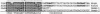Abstract
The Bacillus subtilis mrgA gene encodes an abundant DNA-binding protein that protects cells against the lethal effects of H2O2. Transcription of mrgA is induced by H2O2 or by entry into stationary phase when manganese and iron levels are low. We have selected for strains derepressed for transcription of mrgA in the presence of Mn(II). The resulting cis-acting mutants define an operator site just upstream of the mrgA promoter. Similar sequences flank the promoters for the catalase gene, katA, and the heme biosynthesis operon, hemAXCDBL. Like mrgA, transcription of the katA and hem genes is repressed by Mn(II), which thereby potentiates the killing action of H2O2. We identified two classes of trans-acting mutants derepressed for mrgA transcription in the presence of Mn(II): some exhibit a coordinate derepression of MrgA, catalase, heme biosynthesis, and alkyl hydroperoxide reductase and are H2O2 resistant, while others have reduced catalase activity and are H2O2 sensitive. These data indicate that the peroxide stress response of B. subtilis is regulated by a repressor that senses both metal ion levels and H2O2.
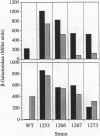
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almirón M., Link A. J., Furlong D., Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992 Dec;6(12B):2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
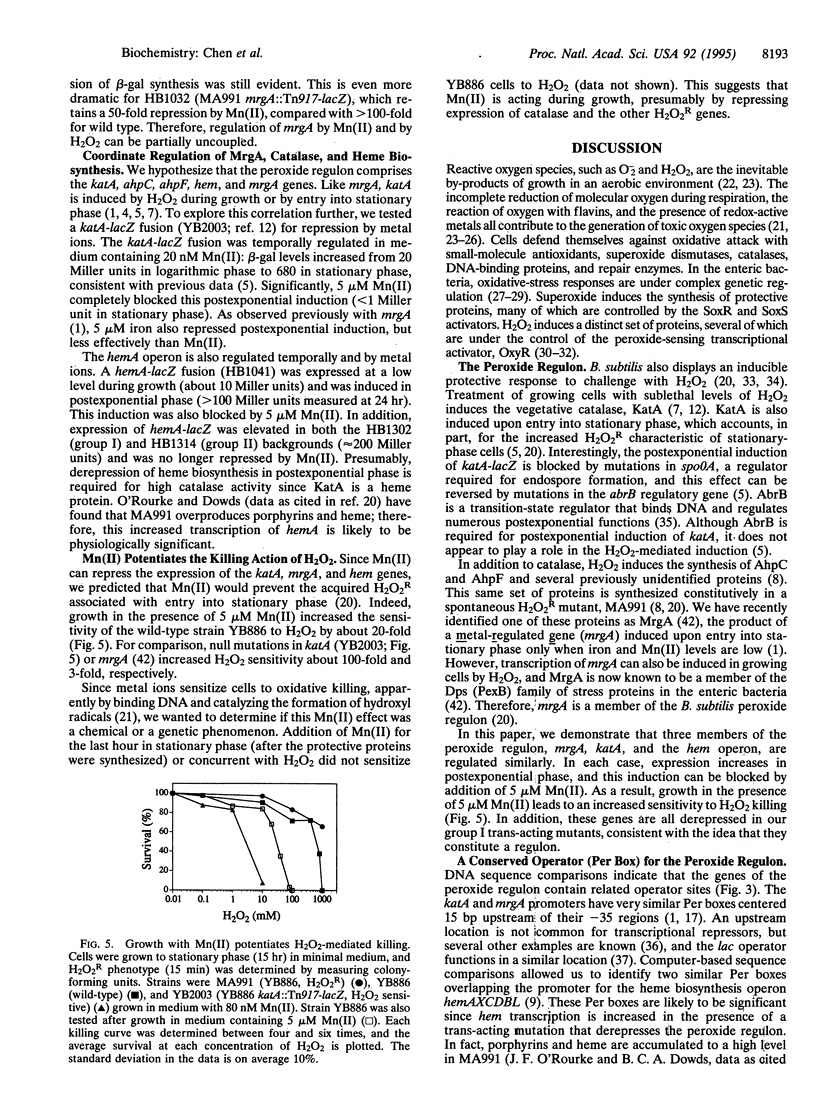
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlett B. S., Chock P. B., Yim M. B., Stadtman E. R. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jan;87(1):389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol D. K., Yasbin R. E. Analysis of the dual regulatory mechanisms controlling expression of the vegetative catalase gene of Bacillus subtilis. J Bacteriol. 1994 Nov;176(21):6744–6748. doi: 10.1128/jb.176.21.6744-6748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol D. K., Yasbin R. E. Characterization of an inducible oxidative stress system in Bacillus subtilis. J Bacteriol. 1990 Jun;172(6):3503–3506. doi: 10.1128/jb.172.6.3503-3506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol D. K., Yasbin R. E. The isolation, cloning and identification of a vegetative catalase gene from Bacillus subtilis. Gene. 1991 Dec 20;109(1):31–37. doi: 10.1016/0378-1119(91)90585-y. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Chen L., James L. P., Helmann J. D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993 Sep;175(17):5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Collado-Vides J., Magasanik B., Gralla J. D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991 Sep;55(3):371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Demple B., Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet. 1991;25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- Dowds B. C., Murphy P., McConnell D. J., Devine K. M. Relationship among oxidative stress, growth cycle, and sporulation in Bacillus subtilis. J Bacteriol. 1987 Dec;169(12):5771–5775. doi: 10.1128/jb.169.12.5771-5775.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowds B. C. The oxidative stress response in Bacillus subtilis. FEMS Microbiol Lett. 1994 Dec 15;124(3):255–263. doi: 10.1111/j.1574-6968.1994.tb07294.x. [DOI] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Brehm K., Kreft J., Goebel W. Cloning, characterization, and expression in Escherichia coli of a gene encoding Listeria seeligeri catalase, a bacterial enzyme highly homologous to mammalian catalases. J Bacteriol. 1991 Aug;173(16):5159–5167. doi: 10.1128/jb.173.16.5159-5167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartford O. M., Dowds B. C. Cloning and characterization of genes induced by hydrogen peroxide in Bacillus subtilis. J Gen Microbiol. 1992 Oct;138(10):2061–2068. doi: 10.1099/00221287-138-10-2061. [DOI] [PubMed] [Google Scholar]
- Hartford O. M., Dowds B. C. Isolation and characterization of a hydrogen peroxide resistant mutant of Bacillus subtilis. Microbiology. 1994 Feb;140(Pt 2):297–304. doi: 10.1099/13500872-140-2-297. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Genetic evidence for interaction of sigma A with two promoters in Bacillus subtilis. J Bacteriol. 1991 Jun;173(11):3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzer M., Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Switala J. Multiple catalases in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3601–3607. doi: 10.1128/jb.169.8.3601-3607.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya O. L., Kidwell J. P., Matin A. Characterization of the sigma 38-dependent expression of a core Escherichia coli starvation gene, pexB. J Bacteriol. 1994 Jul;176(13):3928–3935. doi: 10.1128/jb.176.13.3928-3935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994 Sep 9;269(36):22459–22462. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricek M., Rutberg L., Schröder I., Hederstedt L. Cloning and characterization of the hemA region of the Bacillus subtilis chromosome. J Bacteriol. 1990 May;172(5):2250–2258. doi: 10.1128/jb.172.5.2250-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder I., Johansson P., Rutberg L., Hederstedt L. The hemX gene of the Bacillus subtilis hemAXCDBL operon encodes a membrane protein, negatively affecting the steady-state cellular concentration of HemA (glutamyl-tRNA reductase). Microbiology. 1994 Apr;140(Pt 4):731–740. doi: 10.1099/00221287-140-4-731. [DOI] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Sonenshein A. L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993 Aug;175(15):4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Berlett B. S., Chock P. B. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc Natl Acad Sci U S A. 1990 Jan;87(1):384–388. doi: 10.1073/pnas.87.1.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Strauch M. A., Hoch J. A. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol. 1993 Feb;7(3):337–342. doi: 10.1111/j.1365-2958.1993.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Toledano M. B., Kullik I., Trinh F., Baird P. T., Schneider T. D., Storz G. Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell. 1994 Sep 9;78(5):897–909. doi: 10.1016/s0092-8674(94)90702-1. [DOI] [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Gibbs D. F., Mukhopadhyay P. S., Sulavik C., Johnson K. J., Weinberg J. M., Ryan U. S., Ward P. A. Hydrogen peroxide-induced cell and tissue injury: protective effects of Mn2+. Inflammation. 1991 Aug;15(4):291–301. doi: 10.1007/BF00917314. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Cheo D. L., Bayles K. W. Inducible DNA repair and differentiation in Bacillus subtilis: interactions between global regulons. Mol Microbiol. 1992 May;6(10):1263–1270. doi: 10.1111/j.1365-2958.1992.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Berlett B. S., Chock P. B., Stadtman E. R. Manganese(II)-bicarbonate-mediated catalytic activity for hydrogen peroxide dismutation and amino acid oxidation: detection of free radical intermediates. Proc Natl Acad Sci U S A. 1990 Jan;87(1):394–398. doi: 10.1073/pnas.87.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]