control vector or the HSF-1 vector, and the Slug luciferase reporter for 48 hrs and subjected to luciferase assay. All cells were co-transfected with the Renilla luciferase expression vector, pRL-CMV, to control for transfection efficiency. The results represent means and standard deviations from at least three experiments, and were analyzed by the student t-test to compute p-values. * indicates p-values < 0.05.
(d) Ectopic HSF-1 expression enhanced Slug expression. BT-474 and MDA-MB-453 cells transfected with the control vector or the HSF-1 vector were analyzed by WB to determine Slug and HSF-1 expression levels.
(e)(f) Identified HSEs are important for heregulin- and HSF-1-mediated induction of Slug promoter activation. MDA-MB-453 cells transfected with the wild-type and mutant slug reporters were serum-starved and treated with heregulin for 2 hrs, and then subjected to luciferase assay. All cells were co-transfected with the Renilla luciferase reporter, pRL-CMV, to control for transfection efficiency. The results were derived from at least three experiments, and analyzed by the student t-test to compute p-values. * indicates p-values < 0.05.
(g) Levels of p-HSF-1 (S326) were directly associated with those of Slug in invasive breast carcinoma specimens. IHC was conducted to analyze 100 invasive carcinomas. Linear regression was used to compute R and p values (R=0.56, p<0.000001). Right, representative images.
To determine whether the putative HSEs are essential for HSF-1-mediated Slug expression, we created two mutant promoters, each with mutations at two of the four putative sites, in order to destroy the three tandem repeats required for binding to HSF-1 trimers (Figure 3a). And the analysis of the wild-type and mutant slug promoter reporters showed that both mutant reporters lost the ability to respond to heregulin induction, indicating the identified HSEs are important for heregulin induction of Slug promoter activation (Figure 3e). Both mutant reporters also lost substantial responsiveness to HSF-1 (Figure 3f). Finally, we showed that levels of p-HSF-1 (S326) were directly associated with those of Slug in invasive breast carcinoma specimens (Figure 3g; N=100, R=0.56, p<0.000001). In summary, results in Figure 3 indicate that HSF-1 transcriptionally upregulates Slug gene expression in breast cancer cells.
HSF-1 knockdown prevents heregulin-induced EMT and suppresses growth of breast cancer cells
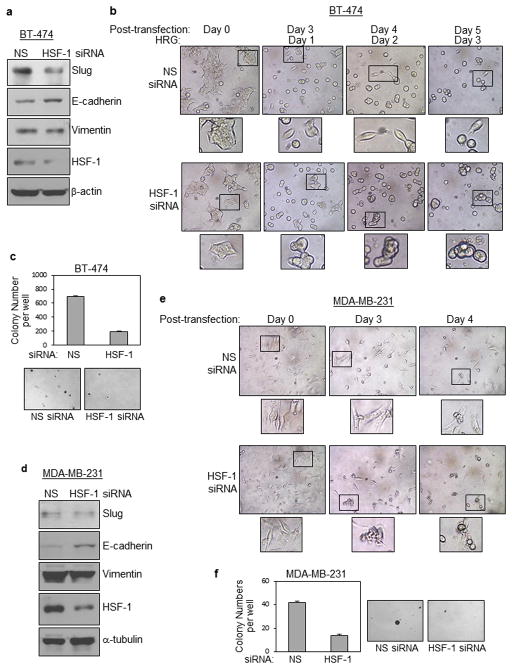
To complement the HSF-1 upregulation results, we knocked down HSF-1 expression using siRNA to determine its impact on Slug expression and EMT. Our results showed that HSF-1 siRNA reduced expression of Slug in BT-474 cells (Figure 4a). The reduction was accompanied with increased expression of E-cadherin and decreased expression of Vimentin. HSF-1 expression was effectively downregulated by HSF-1 siRNA. Moreover, HSF-1 knockdown partially prevented heregulin-induced EMT of BT-474 cells, as indicated by the presence of both clustered epithelial-like cells and spindle-shaped mesenchymal-like cells (Figure 4b). Using the soft agar colony formation assay, we further found that HSF-1 siRNA reduced the propensity of BT-474 cells to grow in an anchorage-independent fashion (Figure 4c).
Figure 4. HSF-1 expression knockdown prevents heregulin-induced EMT and suppresses growth of breast cancer cells.
(a) HSF-1 siRNA reduced expression of Slug in epithelial BT-474 cells. BT-474 cells transfected with non-specific (NS) siRNA or Slug siRNA were analyzed by WB.
(b) HSF-1 knockdown has in part prevented heregulin-induced EMT. BT-474 cells transfected NS siRNA or Slug siRNA were serum-starved and treated with heregulin (100 ng/ml) for 0–3 days. Representative images are shown.
(c) HSF-1 siRNA reduced the propensity of BT-474 cells to grow in an anchorage-independent fashion. BT-474 cells transfected NS siRNA or Slug siRNA were seeded into 6-well culture plates with agarose (2000 cells/well). After colonies were formed to the appropriate size, colonies were counted under a microscope. Data represent means and standard deviations of three independent experiments. The student t-test was performed to calculate p-values. * indicates p-values < 0.05.
(d) HSF-1 siRNA reduced Slug expression in mesenchymal MDA-MB-231 cells. MDA-MB-231 cells transfected with NS siRNA or Slug siRNA were examined by WB.
(e) HSF-1 knockdown did not result in MET of mesenchymal, post-EMT MDA-MB-231 cells, but induced significant cell death. MDA-MB-231 cells transfected NS siRNA or Slug siRNA imaged for 0–4 days post transfections. Representative images are shown.
(f) HSF-1 siRNA reduced the ability of MDA-MB-231 cells to grow in an anchorage-independent fashion. MDA-MB-231 cells transfected NS siRNA or Slug siRNA were seeded into 6-well culture plates with agarose (2000 cells/well). After colonies have formed to the appropriate size, colonies were counted. Results represent means and standard deviations of three independent experiments, and were analyzed by the student t-test. * indicates p-values < 0.05.
To further examine whether HSF-1 knockdown will promote mesenchymal-epithelial transition (MET) of mesenchymal post-EMT breast cancer cells, we used MDA-MB-231 cells, a mesenchymal breast cancer cell line. The results showed that HSF-1 siRNA reduced Slug expression, which was accompanied with increased E-cadherin expression and decreased Vimentin levels (Figure 4d). HSF-1 expression was effectively downregulated by HSF-1 siRNA. However, morphological examination of these cells did not indicate changes indicative of MET (Figure 4e), which is likely due to the observation that knockdown of HSF-1 in MDA-MB-231 cells reduced cell viability. This speculation was consistent with the results of the soft agar colony formation assay, in which we found that HSF-1 siRNA significantly compromised the ability of MDA-MB-231 cells to colonize in an anchorage-independent fashion (Figure 4f). Results presented in Figure 4 demonstrate that HSF-1 knockdown prevents heregulin-induced EMT and suppresses anchorage-independent growth of breast cancer cells.
Concurrent activation of Akt and HSF-1 by heregulin/HER2 in breast cancer cells
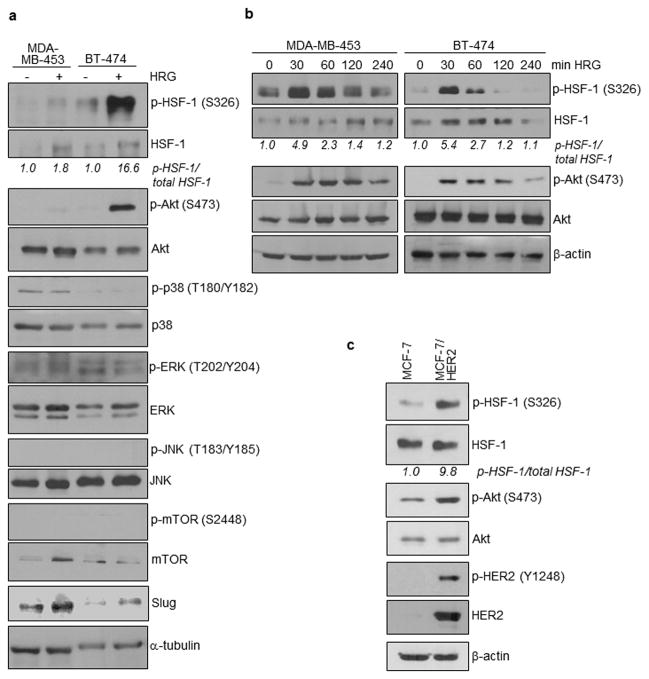
We further investigated the relationship between heregulin/HER2 and HSF-1. It is known that activated HER2 leads to activation of a number of downstream signaling molecules, such as PI3K/Akt, p38, ERK, JNK, and mTOR. Thus, we asked which of the HER2 downstream signaling molecule(s) are activated by heregulin in concordance with HSF-1 activation. Our results indicated that heregulin induced HSF-1 phosphorylation in both HER2-amplified breast cancer cell lines, MDA-MB-453 and BT-474. HSF-1 phosphorylation at S326 has been shown to activate HSF-1 transcriptional activity.20 HSF-1 phosphorylation status is in concordance with Akt phosphorylation, but not with other kinases we examined. As expected, Slug expression was enhanced by heregulin in both cell lines.
To confirm the results with concurrent activation of HSF-1 and Akt, we treated the two cell lines with heregulin for 0–240 min and determined levels of p-HSF-1 (S326) and p-Akt (S473). The results showed that the kinetics for HSF-1 activation is in concordance with that for Akt (Figure 5b). Using MCF-7 and MCF-7/HER2 cell lines, we further observed that ectopic HER2 expression led to increased activation of both HSF-1 and Akt in the cells (Figure 5c). Results in Figure 5 indicate, for the first time, that HSF-1 and Akt are concurrently activated by heregulin/HER2 in breast cancer cells.
Figure 5. Concurrent activation of Akt and HSF-1 by heregulin/HER2 in breast cancer cells.
(a) Heregulin induced phosphorylation of both HSF-1 and Akt in HER2-amplified breast cancer cell lines. MDA-MB-453 and BT-474 cells were treated with and without heregulin (100 ng/ml) for 2 hrs and the whole cell lysates were analyzed by WB for levels of HER2 downstream kinases and Slug.
(b) Kinetics for HSF-1 activation is in concordance with that for Akt. The two cell lines were treated with heregulin for 0–240 min and the whole cell lysates were analyzed by WB to determine levels of p-HSF-1 (S326) and p-Akt (S473).
(c) Ectopic HER2 expression led to increased activation of both HSF-1 and Akt. MCF-7 and MCF-7/HER2 cell lines were examined using WB.
Akt directly interacts with and phosphorylates HSF-1 at S326
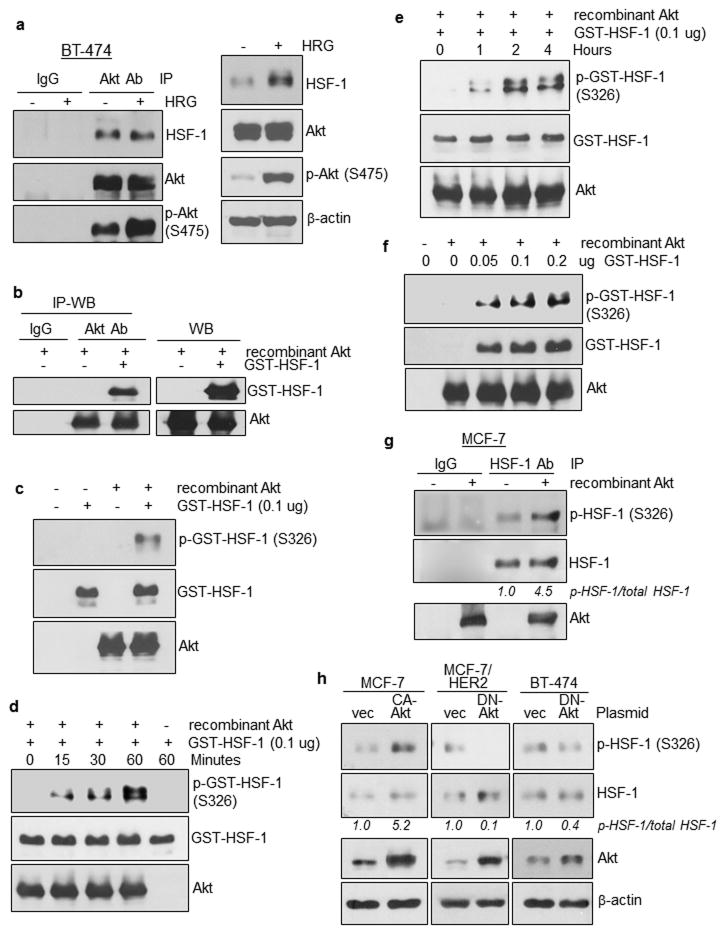
In light of the observation with concurrent activation of Akt and HSF-1, we asked whether these two proteins physically associate. Using immunoprecipitation (IP) followed by WB, we found that Akt constitutively interacted with HSF-1 independent of heregulin treatment (Figure 6a). Recombinant Akt directly interacted with recombinant HSF-1 (Figure 6b). Next, we investigated whether the Akt-HSF-1 interaction resulted in HSF-1 phosphorylation. Using the cell-free Akt kinase assay followed by WB, we showed that recombinant Akt directly phosphorylated recombinant GST-HSF-1 protein at S326 (Figure 6c). The same assay further showed that GST-HSF-1 protein was phosphorylated by Akt in time- and dose-dependent fashions (Figures 6d–6f).
Figure 6. Akt directly interacts with and phosphorylates HSF-1 at S326.
(a) Akt interacts with HSF-1 constitutively, independent of heregulin treatment. IP-WB was conducted using whole cell lysates extracted from BT-474 cells treated with and without heregulin. An Akt Ab was used to immunoprecipitate Akt whereas IgG was used as negative controls. WB results are shown in the right panel.
(b) Recombinant Akt directly interacts with recombinant HSF-1. IP-WB was conducted.
(c) Recombinant Akt directly phosphorylates recombinant HSF-1 protein at S326. Cell-free Akt kinase assay was conducted followed by WB.
(d,e) HSF-1 protein was phosphorylated by Akt in a time-dependent fashion.
(f) HSF-1 protein was phosphorylated by Akt in a dose-dependent fashion.
(g) Cellular HSF-1 is directly phosphorylated by Akt at S326. HSF-1 immunoprecipitated from MCF-7 cells was subjected to the cell-free Akt kinase assay followed by WB.
(h) Ectopic expression of constitutively activated Akt (CA-Akt) significantly enhanced HSF-1 phosphorylation, whereas ectopic expression of dominant-negative Akt (DN-Akt) substantially reduced HSF-1 phosphorylation. Transfected cells were lysed and subjected to WB.
We further investigated whether HSF-1 immunoprecipitated from MCF-7 cells was phosphorylated by recombinant Akt, and the results indicated that cellular HSF-1 was directly phosphorylated by Akt at S326 (Figure 6g). In agreement with these observations, we found that ectopic expression of constitutively activated Akt (CA-Akt) significantly enhanced HSF-1 phosphorylation in MCF-7 cells (Figure 6h). Conversely, ectopic expression of dominant-negative Akt (DN-Akt) substantially reduced HSF-1 phosphorylation in MCF-7/HER2 and BT-474 cells (Figure 6h). Taken together, these results demonstrate, for the first time, that Akt directly interacts with HSF-1 and phosphorylates HSF-1 at S326.
Slug expression is suppressed by blocking the HER2-Akt-HSF-1 signaling axis; HSF-1 is essential for Akt-induced Slug expression
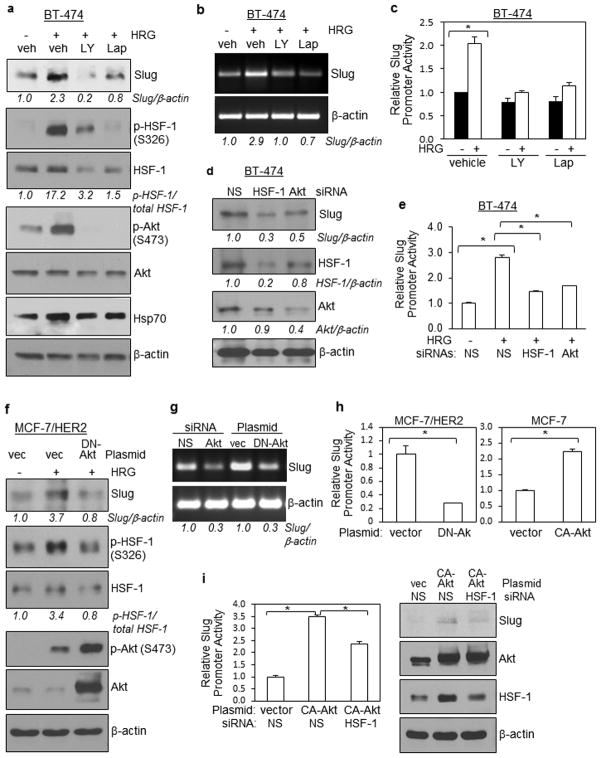
We further investigated the link between Slug and the HER2-Akt-HSF-1 signaling axis. We observed that small molecule inhibitors to PI3K (LY294002; LY) and HER2 (Lapatinib; Lap) suppressed Slug protein and transcription expression in BT-474 cells, as shown in Figures 7a and 7b respectively. Both LY294002 and lapatinib effectively inhibited phosphorylation Akt and HSF-1 (Figure 7a), which is consistent with our earlier observation that Akt phosphorylates HSF-1. As positive controls for HSF-1 activity, we found that expression of Hsp70, a known HSF-1 target gene, was reduced in cells with lower levels of p-HSF-1 (Figure 7a). Using Slug promoter luciferase reporter assay, we further found that both LY294002 and Lapatinib blocked heregulin induction of Slug promoter activity (Figure 7c).
Figure 7. Slug expression is suppressed by blocking the HER2-Akt-HSF-1 signaling axis; HSF-1 is essential for Akt-induced Slug expression.
In luciferase assays (panels b, d and e), three independent experiments were performed to derive means and standard deviations. All cells were co-transfected with the Renilla luciferase reporter, pRL-CMV, to control for transfection efficiency. The results were analyzed by the student t-test to compute p-values. * indicates p-values < 0.05.
(a)(b) Small molecule inhibitors to PI3K/Akt (LY294002; LY; 50 uM) and HER2 (Lapatinib; Lap; 25 uM) pre-treatment for 1 2 hrs suppressed heregulin-induced Slug expression in BT-474 cells. In panel a, WB was conducted. Hsp70 served as positive controls for HSF-1 activity. Heregulin exposure was for 1 hr at 100 ng/ml. In panel b, total RNA was analyzed by RT-PCR. Veh, vehicle (1% DMSO)
(c) Both LY294002 and Lapatinib blocked heregulin induction of Slug promoter activity. BT-474 cells transfected with the Slug luciferase reporter were serum-starved, treated with vehicle or indicated inhibitor (50 uM LY294002 or 25 uM Lapatinib) for 2 hrs, and then treated with and without heregulin for 4 hrs. Harvested cells were lysed and subjected to luciferase assay.
(d) HSF-1 and Akt siRNAs reduced Slug expression in BT-474 cells, as shown by WB.
(e) HSF-1 and Akt siRNAs prevented heregulin-induced activation of Slug promoter in BT-474 cells. BT-474 cells co-transfected with siRNA and the Slug luciferase reporter were serum-starved and treated with heregulin for 4 hrs, and then subjected to luciferase assay.
(f) DN-Akt blocked heregulin-induced Slug expression and HSF-1 activation in MCF-7/HER2 cells, as shown by WB.
(g) Akt siRNA and DN-Akt inhibited Slug transcription in BT-474 cells as shown by RT-PCR.
(h) DN-Akt reduced activity of the Slug promoter in MCF-7/HER2 cells while CA-Akt enhanced its activity in MCF-7 cells. Luciferase assay was conducted to measure Slug promoter activity.
(i) HSF-1 is essential for CA-Akt-induced Slug expression. MCF-7 cells were used. Left, luciferase assay. Right, WB.
HSF-1 and Akt siRNAs reduced Slug protein expression in BT-474 cells (Figure 7d). Consistent with this observation, HSF-1 and Akt siRNAs prevented heregulin-induced activation of Slug promoter in BT-474 cells (Figure 7e). Ectopic expression of DN-Akt blocked heregulin-induced Slug protein expression and HSF-1 activation (Figure 7f). Both Akt siRNA and DN-Akt inhibited Slug transcription in BT-474 cells (Figure 7g). DN-Akt reduced activity of the Slug promoter in MCF-7/HER2 cells while CA-Akt enhanced its activity in MCF-7 cells (Figure 7h). Finally, we observed that HSF-1 siRNA significantly reduced the ability of CA-Akt to induce Slug promoter activity (Figure 7i; left panel) and Slug protein expression (right panel), indicating that HSF-1 plays an essential role in Akt-induced Slug expression. Together, results presented in Figure 7 indicate that a novel HER2-Akt-HSF-1 signaling axis positively regulates expression of the EMT-promoting transcription factor Slug, and that HSF-1 plays an essential role in Akt-induced Slug expression.
Discussion
Slug participates in several physiological and pathological processes from development to re-epithelialization during wound healing, and to EMT and tumor progression.7 However, the regulation of Slug expression is still not well understood. In this study, we provide evidence uncovering a novel HER2-Akt-HSF-1 signaling axis that acts to promote Slug expression and EMT whereby HSF-1 directly binds to the Slug promoter to induce transcription.
Our results indicate HSF-1 directly upregulates Slug transcription to promote EMT. This novel observation is in agreement with a recent study suggesting an association between HSF-1 and Slug expression as HER2+HSF-1+/+ mice showed greater Slug expression in mammary tumors than HER2+HSF-1+/− mice, which was also accompanied with reduced E-cadherin.25 We further confirmed this in vitro observation in vivo as we found a strong correlation between active HSF-1 and Slug expression in a sample of 100 invasive breast carcinoma specimens. These experimental findings led us to propose a model whereby overexpression of HER2 activates the PI3K-Akt pathway, which leads to HSF-1 activation/phosphorylation that upregulates Slug expression and promotes EMT of breast cancer cells.
Our results demonstrated that HER2-induced Akt activation leads to Akt phosphorylation of HSF-1 at S326, which results in enhanced HSF-1 transcriptional activity. Interestingly, heregulin has been shown to upregulate expression of HSF-126, although the underlying mechanisms are still unclear. It has also been observed that activation of HER2 can upregulate HSF-1 expression whereas loss of HSF-1 reduces HER2-driven tumorigenesis.26, 27 Based on these observations, the relationship between HER2 and HSF-1 appears to be complex warranting further investigations.
Our discovery of HSF-1 being a target of Akt is an important finding. Akt is frequently dysregulated in human cancers and is consequently an important target for cancer therapeutics. Although Akt-targeted therapy has shown promising clinical results, identification of new Akt downstream effectors will help us develop new biomarkers for the aberrant Akt pathway and potentially, novel drug targets for tumors with hyperactive Akt signaling. In light of these notions, the HSF-1 pathway could serve as a new biomarker and a novel therapeutic target for human cancers with high Akt activity, such as, those with PTEN loss, constitutively activated PI3K, or with overexpression of receptor tyrosine kinases, HER2 and EGFR.
In addition to Akt, several other Ser/Thr kinases have been shown to phosphorylate HSF-1 to regulate HSF-1 transcriptional activity, stability and intracellular trafficking.28 Phosphorylation HSF-1 at S326 was reported to be a key step in the ability of HSF-1 to regulate gene expression in response to heat stress 20. However, to date, little evidence has been provided as to what kinases mediate phosphorylation at S326. In this study, we provide conclusive evidence demonstrating that Akt can directly phosphorylate HSF-1 on S326. Interestingly, it was recently reported that mTOR could phosphorylate HSF-1 on S326 in HeLa cells under proteotoxic stress.29 However, it is worth noting that we did not observe detectable activation of mTOR in the context of HER2 activation in the breast cancer cells we examined. Although Akt has been shown to activate mTOR30, we did not observe substantial involvement of mTOR in the Akt-HSF-1-Slug mediated promotion of EMT in HER2-amplified breast cancer cells we have examined. These differences could be explained by several potential mechanisms. First, mTOR-induced phosphorylation of HSF-1 may require mTOR activation from increased cell stress via heat stress or exposure to noxious substances as was observed in a previous study.29 Second, Akt may act as the predominant regulator of HSF-1 over mTOR in cancer cells with HER2 amplification, as suggested by the data presented in this study. Third, the choice of Akt or mTOR for HSF-1 S326 phosphorylation may differ in a cancer- and/or cell-type dependent fashion. Finally, it is likely that HSF-1 S326 residue can be targeted by other Ser/Thr kinases besides Akt and mTOR, which may influence the accessibility of HSF-1 by Akt and/or mTOR.
Our results showed the transcriptional activity of HSF-1 can be activated by the HER2-Akt signaling axis independent of heat shock. This is in agreement with emerging evidence suggesting that HSF-1 can induce a transcriptional program independent of the heat shock program.23 Importantly, our results further linked HSF-1 to EMT, independent of heat shock, which is clearly a novel, important observation. This observation plus previous reports showing that HSF-1 promotes cell cycle progression and antagonizes apoptosis23, together, could help define HSF-1 as a mediator of aggressive tumor phenotypes. These observations also raise the possibility that HSF-1 may have wide-ranging oncogenic functions, thus warranting future in-depth investigations. In support of this possibility, our data suggest that HSF-1 may support HER2-mediated breast cancer cell growth (Figures. 4c and 4f). This interesting observation is in accordance with several earlier studies25, 27, 31. We speculate that the effects of HSF-1 on tumor growth and progression are likely due to a combination of the effects of HSF-1 on the induction of the heat shock program22, the induction of genes unrelated to heat shock that support malignant growth23, and the promotion of EMT as indicated by our results, all three of which can support cell survival and resistance to therapy.
In summary, our study uncovered a novel role for HSF-1 in promoting EMT via direct upregulation of Slug in HER2-amplified breast cancer cells independent of heat shock. Our data also revealed, for the first time, that Akt can directly phosphorylate and activate HSF-1 to induce the expression of Slug. Understanding the other post-translational modifications of HSF-1, along with which proteins mediate those modifications, and what role they play in tumor progression, will further elucidate the role that HSF-1 plays in the progression of cancer. It is becoming increasingly apparent that HSF-1 can regulate a vast array of genes outside of the heat shock program. Identification and functional investigations into these HSF-1-targeted genes and cellular mechanisms are certainly warranted to fully understand the role of HSF-1 in cancer. Investigation into the impact of inhibition of HSF-1 is also needed. There are several compounds that have been developed that have been observed to inhibit HSF-1, including, KRIBB1132, KNK43733, and triptolide.34 However, the potential impact of these compounds in HER2-positive breast cancer models is largely unknown. Thus, there is much left to understand regarding the role of HSF-1 in cancer as evidence continues to suggest HSF-1 has a much broader biological function than mediating the cellular heat shock response.
Materials and Methods
Reagents, Cell Lines and Primary Specimens
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise stated. All human breast cancer cell lines used in this study were obtained from ATCC (Manassas, VA). All cell lines were maintained according to ATCC’s instructions. Tissue microarray slides (BC081116) were purchased from US Biomax (Rockville, MD) that contained 100 invasive breast carcinomas. MCF-7/HER2 stable transfectant cell line was a generous gift from Dr. Mien-Chie Hung at MD Anderson Cancer Center and was maintained in MEM medium supplemented with 10% FBS, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 10 μg/mL bovine insulin, and 350 μg/ml G418. All siRNAs were ordered from Bioneer (Alameda, CA). The siRNA sequences are 5′-CCUACGCCA CCACUUUCGU(dTdT)-3′ (non-specific control), 5′-GACAACCGCCAUCCAGACU(dTdT)-3′ (Akt), and 5′-GAGAUCUAUAAACAGACAG(dTdT)-3′ (HSF-1). Lapatinib was purchased from LC Laboratories (Woburn, MA). LY294002 was obtained from Cayman Chemical (Ann Arbor, MI).
RT-PCR and qPCR
Total RNA isolation and RT were conducted using SV Total RNA Isolation System (Promega) and Superscript II First-Strand cDNA synthesis system (Invitrogen), retrospectively. The forward and reverse primers used for regular PCR were: 5′-TGATGAAGAGGAAAGACTACAG-3′ and 5′-GCTCA CATATTCCTTGTCACAG-3′ (Slug), 5′-GGAGTCCGCAGTCTTACGAG-3′ and 5′-TCTGGAGGA CCTGGTAGAGG-3′ (TWIST), 5′-CGAAAGGCCTTCAACTGCAAA-3′ and 5′-ACTGGTACTTCTT GACATCTG-3′ (Snail), 5′-GTCCTGGGCAGAGTGAATTTT-3′ and 5′-ATTCAGCGTGACTTTGG TGGA-3′ (E-cadherin), 5′-ACCAACGAGAAGGTGGAGCTG-3′ and 5′-TCGTTGGTTAGCTGGTC CACC-3′ (Vimentin), and 5′-GGCGGCACCACCATGTACCC-3′ and 5′-AGGGGCCGGACTCG TCATACT-3′ (β-actin). Primers used in qPCR included 5′-TCGGACCCACACATTACCTT-3′ and 5′-TGACCTGTCTGCAAATGCTC-3′ (Slug), 5′-CTCAGCTACGCCTTCTCG-3′ and 5′-ACTGTCCATTTTCTCCTTCTCTG-3′ (TWIST), 5′-GGAAGCCTAACTACAGCGAG-3′ and 5′-CAGAGTCCCAGATGAGCATTG-3′ (Snail), and 5′-ACCCCTGAAGTACCCCAT-3′ and 5′-CCACACGCAGCTGATTGT-3′ (β-actin). In qPCR, β-actin gene was used as normalization controls and all experiments were done in triplicates. qPCR master mix was purchased from Apex Bioresearch Products.
Western Blotting (WB) and Immunoprecipitation (IP)
This was performed as described previously.35, 36 Antibodies used in WB included mouse monoclonal antibodies against β-actin (Sigma), α-tubulin (Sigma), E-cadherin (610404, BD), p-HSF-1/S326 (ab76076, Epitomics/Abcam), p-JNK/S63/73 (sc-6254; Santa Cruz), as well as, rabbit antibodies against Slug (AP2053a,ABGENT), HSF-1 (4356, Cell Signaling), Hsp70 (4876, Cell Signaling), p38 (9212, Cell Signaling), p-p38/T180/Y182 (4511, Cell Signaling), pERK/T202/Y204 (9109, Cell Signaling), Akt/pan (4691, Cell Signaling), p-Akt/S473 (4060, Cell Signaling), mTOR (2983, Cell Signaling), p-mTOR/S2448 (5536, Cell Signaling), JNK (sc-571, Santa Cruz), HER2 (2165, Cell Signaling), p-HER2/Y1278 (2247, Cell Signaling). Rabbit polyclonal HSF-1 antibody used in IP and ChIP assay was from Cell signaling (4356).
Plasmids, Transfection and Luciferase Assay
Constitutively activated and dominant-negative Akt constructs were generous gifts from Dr. Mong-Hong Lee at MD Anderson Cancer Center.37 The FLAG-HSF-1 plasmid was purchased from Addgene (ID 32537, Cambridge MA), which was originally established by Dr. Stuart Calderwood.38 Slug_pGL2 luciferase reporter construct was obtained from Addgene (ID 31695), originally cloned by Dr. Paul Wade.39 HER2-WT plasmid was obtained from Addgene (ID 16257), which was generated by Dr. Mien-Chie Hung.40 All transfections were performed with cells in exponential growth using lipofectamine 2000 (Invitrogen) or XtremeGene HP (Roche). A Renilla luciferase expression vector, pRL-CMV was used to control for transfection efficiency. Forty-eight hrs after transfection, the cells were lysed and luciferase activity measured using the Firefly and Renilla Luciferase Assay Kit (Biotium, Hayward, CA), as we previously described.10, 35, 36, 41 Relative promoter activity was computed by normalizing the Firefly luciferase activity against that of the Renilla luciferase.
Mutagenesis
Generation of mutant Slug promoter reporter vectors was done using a QuikChange Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA) per manufacturer’s instructions. Primers used for mutagenesis were: 5′-CCTTTGTCTTCCCGCTACCCCCTACCTTTTTCAAAAGC-3′ and 5′-GCTTTTGAAAAAGGTAGGGGGTAGCGGGAAGACAAAGG-3′ (m1-Slug_pGL2) and 5′-GTCTTC CCGCTACCCCCTTCCTTTTACAAAAGCCAAG-3′ and 5′-CTTGGCTTTTGTAAAAGGAAGGGGG TAGCGGGAAGAC-3′ (m2-Slug-pGL2) Mutation was confirmed by sequencing.
ChIP Assay to Determine Binding of HSF-1 to the Slug gene Promoter
This was performed using a ChIP Assay Kit (Upstate, Billerica, MA) as we described previously 41. Rabbit polyclonal HSF-1 antibody was used (4356, Cell Signaling). DNA sequences for the primers used to amplify the Slug promoter are 5′-TGGAAGTGGCATCTGGAGAG-3′ (forward) and 5′-GCTAACACGGTGACATGAGT-3′ (reverse), and for the Hsp70 promoter, 5′-CACTCCCCCTTCCTCTCAG-3′ (forward) and 5′-TTCCCTTCTGAGC CAATCAC-3′ (reverse).
Immunohistochemistry (IHC)
This was conducted as we described previously.42 The slides were incubated with p-HSF-1 (S326) (Abcam; Cambridge, MA) and Slug (Abgent; San Diego, CA) antibodies. Histologic scores (H-Scores) were computed from both percent positivity (A%, A=1–100) and intensity (B=0–3) using the equation, H-Score=A × B.
Determination of Anchorage-Independent Growth by Colony Formation Assays
Clonogenic growth assays were performed in 6-well cell culture plates with 2,000 cells per well, as we previously described.41, 43 All wells were pre-coated with 0.5% agarose as the bottom layer whereas the top layer is consisted of 0.3% agarose and tumor cells. After 6–8 weeks, colonies were stained with crystal violet blue solution (Sigma) for 1 hr and counted under a microscope. Triplicate wells were used for each cell line and three independent experiments were performed.
Akt Kinase Assay
Recombinant AKT and recombinant HSF-1 were purchased from Sigma. Indicated amounts of recombinant HSF-1 (0.1–0.4 μg) was incubated with or without 0.1 μg recombinant AKT for up to 60 min at 37°C in the presence of ATP in kinase assay buffer (20 mM HEPES, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT). Samples were then boiled and subjected to SDS-PAGE and WB with indicated antibodies.
Statistical Analysis
The student t-test and linear regression analysis were performed using STATISTICA (StatSoft Inc., Tulsa, OK) and Microsoft Excel, as we previously described.35, 42, 44
Acknowledgments
We are thankful to Dr. Mong-Hong Lee at MD Anderson Cancer Center who provided CA-Akt and DN-Akt constructs. This study was supported by the NIH grant K01-CA118423, and W81XWH-11-1-0600 from the U.S. Department of Defense, the Beez Foundation and the Intramural Division of Surgical Sciences grants, Dani P. Bolognesi, Ph.D. Award and Clarence Gardner, Ph.D. Award.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 3.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- 5.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 6.Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol. 1997;137:1403–1419. doi: 10.1083/jcb.137.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobaleda C, Perez-Caro M, Vicente-Duenas C, Sanchez-Garcia I. Function of the zinc-finger transcription factor SNAI2 in cancer and development. Annu Rev Genet. 2007;41:41–61. doi: 10.1146/annurev.genet.41.110306.130146. [DOI] [PubMed] [Google Scholar]
- 8.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 9.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 10.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 15.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 16.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JC, Qiu X, Chang HM, Leung PC. HER2 mediates epidermal growth factor-induced down-regulation of E-cadherin in human ovarian cancer cells. Biochem Biophys Res Commun. 2013;434:81–86. doi: 10.1016/j.bbrc.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 18.D’Souza B, Taylor-Papadimitriou J. Overexpression of ERBB2 in human mammary epithelial cells signals inhibition of transcription of the E-cadherin gene. Proc Natl Acad Sci U S A. 1994;91:7202–7206. doi: 10.1073/pnas.91.15.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN, et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012;11:2526–2534. doi: 10.1158/1535-7163.MCT-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroeger PE, Morimoto RI. Selection of new HSF1 and HSF2 DNA-binding sites reveals difference in trimer cooperativity. Mol Cell Biol. 1994;14:7592–7603. doi: 10.1128/mcb.14.11.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2013;87:19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santagata S, Hu R, Lin NU, Mendillo ML, Collins LC, Hankinson SE, et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci U S A. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xi C, Hu Y, Buckhaults P, Moskophidis D, Mivechi NF. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J Biol Chem. 2012;287:35646–35657. doi: 10.1074/jbc.M112.377481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 27.Meng L, Gabai VL, Sherman MY. Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene. 2010;29:5204–5213. doi: 10.1038/onc.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anckar J, Sistonen L. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 2011;80:1089–1115. doi: 10.1146/annurev-biochem-060809-095203. [DOI] [PubMed] [Google Scholar]
- 29.Chou SD, Prince T, Gong J, Calderwood SK. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7:e39679. doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 31.Gabai VL, Meng L, Kim G, Mills TA, Benjamin IJ, Sherman MY. Heat shock transcription factor Hsf1 is involved in tumor progression via regulation of hypoxia-inducible factor 1 and RNA-binding protein HuR. Mol Cell Biol. 2012;32:929–940. doi: 10.1128/MCB.05921-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon YJ, Kim JA, Shin KD, Shin DS, Han YM, Lee YJ, et al. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J Biol Chem. 2011;286:1737–1747. doi: 10.1074/jbc.M110.179440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol. 2004;80:607–614. doi: 10.1080/09553000412331283470. [DOI] [PubMed] [Google Scholar]
- 34.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 35.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14:6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo HW, Stephenson L, Cao X, Milas M, Pollock R, Ali-Osman F. Identification and Functional Characterization of the Human Glutathione S-Transferase P1 Gene as a Novel Transcriptional Target of the p53 Tumor Suppressor Gene. Mol Cancer Res. 2008;6:843–850. doi: 10.1158/1541-7786.MCR-07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao R, Yang HY, Shin J, Phan L, Fang L, Yeung SC, et al. CDK inhibitor p57 (Kip2) is downregulated by Akt during HER2-mediated tumorigenicity. Cell cycle. 2013:12. doi: 10.4161/cc.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Grammatikakis N, Siganou A, Calderwood SK. Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Molecular and cellular biology. 2003;23:6013–6026. doi: 10.1128/MCB.23.17.6013-6026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113:207–219. doi: 10.1016/s0092-8674(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 40.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Lo H-W, Hsu S-C, Ali-Seyed M, Gunduz M, Xia W, Wei Y, et al. Nuclear Interaction of EGFR and STAT3 in the Activation of iNOS/NO Pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Lo HW, Zhu H, Cao X, Aldrich A, Ali-Osman F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69:6790–6798. doi: 10.1158/0008-5472.CAN-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–245. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu H, Cao X, Ali-Osman F, Keir S, Lo HW. EGFR and EGFRvIII interact with PUMA to inhibit mitochondrial translocalization of PUMA and PUMA-mediated apoptosis independent of EGFR kinase activity. Cancer Lett. 2010;294:101–110. doi: 10.1016/j.canlet.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]