Abstract
Background:
Early-onset Alzheimer’s disease (EOAD) has been overshadowed by the more common late-onset AD (LOAD). Yet, the literature indicates EOAD may have less hippocampal-memory presentations and more focal neocortical localization early in the disease.
Objective:
To evaluate these proposed differences between these 2 forms of AD and to explore what they inform about differences in AD pathophysiology.
Methods:
In all, 21 patients with EOAD and 24 patients with LOAD matched for disease progression and severity were compared on neurocognitive measures and resting state fluorodeoxy-glucose positron–emission tomography (FDG-PET).
Results:
Patients with EOAD had worse executive functions with greater hypometabolism in the parietal regions; whereas patients with LOAD had worse confrontation naming and verbal recognition memory with greater hypometabolism in inferior frontotemporal regions.
Conclusions:
In addition to highlighting significant differences between EOAD and LOAD, these results reveal dissociation between executive deficits in AD and frontal hypometabolism, suggesting early disturbances of the parietal–frontal network in EOAD.
Keywords: early onset, late onset, neuropsychology, Alzheimer’s disease, dementia, neuroimaging, FDG-PET
Introduction
In 1901, Alois Alzheimer observed a patient at the Frankfurt Asylum named Auguste Deter; a 51-year-old patient with strange behavioral symptoms and a memory disturbance. Five years later, when this patient passed away, neuropathology revealed neuritic plaques and neurofibrillary tangles consistent with what became known as Alzheimer’s disease (AD). Over the years, the much more common late-onset AD (LOAD) overshadowed this early-onset AD (EOAD) characterized by an onset before age 65. The study of EOAD is important, not only because it affects up to 2.4 out of 100 000 persons, 1 but also because it tends to strike people when they are in the prime of their career and family responsibilities.
The literature suggests that EOAD differs substantially from LOAD. 2 -4 There is general agreement that patients with EOAD have greater cortical atrophy and hypometabolism compared to patients with LOAD at a similar stage of disease. 5 Functional neuroimaging studies have demonstrated significant cortical atrophy, hypoperfusion, and hypometabolism in EOAD, particularly in parietal and lateral temporal cortices, and significant lesions or hypometabolism in medial temporal and hippocampal regions in LOAD. 5 -14 There are conflicting studies, however, on the cognitive aspects of EOAD. Some studies find that patients with EOAD show greater impairments in attention, language, visuospatial and executive functions; and patients with LOAD show comparatively greater deficits in episodic memory. 2 -5,15 -22 In contrast, other studies find no differences in cognition according to age of onset. 7,23 Given the differences on functional neuroimaging, it would be important to know whether the cognitive aspects correspond to the differences in functional neuroimaging results in EOAD versus LOAD.
This study compares EOAD with LOAD on both cognitive features and functional neuroimaging with positron-emission tomography (PET). There have been few direct comparisons between patients with EOAD and patients with LOAD, and much of the literature has been restricted to examination of solely neuropsychological differences between the groups 2,3,15,24 -27 ; or solely neuroimaging differences. 6,8 -11,13,28 -30 We hypothesize that such comparison would confirm the presence of greater nonmemory deficits and more prominent neocortical hypometabolism, particularly in the parietal lobes, in EOAD as compared to LOAD. Finally, this study has implications for understanding differences in the underlying pathophysiology of AD.
Methods
Participants
Participants were recruited from the Departments of Neurology and Geriatric Psychiatry at the Veterans Affairs (VA) Greater Los Angeles Healthcare Center and the University of California, Los Angeles (UCLA) Geffen School of Medicine. All participants were native English speakers. Diagnosis of AD was determined by the National Institute of Communicable Diseases and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA) criteria for clinically probable AD. 31 Whether one was classified as “early onset” versus “late onset” was based on age of onset; those with a disease onset prior to age 65 were early onset and those with a disease onset subsequent to age 65 were late onset. Individuals with major medical illnesses (except hypertension or diabetes) or psychiatric illnesses not due to the dementia process were excluded from the study. The study was reviewed and approved by the local institutional review board (IRB), and study participants were enrolled according to the IRB guidelines.
The participants in this study were specifically selected in order to match the EOAD and LOAD groups on 5 variables that are well-established confounds in previous studies. The participants were group matched on education, time since diagnosis, gender distribution, functional status (eg, activities of daily living [ADLs]/instrumental activities of daily living [IADLs]) and global cognitive severity (as measured by the Mini-Mental State Examination [MMSE]).
Procedures
The patients underwent neuropsychological testing, and neuroimaging with FDG-PET of the brain. Caregivers (study partners) who accompanied patients to appointment also filled out questionnaires to provide information regarding their experiences/impressions of the patient.
Measures
Demographics
The study partners (caregivers) completed a demographic questionnaire on behalf of the participants, which included information such as age, education level, marital and living situation status, and occupation. Disease-related information including diagnosis and time of onset was also provided by caregivers.
Global Cognitive Severity and Functional Status (ADLs/IADLs)
All participants completed the MMSE as a measure of global cognitive severity. Data were normed, adjusting for age and education level. 32
Alzheimer’s Disease Cooperative Study–Activities of Daily Living Inventory (ADCS-ADLs). The ADCS-ADLs is a 23-item inventory wherein the caregiver is asked to focus onthe patient’s observed performance over the past month.
Language
Boston Naming Test. This is a 60-item confrontation naming task measuring the word retrieval process of patients when presented with a picture of an item. Data were normed, adjusting for age, education, and gender. 33
Controlled Oral Word Association Test (COWAT), wherein participants are given 60 seconds to produce as many words as possible from a semantic and phonemic category. Data were normed, adjusting for age, education, and gender. 34
Visuospatial Skills
A subindex from the intersecting pentagon task on the MMSE 35 , 36 was utilized to evaluate the types of visuospatial errors made in this task. The 8 errors in this index were relating to size of the figures, number of figures drawn, intersection of the pentagons, tremor or segmentation errors, angle errors, rotation, motor perseveration, and pull-to-stimulus tendencies.
Attention and Executive Control
Digit span subtest of the Wechsler Adult Intelligence Scale, third edition (WAIS-III). Participants are presented with a number of auditory series and asked to repeat these numbers back to examiner in the exact order read to them (digits forward) or in the reverse order the digits were read to them (digits backward). Normative data adjusting for age were obtained from the WAIS-III manual.
Stroop Task, which provides participants with 3 different conditions (word reading, color naming, and interference task). In each condition, participants are timed to see how many words they can successfully read/name within a given amount of time. Normative data adjusting for age was obtained from the Golden manual.
Trail Making Test (TMT, Part A only). This is a test of visual attention and psychomotor speed, where the participant is provided with a sheet of paper with numbers from 1 to 25 within circles and instructed to connect the numbers in consecutive order using straight lines. Data were normed, adjusting for age and education. 37
COWAT (“FAS”). Participants are given 60 seconds to provide as many words that begin with the letter “F”, “A”, and “S”, respectively.
Verbal Learning and Memory
Neuropsychological battery of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD); 10-item word list learning and recognition only). The list learning/recognition task on the CERAD includes 10 common nouns presented consecutively and read aloud by the participant, with a different order used on each of the 3 successive occasions. Following this task, there is a free recall condition and recognition paradigm presented. Data were normed, adjusting for age, gender, and education level. 38
The (FDG) PET Imaging: Data Acquisition and Processing
All patients underwent resting state functional neuroimaging with PET scans as part of this study in accordance with the technique of Hamacher, Coenen, and Stocklin. 39 They received an intravenous infusion of 5 to 10 mCi of [18F]-FDG and then waited 40 minutes quietly in a dimly lighted room with eyes open (uptake phase). Participants were next placed in the scanner with the imaging plane parallel to the canthomeatal plane and metabolic data were acquired for 40 minutes. Patients with LOAD were scanned on different tomographs over the course of the study. A total of 3 patients were scanned on a Siemens 953/31 tomographic scanner (voxel size 1.96 × 1.96 × 3.38 mm3, Siemens Medical Solutions, Hoffman Estates, Illinois), 11 on a mobile GE Advance (voxel size 2.34 × 2.34 × 4.25 mm3, 2.73 × 2.73 × 4.25, or 2.34 × 2.34 × 4.25 m3, or 2.34 × 2.34 × 3.27 GE Medical Systems, Milwaukee, Wisconsin), and 7 on a Philips Gemini TF-PET-CT (2 × 2 × 2). All early patients with AD (n = 20) were scanned on a Philips Gemini TF-PET-CT (2 × 2 × 2). Most participants completed PET imaging on the same day as the cognitive assessment; however, all of the PET scans were done within 2 months of the respective cognitive testing. Raw data in Digital Imaging & Communications in Medicine (DICOM) format were converted to ANALYZE format. In all images, origins were manually set to the anterior cingulate cortex, before the spatial normalization step. Images were normalized to Montreal Neurological Institute (MNI) space using trilinear interpolation and resampled to 2 × 2 × 2 mm3 voxels, and smoothed using a 6-mm Full width at half maximum (FWHM) smoothing kernel, using SPM8 (Welcome Trust Centre for Neuroimaging, London, United Kingdom) in MATLAB R2010b (MathWorks, Inc., Massachusetts USA).
We investigated the data to determine whether there were any systematic differences between groups with respect to global metabolism. We obtained mean values of the entire brain using custom MATLAB software and of cerebellum gray matter using Automatic Anatomic Labeling (AAL) toolkit in Statistical Parametric Mapping, 8th edition (SPM8). The ratio of whole brain/cerebellar gray matter was calculated for each participant.
Analyses
Demographic and neuropsychological variables were compared in SPSS 17.0 using chi-Square analyses as well as independent samples t tests to evaluate the between-group differences. Both raw scores and normed standard scores (adjusting for age, education, gender in order to directly compare levels of performance by expressing each patient’s scores in standard deviations from the mean of normal control performance) were compared. Group differences were also evaluated with analysis of covariance (ANCOVA), using global cognitive severity (MMSE scores) as a covariate. The significance level was set at the P < .05 level.
Voxel-Based Statistical Analysis
Statistical maps of the FDG-PET images were generated using SPM8. Two-sample t test was performed between EOAD and LOAD groups. Participant images were normalized to the global mean. Threshold masking was used to remove signal from structures outside of the brain (set to 0.8). Between-group difference maps were set at a voxel threshold of P < .01 (uncorrected for multiple comparisons) and considered significant at the cluster level at P < .05, corrected for multiple comparisons using the family-wise error (FWE) procedure.
Results
Demographics
Table 1 depicts the demographic makeup of our sample. The only demographic variables that significantly differed between the EOAD and LOAD groups was age (as anticipated) and ethnicity, wherein the EOAD group had more nonwhite individuals.
Table 1.
Demographics of Participants With Early- and Late-Onset Alzheimer's Disease
| Demographic | Early-onset Alzheimer's disease | Late-onset Alzheimer's disease |
|---|---|---|
| Variable | N = 21 | N = 24 |
| Ageb (years) | M = 57.78 (SD = 4.35) | M = 80.32 (SD = 5.89) |
| Gender | ||
| Male | N = 15 (65%) | N = 15 (68%) |
| Female | N = 8 (35%) | N = 7 (32%) |
| Education (years) | M = 15.95 (SD = 2.63) | M = 16.05 (SD = 2.38) |
| Handedness | ||
| Right | N = 20 (91%) | N = 21 (95%) |
| Left | N = 1 (4%) | N = 1 (4.5%) |
| Ambidextrous | N = 1 (5%) | N = 0 |
| Time since onset (years) | M = 3.12 (SD = 1.61) | M = 3.91 (SD = 2.76) |
| Family history of | ||
| dementia | ||
| Yes | N = 8 (35%) | N =11(50%) |
| No | N = 12 (52%) | N =11(50%) |
| Unsure | N = 3 (13%) | N = 0 |
| Ethnicitya | ||
| White | N = 21 (91%) | N = 15 (68%) |
| Nonwhite | N = 2 (9%) | N = 7 (32%) |
Abbreviation: SD, standard deviation.
a Indicates significant between-group difference at the P < .05 level.
b Indicates significant between-group difference at the P < .001 level.
Neuropsychological Comparisons of Patients With EOAD and LOAD
Global Cognitive Severity and Functioning
Patients with EOAD and LOAD were matched in regard to global cognitive functioning as measured by the MMSE (see Table 2). When normed (age and education adjusted) data were compared, the nonsignificant between-group differences remained. There were no significant group differences on the Alzheimer’s Disease Cooperative Study Scale (measuring ADL/IADL functionality, t(34) = −0.15, ns). Similarly, when broken down and analyzed by subscales indicating degrees of daily functioning, no significant group differences were documented (basic: t(27) = −1.18, n.s.; autonomy: t(32) = 0.04, ns; higher level function, t(20) = 0.26, ns).
Table 2.
Neuropsychological Comparisons of Patients With EAD and LADa
| EAD | LAD | ||||||
|---|---|---|---|---|---|---|---|
| Neuropsychological measure | t | df | Sig | M | SD | M | SD |
| Global cognitive severity | |||||||
| Mini-Mental State Examination (MMSE) | −1.13 | 41 | NS | 19.19 | 6.35 | 21.09 | 4.03 |
| Language | |||||||
| Boston Naming Test (with or without stimulus cue) | 3.15 | 38 | A, B, C | 49.85 | 15.58 | 35.30 | 13.57 |
| FAS (phonemic fluency) | −1.23 | 42 | B, C | 21.55 | 14.64 | 26.68 | 13.08 |
| Animals (semantic fluency) | 0.58 | 35 | NS | 8.55 | 6.51 | 7.59 | 3.99 |
| Visuospatial skills | χ 2 | df | Sig | % Positive EAD | % Positive LAD | ||
| MMSE pentagons | |||||||
| Pull-to-stimulus copy | 6.59 | 1 | P = .01 | 26.3% | 0% | ||
| Size of figure | 0.48 | 1 | NS | 10.5% | 18.2% | ||
| Number of figures | 1.92 | 1 | NS | 31.6% | 13.6% | ||
| Pentagon intersection | 2.18 | 1 | NS | 68.4% | 45.5% | ||
| Tremor or segmentation | 0.98 | 1 | NS | 36.8% | 22.7% | ||
| Five angles | 0.11 | 1 | NS | 36.8% | 31.8% | ||
| Rotation | 0.02 | 1 | NS | 10.5% | 9.1% | ||
| Motor perseveration | 0.01 | 1 | NS | 26.3% | 27.3% | ||
| EAD | LAD | ||||||
| Attention/executive control | t | df | Sig | M | SD | M | SD |
| Stroop task | |||||||
| Word reading | 0.306 | 35 | NS | 55.56 | 21.76 | 58.58 | 19.49 |
| Color naming | 0.294 | 35 | NS | 32.78 | 16.06 | 32.63 | 18.03 |
| Interference (color and word) | −1.79 | 35 | B, C | 7.83 | 5.89 | 13.32 | 11.59 |
| Trail Making Test–Part A | 1.16 | 13.82 | B | 165.07 | 240.66 | 99.05 | 52.87 |
| Wechsler adult intelligence scale digit span | |||||||
| Forward | −0.831 | 41 | NS | 6.77 | 2.33 | 7.33 | 2.08 |
| Backward | −2.36 | 41 | A, B, C | 3.00 | 1.75 | 4.42 | 2.20 |
| Total | −1.74 | 41 | NS | 9.77 | 3.83 | 11.76 | 3.65 |
| Verbal learning/memory | |||||||
| CERAD | |||||||
| Word list memory (trials 1 -3) | .131 | 32 | NS | 9.00 | 5.49 | 8.77 | 4.44 |
| Word list recall | 1.92 | 32 | NS | 1.33 | 1.78 | 0.32 | 1.29 |
| Word list recognition | 2.94 | 33 | A, B, C | 7.77 | 2.55 | 4.95 | 2.84 |
| Savings [(Delayed recall/trial 3) × 100] | 1.67 | 33 | NS | 28.27 | 34.49 | 8.10 | 32.81 |
Abbreviations: CERAD stands for Consortium to Establish a Registry for Alzheimer's Disease; EOAD, early-onset Alzheimer’s disease; LOAD, late-onset Alzheimer’s disease; M, mean; SD, standard deviation.
a “A”,“B”, and “C” denote statistically significant findings: A denotes significance in raw data analyses (t tests) ; B indicates significance when comparing normed data; NS denotes not significant in raw data or normed data analyses; and C indicates maintained significance when adjusting for global cognitive severity scores.
Language
Patients with LOAD performed significantly worse than patients with EOAD on the Boston Naming Test (number correct with or without stimulus cue), t(38) = 3.15, P < .01. This finding remained significant when comparing normed data (adjusting for age, gender, and education). On verbal fluency raw scores, there were no significant differences with respect to either phonemic or semantic fluency. When normed data were compared, patients with EOAD had poorer performances when compared to patients with LOAD on the phonemic fluency measure.
Visuospatial Skills
Although there were no statistically significant differences in gross visuoconstruction skills as assessed by the overlapping pentagons portion on the MMSE, there were differences between the EOAD and LOAD groups in type of errors made on visuoconstruction task. 35,36 More of the patients with EOAD (26.3%) made pull-to-stimulus errors (eg, patient drew, wrote, or copied over the figure model), compared to the patients with LOAD (0%; χ 2 (1) = 6.59, P = .01).
Attention and Executive Control
On a measure of simple attention, there were no significant between-group differences; WAIS Digit Span Forward, t(41) = −0.83, ns; overall digit span total score, t(41) = −1.74, ns. In contrast, on measures of complex attention or working memory, significant between-group differences were found on the Wechsler Adult Intelligence Scale Digit Span (backward, raw) subtest, t(41) = −2.36, P < .05. Patients with EOAD had lower performances (M = 3.0, SD = 1.75) on this subtest when compared to their LOAD counterparts (M = 4.42, SD = 2.20). Measures of visual attention and psychomotor speed did not yield significant differences when raw scores were compared; however, a significant difference emerged on Trails A performance when normed data (adjusting for age and education levels) were compared, t(16.10) = −2.16, P < .05. patients with LOAD tended to perform comparatively better than patients with EOAD.
On executive measures, the EOAD group consistently performed worse than the LOAD group on a number of tasks. Although raw score differences were null, normed data comparisons (controlling for age) revealed significant between-group differences on the Stroop interference or response inhibition task (t(35) = −4.48, P < .001), with LOAD patients performing comparatively better than patients with EOAD. Additionally, although semantic fluency scores were comparable, a phonemic fluency task indicated significant differences with poorer performances for the EOAD group compared to the LOAD group when analyses were adjusted for demographics (FAS, t(42) = −2.15, P < .05). The other “executive” deficits in EOAD, compared to LOAD, have been noted and include problems with backward digits and “pull-to-stimulus” visuospatial functioning.
Verbal Learning and Memory
There was no significant group differences on Trials 1 to 3, CERAD total recall, t(32) = 0.13, ns; however, the delayed recall nearly reached significance with patients with LOAD performing the worst, t(32) = 1.92, P = .06. In addition, there was a significant difference between true-positive hits on a recognition paradigm; patients with LOAD performed worse on this task than patients with EOAD patients.
Normed data comparisons (controlling for age, gender, and education level) yielded similar findings, with only the age-, gender-, and education-corrected recognition scores yielding significant differences (CERAD recognition, t(31.84) = 4.17, P ≤ .001).
Covariate-Adjusted Analyses: Controlling for Global Cognitive Functioning
When the MMSE scores were entered as a covariate in analyses examining between-group differences, all previously significant findings persisted with the sole exception of the Trail Making Test, Part A. This test was no longer significant when controlling for MMSE scores, F(2, 31) = 2.67, ns.
The FDG-PET Imaging
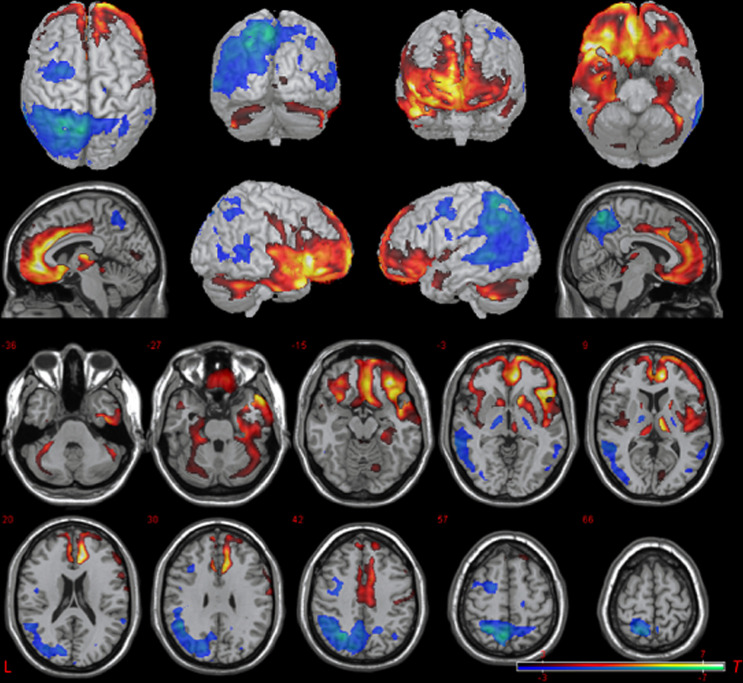
SPM8 was employed to compare FDG-PET images of the EOAD and LOAD groups. Results are illustrated in Figure 1 and see Table 3 for coordinates and statistics. There were no differences between groups with respect to the whole brain/cerebellum ratio. Between-group 2-sample t test (P < .05, corrected at the cluster level using the FWE procedure) revealed relative hypometabolism in the EOAD group compared to the LOAD group in a large cluster in the posterior cortex, including bilateral precuneus, bilateral superior parietal lobule (L>R), left supramarginal gyrus, left angular gyrus, posterior regions of the left lateral temporal cortex (superior, middle, and inferior temporal gyri), and left occipital lobe (P < .05, FWE corrected at the cluster level). There was a trend toward relative hypometabolism in left lateral frontal cortex, primarily encompassing the left precentral gyrus and extending anteriorly to include posterior middle frontal and inferior frontal gyri (P = .07 FWE corrected at the cluster level). In contrast, relative hypometabolism in the LOAD group compared to the EOAD group was observed in inferior portions of the brain, including inferior frontal lobe and temporal cortex. Specifically, there was a large cluster encompassing bilateral orbitofrontal gyri and medial frontal lobe (including the cingulate, gyrus rectus, and superior frontal gyrus). Moving posteriorly it included parts of bilateral inferior frontal gyrus. This also included the right superior, middle, and inferior temporal gyri, fusiform, parahippocampal gyrus, and hippocampus. A second cluster revealed relative hypometabolism in LOAD versus EOAD in left parahippocampal gyrus and fusiform. Additional between-group differences were observed in bilateral cortex surrounding the Sylvian fissure and cerebellum. Figure 2 provides the view of 1 representative patient from the EOAD and LOAD group, respectively, to demonstrate the pattern of parietal hypometabolism in the EOAD group, while showing the pattern of inferior frontal hypometabolism in the LOAD group. The frontal pattern within the LOAD group, however, is more subtle, as the true differences are more apparent in the between-group comparisons.
Figure 1.
Base comparison of EOAD relative to LOAD. Two-sample t test comparison shows relative hypometabolism in the EAD group (blue) and relative hypometabolism in the LAD group (red; uncorrected P = .01, T = 2.43, kc = 10 voxels). Picture is in neurological convention; brain’s left is your left. EOAD indicates early-onset Alzheimer’s disease; LOAD, late-onset Alzheimer’s disease.
Table 3.
Between-Group Differences in Metabolism
| Cluster P (FWE corrected) | kc | Peak voxel MNI (x, y, z) | Peak T | |
|---|---|---|---|---|
| EAD < LAD; bilateral Precuneus and superior parietal lobule, left inferior parietal lobule, left posterior temporal cortex, left occipital lobe | <.0005 | 12 311 | −8, −64, 54 | 6.6 |
| LAD < EAD; bilateral inferior and medial frontal cortex, right temporal cortex | <.001 | 29 872 | 52, 24, −2 | 8.24 |
| Left parahippocampal/fusiform gyri, cerebellum | .016 | −44, −54, −46 | 5.69 |
Abbreviations: FEW, family-wise error; MNI, Montreal Neurological Institute; EAD, early-onset Alzheimer’s disease; LAD, late-onset Alzheimer’s disease.
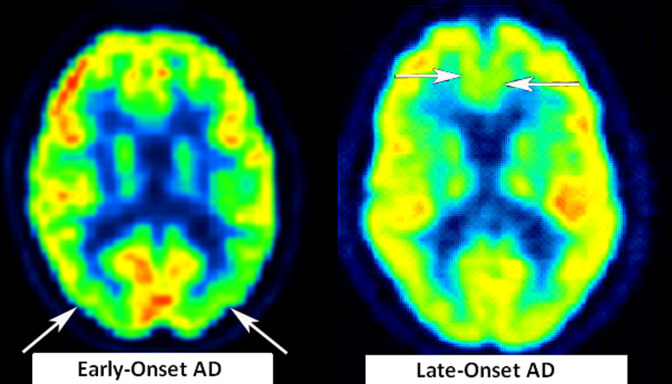
Figure 2.
Axial image of regional hypometabolism in representative patients with EOAD and LOAD. Arrows highlight areas of hypometabolism for each respective patient; for patients with EOAD, hypometabolism seen in the parietal region, whereas in LOAD, hypometabolism seen in the inferior frontal region. Picture is in neurological convention; brain’s left is your left.
Discussion
This study found neuropsychological and functional imaging differences between patients with AD of early onset compared to those with AD of late onset. Compared to the patients with LOAD, the patients with EOAD performed worse on several measures of executive functioning (working memory on digit span backward; phonemic vs. semantic fluency; a pull-to-stimulus copy performance, and the Stroop interference task). This finding of greater executive dysfunction in the EOAD cohort has been inconsistent in previous studies; while some studies have confirmed this finding, 21 others have not found group differences on executive tasks. 7 In contrast, the patients with LOAD, compared to the patients with EOAD, had worse confrontation naming and recognition memory scores, findings more typical of AD. 2 -4,15,22,25 These changes correspond with relative parietal hypometabolism among the patients with EOAD and relative frontotemporal hypometabolism among the patients with LOAD.
These results are consistent with the literature in some findings but not others. Similar to other studies, 26 this study finds that patients with EOAD perform worse than patients with LOAD on executive tasks, including working memory as reflected in digit span backward. The finding that EOAD are significantly worse on a measure of phonemic fluency (but not semantic fluency) further supports the pattern of executive dysfunction, as research has demonstrated that disproportionate letter fluency correlates with frontal-executive dysfunction. 40,41 In addition, a pull-to-stimulus (eg, patient wrote, drew, or copied over ssure model) error on the visuoconstruction task is much more common in patients with EOAD rather than in patients with LOAD. This type of visuospatial error is consistent with an executive control problem in that it indicates environmental dependency. 42 In contrast, the patients with LOAD, as compared to the patients with EOAD, have worse language functioning as evidenced by poorer performance on a confrontation naming task. This finding differs from some previous studies which report more severe language impairment as a feature of EOAD. 4,17,43 Similarly, there have been different findings regarding worse memory disturbances in LOAD versus EOAD. 7 ,16,17 This study reports worse recognition memory for the LOAD group compared to the EOAD group.
On FDG-PET, the patients with EOAD, compared to the patients with LOAD, have lower metabolic activity in the parietal (left worse than right) lobes. Other studies of EOAD indicate the presence of significant parietal lobe involvement along with additional changes in other neocortical regions early in the disease, 6 -14 whereas prior neuroimaging studies of LOAD show early changes in hippocampal and inferior temporal regions. 5 In terms of laterality, although the current study suggests that patients with EOAD have greater lateralization of Alzheimer changes to the left hemisphere, other studies are inconsistent on whether AD affects the left or right hemisphere disproportionately. 5,14 Finally, the degree of underlying atrophy can impact regional hypometabolism, but it would still reflect selective, disproportionate disease of the parietal lobes in EOAD compared to the frontotemporal lobes in LOAD.
A significant finding of this study is the presence of worse executive functions without worse frontal metabolism in EOAD as compared to LOAD. This dissociation has been previously noted but not specifically studied. 41 Such a dissociation supports recent views of regional dysfunction in that executive skills are not exclusively frontally mediated and are rather the product of an interconnected network. Namely, the dorsolateral frontal cortex has dense connections with the parietal lobe; a network that has been implicated in tasks of executive functioning, 40,41 especially in early-onset dementia. 41 There is an increasing understanding that cognition is a product of these networks and tracts, and investigations of white matter pathways indicate disruption of important parietal lobe–dorsolateral frontal tracts in AD. 40 Early involvement of the parietal lobe in EOAD would affect gray matter as well as its main dorsolateral frontal networks. In addition, in this study there was a trending pattern of lower metabolic activity in the dorsolateral frontal regions in EOAD.
This study has potential limitations. First, sample size is relatively small, which has implications for generalizability of these findings as well as statistical limitations. Nevertheless, this report revealed several important differences between EOAD and LOAD, which were generally adequately powered (post hoc power analyses ranged from .66 to .99 for significant group differences reported herein). Thus, the results herein, which may be characterized as exploratory, may require confirmatory analyses with a larger sample size in future research. Second, some cognitive domains, in particular visuospatial functioning, had limited assessment. Future research may include a more comprehensive cognitive comparison of both early- and late-onset groups. Third, the presence of greater hypometabolism in the medial/inferior frontal lobe in the LOAD group may reflect age-related changes in this older cohort. Previous work has found that medial prefrontal regions are sensitive to normal aging 44,45 . This could not be addressed in the current study, given the lack of normal controls. However, prior investigations comparing participants with EOAD or LOAD with normal controls report the same pattern of metabolic changes (eg, 5,11 ). Thus, although not directly compared in the present study, the patterns of worse parietal metabolism in EOAD and frontal metabolism in LOAD are consistent with qualitatively different patterns than would be observed from the normal aging profile. Furthermore, given the lack of overlap in age between the 2 cohorts, controlling for age would remove innate variance. Finally, the LOAD group included FDG-PET data from participants scanned on multiple PET tomographs. The between-group differences were analyzed using a normalization procedure in SPM, which yields a ratio scale for each participant (voxel divided by global mean) and ratio values appear to vary by less than 5% across scanners. 46 We assessed the global ratio values in each group and observed a nearly identical mean and range of values in each group. This suggests that while the use of multiple scanners may have impacted our ability to detect differences between groups, it is unlikely a large effect.
In conclusion, these findings suggest differences in AD pathophysiology between EOAD and LOAD; a notion that has been debated since Auguste Deter was first encountered by Alois Alzheimer. This study confirms some previous findings and reports new findings with respect to differences between EOAD and LOAD. Specifically, the results implicate the differential early involvement in parietal lobes with probable dysfunction of, not only cognition from parietal gray matter disturbances but also executive dysfunction presumably from the parietal–frontal network. Much more research is needed in order to clarify this finding and the differences in AD pathophysiology in EOAD as compared to the much more common, and typical, LOAD. Future studies could profitably evaluate other important areas, such as white matter integrity in order to assess the fronto–parietal network and gene status, as this has a large impact on the development of early-onset dementias. 47
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH Grant #R01AG034499-3 (M. Mendez PI); Department of Veterans Affairs VA Merit Review (2 separate awards; 1 to M. Mendez and another to D. Sultzer); Career Development Award to R. Melrose; NIMH #R01-MH56031 (D. Sultzer,) PI.
References
- 1. Renvoize E, Hanson M, Dale M. Prevalence and causes of young onset dementia in an English health district. Int J Geriatr Psychiatry. 2011;26(1):106–107. [DOI] [PubMed] [Google Scholar]
- 2. Loring DW, Largen JW. Neuropsychological patterns of presenile and senile dementia of the Alzheimer type. Neuropsychologia. 1985;23(3):351–357. [DOI] [PubMed] [Google Scholar]
- 3. Filley CM, Kelly J, Heaton RK. Neuropsychologic features of early- and late-onset Alzheimer's disease. Arch Neurol. 1986;43(6):574–576. [DOI] [PubMed] [Google Scholar]
- 4. Seltzer B SI. A. comparison of clinical features in early-and-late-onset primary degenerative dementia. One entity or two? Archives of Neurology. 1983;40(3):143–146. [DOI] [PubMed] [Google Scholar]
- 5. Kim EJ, Cho SS, Jeong Y, et al. Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Brain. 2005;128(pt 8):1790–1801. [DOI] [PubMed] [Google Scholar]
- 6. Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Structural correlates of early and late onset Alzheimer's disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2005;76(1):112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grady CL, Haxby JV, Horwitz B, Berg G, Rapoport SI. Neuropsychological and cerebral metabolic function in early vs late onset dementia of the Alzheimer type. Neuropsychologia. 1987;25(5):807–816. [DOI] [PubMed] [Google Scholar]
- 8. Jagust WJ, Reed BR, Seab JP, Budinger TF. Alzheimer's disease. Age at onset and single-photon emission computed tomographic patterns of regional cerebral blood flow. Arch Neurol. 1990;47(6):628–633. [DOI] [PubMed] [Google Scholar]
- 9. Yasuno F, Imamura T, Hirono N, et al. Age at onset and regional cerebral glucose metabolism in Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9(2):63–67. [DOI] [PubMed] [Google Scholar]
- 10. Salmon E, Collette F, Degueldre C, Lemaire C, Franck G. Voxel-based analysis of confounding effects of age and dementia severity on cerebral metabolism in Alzheimer's disease. Hum Brain Mapp. 2000;10(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakamoto S, Ishii K, Sasaki M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer's disease. J Neurol Sci. 2002;200(1-2):27–32. [DOI] [PubMed] [Google Scholar]
- 12. Kemp PM, Holmes C, Hoffmann SM, et al. Alzheimer's disease: differences in technetium-99m HMPAO SPECT scan findings between early onset and late onset dementia. J Neurol Neurosurg Psychiatry. 2003;74(6):715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer's disease and assessment of diagnostic performance of z score images. AJNR Am J Neuroradiol. 2005;26(2):333–340. [PMC free article] [PubMed] [Google Scholar]
- 14. Karas G, Scheltens P, Rombouts S, et al. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49(12):967–976. [DOI] [PubMed] [Google Scholar]
- 15. Binetti G, Magni E, Padovani A, Cappa SF, Bianchetti A, Trabucchi M. Neuropsychological heterogeneity in mild Alzheimer's disease. Dementia.1993;4(6):321–326. [DOI] [PubMed] [Google Scholar]
- 16. Jacobs D, Sano M, Marder K, et al. Age at onset of Alzheimer's disease: relation to pattern of cognitive dysfunction and rate of decline. Neurology. 1994;44(7):1215–1220. [DOI] [PubMed] [Google Scholar]
- 17. Koss E, Edland S, Fillenbaum G, et al. Clinical and neuropsychological differences between patients with earlier and later onset of Alzheimer's disease: A CERAD analysis, Part XII. Neurology. 1996;46(1):136–141. [DOI] [PubMed] [Google Scholar]
- 18. Fujimori M, Imamura T, Yamashita H, et al. Age at onset and visuocognitive disturbances in Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12(3):163–166. [DOI] [PubMed] [Google Scholar]
- 19. Imamura T, Takatsuki Y, Fujimori M, et al. Age at onset and language disturbances in Alzheimer's disease. Neuropsychologia. 1998;36(9):945–949. [DOI] [PubMed] [Google Scholar]
- 20. Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007;43(7):835–845. [DOI] [PubMed] [Google Scholar]
- 21. Stopford CL, Snowden JS, Thompson JC, Neary D. Variability in cognitive presentation of Alzheimer's disease. Cortex. 2008;44(2):185–195. [DOI] [PubMed] [Google Scholar]
- 22. Skantzakis E, Tzallas P, Kruse J, Kalpouzos C, Charalambidis D. Coherent continuum extreme ultraviolet radiation in the sub-100-nJ range generated by a high-power many-cycle laser field. Opt Lett. 2009;34(11):1732–1734. [DOI] [PubMed] [Google Scholar]
- 23. Toyota Y, Ikeda M, Shinagawa S, et al. Comparison of behavioral and psychological symptoms in early-onset and late-onset Alzheimer's disease. Int J Geriatr Psychiatry. 2007;22(9):896–901. [DOI] [PubMed] [Google Scholar]
- 24. Licht EA, McMurtray AM, Saul RE, Mendez MF. Cognitive differences between early- and late-onset Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2007;22(3):218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suribhatla S, Baillon S, Dennis M, et al. Neuropsychological performance in early and late onset Alzheimer's disease: comparisons in a memory clinic population. Int J Geriatr Psychiatry. 2004;19(12):1140–1147. [DOI] [PubMed] [Google Scholar]
- 26. Reid W, Broe G, Creasey H, et al. Age at onset and pattern of neuropsychological impairment in mild early-stage Alzheimer disease. A study of a community-based population. Arch Neurol. 1996;53(10):1056–1061. [DOI] [PubMed] [Google Scholar]
- 27. Smits LL, Pijnenburg YA, Koedam EL, et al. Early Onset Alzheimer's Disease is Associated with a Distinct Neuropsychological Profile. J Alzheimers Dis. 2012;30(1):101–108. [DOI] [PubMed] [Google Scholar]
- 28. Small GW, Kuhl DE, Riege WH, et al. Cerebral glucose metabolic patterns in Alzheimer's disease. Effect of gender and age at dementia onset. Arch Gen Psychiatry. 1989;46(6):527–532. [DOI] [PubMed] [Google Scholar]
- 29. Ichimiya A, Herholz K, Mielke R, Kessler J, Slansky I, Heiss WD. Difference of regional cerebral metabolic pattern between presenile and senile dementia of the Alzheimer type: a factor analytic study. J Neurol Sci. 1994;123(1-2):11–17. [DOI] [PubMed] [Google Scholar]
- 30. Hanyu H, Nakano S, Abe S, Arai H, Iwamoto T, Takasaki M. Differences of neuroimaging between early-onset and late-onset Alzheimer-type dementia. Rinsho Shinkeigaku. 1995;35(10):1104–1109. [PubMed] [Google Scholar]
- 31. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 32. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- 33. Heaton RKM, Walden S, Taylor Micheal J, Grant Igor. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Dempgraphically Adjusted Neuropsychological Norms for African American and Caucasian Adults Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 34. Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. [DOI] [PubMed] [Google Scholar]
- 35. Lamar M, Podell K, Carew TG, et al. Perseverative behavior in Alzheimer's disease and subcortical ischemic vascular dementia. Neuropsychology. 1997;11(4):523–534. [DOI] [PubMed] [Google Scholar]
- 36. Jefferson AL, Cosentino SA, Ball SK, et al. Errors produced on the mini-mental state examination and neuropsychological test performance in Alzheimer's disease, ischemic vascular dementia, and Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2002;14(3):311–320. [DOI] [PubMed] [Google Scholar]
- 37. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. [DOI] [PubMed] [Google Scholar]
- 38. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609–614. [DOI] [PubMed] [Google Scholar]
- 39. Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27(2):235–238. [PubMed] [Google Scholar]
- 40. Woo BK, Harwood DG, Melrose RJ, et al. Executive deficits and regional brain metabolism in Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25(11):1150–1158. [DOI] [PubMed] [Google Scholar]
- 41. Schroeter ML, Vogt B, Frisch S, et al. Executive deficits are related to the inferior frontal junction in early dementia. Brain. 2012;135(Pt 1):201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cosentino S, Jefferson A, Chute DL, Kaplan E, Libon DJ. Clock drawing errors in dementia: neuropsychological and neuroanatomical considerations. Cogn Behav Neurol. 2004;17(2):74–84. [DOI] [PubMed] [Google Scholar]
- 43. Chui HC, Teng EL, Henderson VW, Moy AC. Clinical subtypes of dementia of the Alzheimer type. Neurology. 1985;35(11):1544–1550. [DOI] [PubMed] [Google Scholar]
- 44. Kalpouzos G, Chetelat G, Baron JC, et al. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30(1):112–124. [DOI] [PubMed] [Google Scholar]
- 45. Mielke R, Kessler J, Szelies B, Herholz K, Wienhard K, Heiss WD. Normal and pathological aging—findings of positron-emission-tomography. J Neural Transm. 1998;105(8-9):821–837. [DOI] [PubMed] [Google Scholar]
- 46. Grady CL, Berg G, Carson RE, Daube-Witherspoon ME, Friedland RP, Rapoport SI. Quantitative comparison of cerebral glucose metabolic rates from two positron emission tomographs. J Nucl Med. 1989;30(8):1386–1392. [PubMed] [Google Scholar]
- 47. Rogers BS, Lippa CF. A clinical approach to early-onset inheritable dementia. Am J Alzheimers Dis Other Demen. 2012;27(3):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]