Abstract
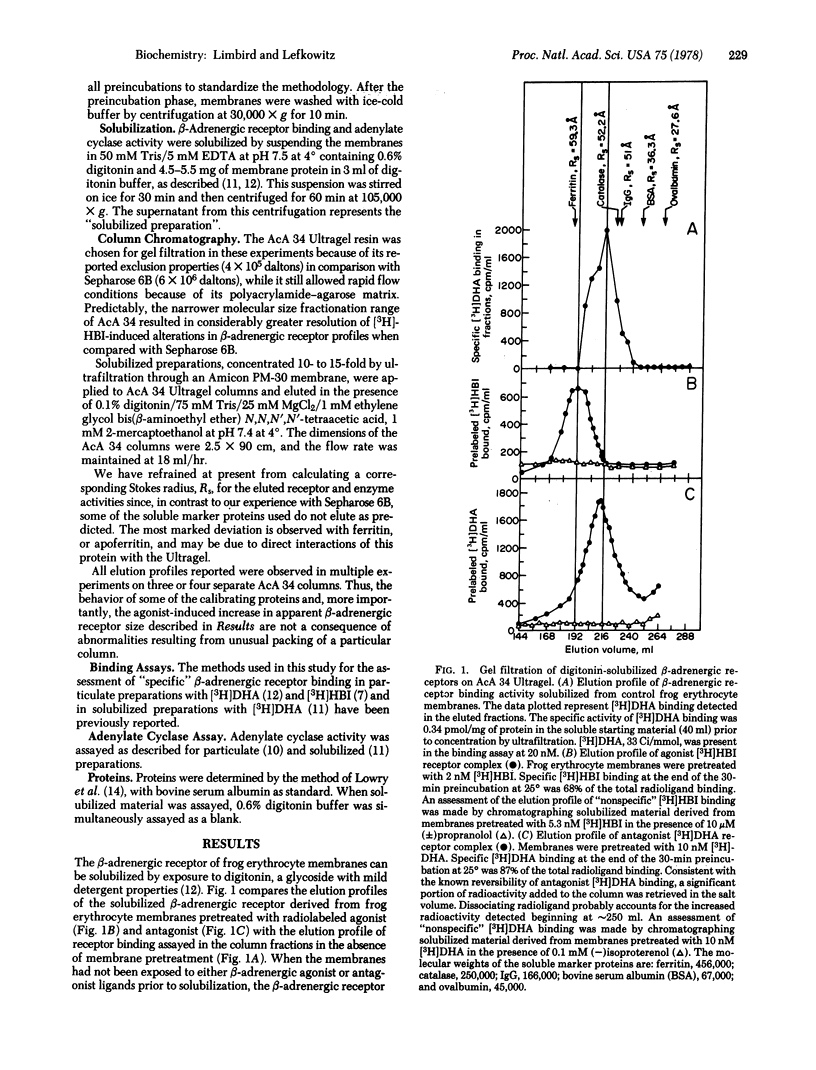
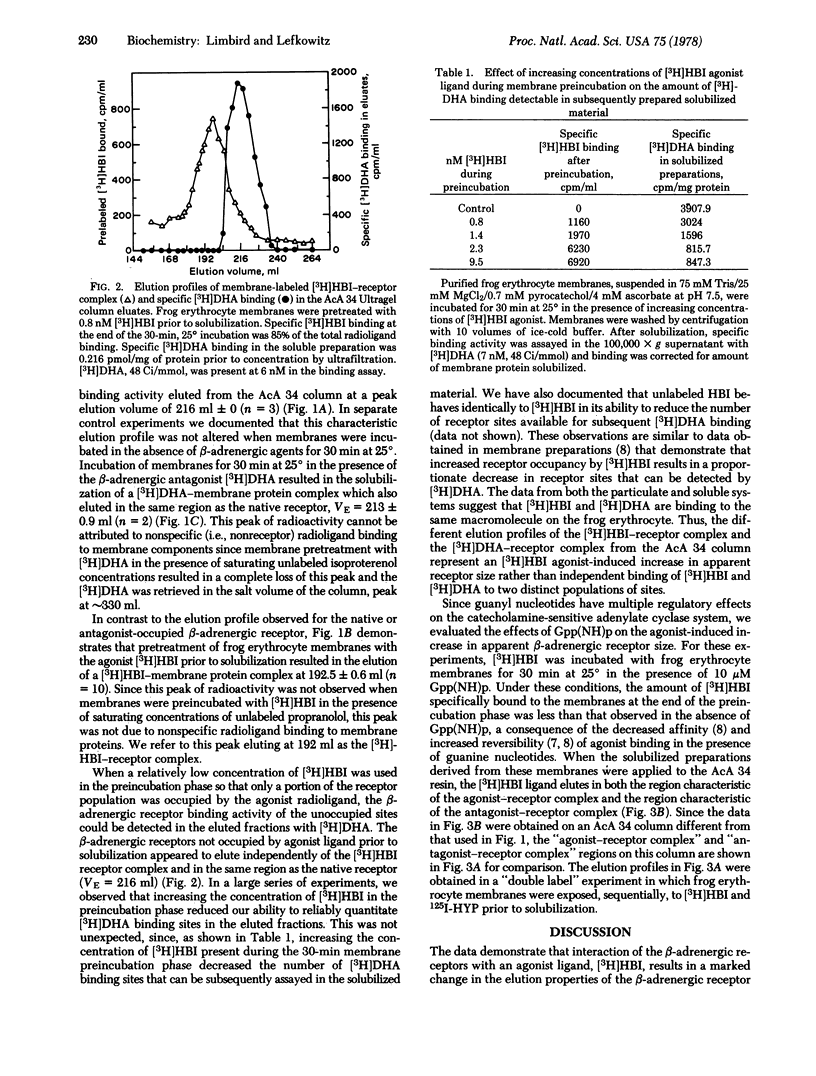
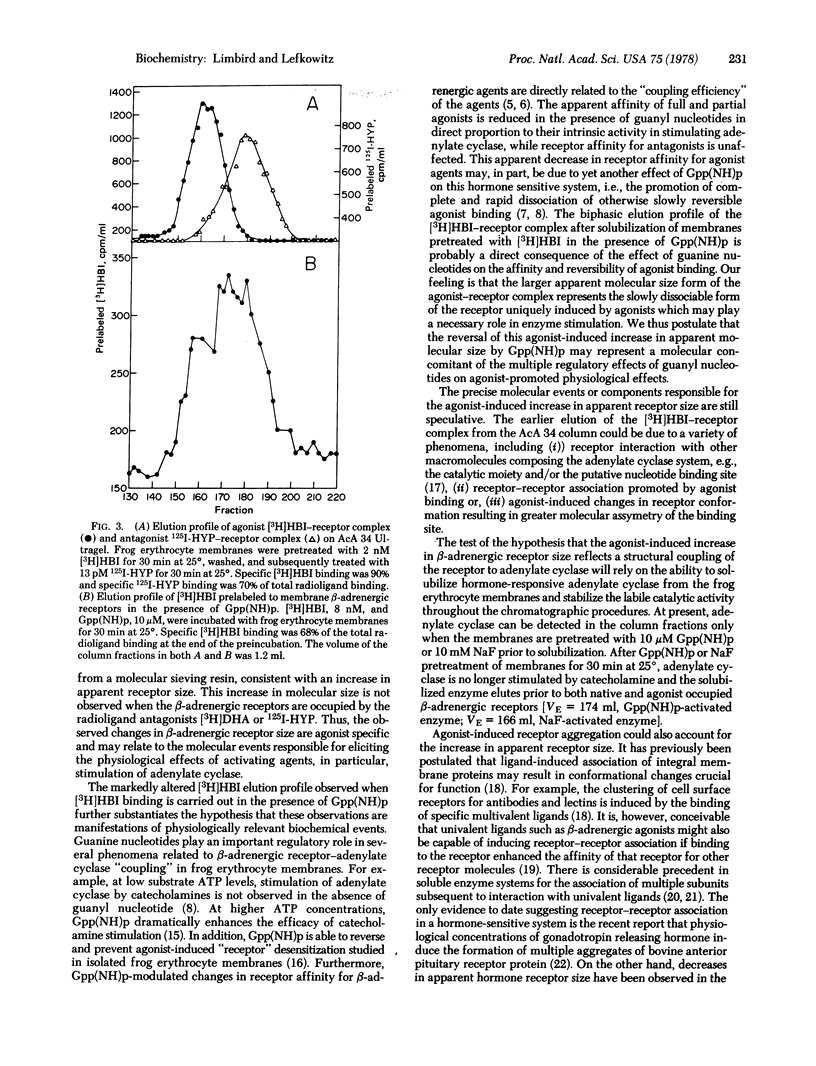
The properties of digitonin-solubilized β-adrenergic receptors from frog erythrocyte membranes were studied by gel exclusion chromatography on AcA 34 Ultragel. β-Adrenergic receptor binding activity in these membranes can be identified by both an agonist ligand, [3H]hydroxybenzylisoproterenol, and the antagonist ligands, [3H]dihydroalprenolol and 125I-labeled hydroxybenzylpindolol. Occupancy of the β-adrenergic receptors with the [3H]hydroxybenzylisoproterenol agonist prior to their solubilization from the membrane leads to an increase in apparent receptor size. Alterations in the molecular size of the receptor cannot be mimicked by occupancy of the binding site with the antagonist ligands. Exposure of frog erythrocyte membranes to [3H]hydroxybenzylisoproterenol agonist in the presence of 10 μM Gpp(NH)p, a guanyl nucleotide analog that exerts multiple regulatory effects on the catecholamine-sensitive adenylate cyclase [ATP pyrophosphate-lyase (cyclizing); EC 4.6.1.1] system, results in the elution of the [3H]hydroxybenzylisoproterenol radioligand in both the region characteristic of the agonist—receptor complex and the region characteristic of the antagonist—receptor complex.
The precise molecular interactions responsible for the agonist-induced increase in apparent β-adrenergic receptor size are still unresolved. However, the low concentrations of agonist that are capable of altering apparent receptor size and the sensitivity of this effect to guanyl nucleotides suggest that these phenomena may be intimately involved in eliciting the physiological effects of β-adrenergic catecholamines at the molecular level.
Keywords: hormone receptor, adenylate cyclase, ligand-induced molecular interconversions, guanine nucleotides
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caron M. G., Lefkowitz R. J. Solubilization and characterization of the beta-adrenergic receptor binding sites of frog erythrocytes. J Biol Chem. 1976 Apr 25;251(8):2374–2384. [PubMed] [Google Scholar]
- Dunne C. P., Wood W. A. L-threonine dehydrase as a model of allosteric control involving ligand-induced oligomerization. Curr Top Cell Regul. 1975;9:65–101. doi: 10.1016/b978-0-12-152809-6.50010-x. [DOI] [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Hammes G. G., Wu C. W. Kinetics of allosteric enzymes. Annu Rev Biophys Bioeng. 1974;3(0):1–33. doi: 10.1146/annurev.bb.03.060174.000245. [DOI] [PubMed] [Google Scholar]
- Klein I., Fletcher M. A., Levey G. S. Evidence for a dissociable glucagon binding site in a solubilized preparation of myocardial adenylate cyclase. J Biol Chem. 1973 Aug 10;248(15):5552–5554. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J., Limbird L. E., Mukherjee C., Caron M. G. The beta-adrenergic receptor and adenylate cyclase. Biochim Biophys Acta. 1976 Apr 13;457(1):1–39. doi: 10.1016/0304-4157(76)90012-5. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Caron M. G. Regulation of beta-adrenergic receptors by guanyl-5'-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976 Aug 10;251(15):4686–4692. [PubMed] [Google Scholar]
- Lefkowitz R. J. Stimulation of catecholamine-sensitive adenylate cyclase by 5'-guanylyl-imidodiphosphate. J Biol Chem. 1974 Oct 10;249(19):6119–6124. [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1977 Feb;74(2):515–519. doi: 10.1073/pnas.74.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Adenylate cyclase-coupled beta adrenergic receptors effect of membrane lipid-perturbing agents on receptor binding and enzyme stimulation by catecholamines. Mol Pharmacol. 1976 Jul;12(4):559–567. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Negative cooperativity among beta-adrenergic receptors in frog erythrocyte membranes. J Biol Chem. 1976 Aug 25;251(16):5007–5014. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Resolution of beta-adrenergic receptor binding and adenylate cyclase activity by gel exclusion chromatography. J Biol Chem. 1977 Jan 25;252(2):799–802. [PubMed] [Google Scholar]
- Limbird L. E., Meyts P. D., Lefkowitz R. J. Beta-adrenergic receptors: evidence for negative cooperativity. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1160–1168. doi: 10.1016/0006-291x(75)90815-3. [DOI] [PubMed] [Google Scholar]
- Maguire M. E., Van Arsdale P. M., Gilman A. G. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol Pharmacol. 1976 Mar;12(2):335–339. [PubMed] [Google Scholar]
- Maguire M. E., Wiklund R. A., Anderson H. J., Gilman A. G. Binding of (125I)iodohydroxybenzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976 Mar 10;251(5):1221–1231. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Coverstone M., Lefkowitz R. J. Identification of adenylate cyclase-coupled beta-adrenergic receptors in frog erythrocytes with (minus)-[3-H] alprenolol. J Biol Chem. 1975 Jul 10;250(13):4869–4876. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]



