Abstract
Basidiomycetous macrofungi play important roles in maintaining forest ecosystems via carbon cycling and the mobilization of nitrogen and phosphorus. To understand the impact of human activity on macrofungi, an ongoing project at the Korea National Arboretum is focused on surveying the macrofungi in unexploited areas. Mt. Oseo was targeted in this survey because the number of visitors to this destination has been steadily increasing, and management and conservation plans for this destination are urgently required. Through 5 field surveys of Mt. Oseo from April to October 2012, 116 specimens of basidiomycetous macrofungi were collected and classified. The specimens were identified to the species level by analyzing their morphological characteristics and their DNA sequence data. A total of 80 species belonging to 57 genera and 25 families were identified. To the best of our knowledge, this is the first study to identify five of these species-Artomyces microsporus, Hymenopellis raphanipes, Pholiota abietis, Phylloporus brunneiceps, and Sirobasidium magnum-in Korea.
Keywords: Basidiomycetous macrofungi, DNA barcoding, Fungal flora, Internal transcribed spacer, Mt. Oseo, Unrecorded species
Macrofungi are fungal species that produce fruiting bodies visible to the naked eye [1]. Most macrofungi are members of Basidiomycota, but some belong to Ascomycota. Basidiomycetous macrofungi, such as bracket fungi, mushrooms, and puffballs, play essential roles in maintaining forest ecosystems. Saprobic macrofungi contribute to carbon cycling by decomposing woody debris on the forest floor [2], while mycorrhizal macrofungi aid the survival of other forest species by mobilizing nitrogen and phosphorus [3]. Additionally, fruiting bodies of basidiomycetous macrofungi can be important sources of food [4, 5] and medicine [6, 7, 8, 9, 10] for humans.
Macrofungi have traditionally been identified based on their fruiting body morphologies and microscopic features. Some macrofungi do not have unique characters, and therefore, it is difficult to distinguish them to the species level using morphology alone. Many of these problems can be overcome by using DNA barcoding [11]. For the kingdom Fungi, the internal transcribed spacer (ITS) region has been formally proposed as the primary fungal barcoding gene, because its high sequence variation resolves closely related species across the fungal tree of life [12]. Coupling morphological data with ITS sequences increases the accuracy of species identification and provides more detailed information on fungal flora. For example, several Amanita species were confirmed as new records in Korea based on their morphological characteristics and ITS and large subunit rDNA sequence data [13, 14]. In addition, 2 similar Korean species of the genus Russula were delimited based on their morphology and ITS sequences [15].
In 2012, the Korea National Arboretum initiated a survey of macrofungi in the unexploited areas of Korea. One of the areas targeted in the survey was Mt. Oseo, located in Chungcheongnam-do, South Korea. In recent years, the number of visitors to Mt. Oseo has steadily increased, and the fear that human activity is damaging the ecosystem is increasing. Management and conservation measures, which rely on baseline data from biodiversity surveys, are needed. Although many flora and fauna surveys have recently been conducted at Mt. Oseo [16, 17, 18, 19, 20, 21], studies on fungal flora have been rare; the only fungal data available are from a single report based on a survey in August 2010 [22].
The goal of our study was to investigate and catalog the basidiomycetous macrofungi of Mt. Oseo. In addition to building a checklist of the basidiomycetous macrofungi of Mt. Oseo, we discovered 5 species previously not recorded in Korea.
MATERIALS AND METHODS
Study area
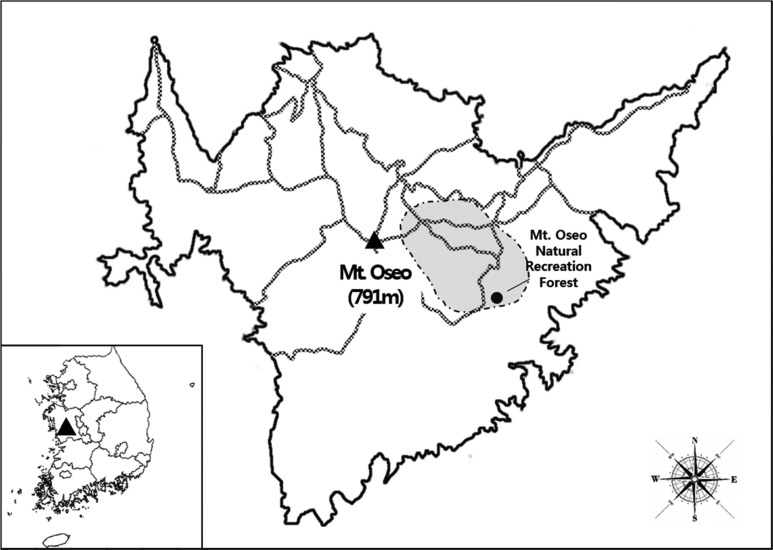
Mt. Oseo (36°27' N, 126°40' W), the highest peak of the Geumbuk Mountains, is located in Cheongnamyeon, Boryeong-si, Chungcheongnam-do, South Korea. The mountain range runs north to south for 12 km and east to west for 18 km and has an average altitude of 790.7 m [23]. The surveys were conducted from April to October 2012 in a region of Mt. Oseo that includes the Natural Recreation Forest (shaded region in Fig. 1). In 2012, the prevailing climate of the area was moderate, with an annual mean temperature of 12.2℃ and temperatures ranging from -1.4℃ (mean minimum temperature in December) to 26.8℃ (mean maximum temperature in August). Precipitation during the spring, summer, and fall of 2012 was 160.3 mm, 681.3 mm, and 272.7mm, respectively. All the meteorological data were collected by the Korea Meteorological Administration at the Boryeong meteorological observatory: http://www.kma.go.kr/weather/climate/data_monthly.jsp (accessed March 2014).
Fig. 1.
Geographical location and sampling regions of Mt. Oseo (Chungcheongnam-do, South Korea). The area surveyed in this study is denoted by the shaded region, which includes the Mt. Oseo Natural Recreation Forest.
Fungal survey and processing
Five surveys were conducted throughout 2012 (April, June, July, August, and October). The surveys spanned spring, summer, and fall-a period during which the macrofungi produce fruiting bodies. In the field, color photographs were obtained and information about the collecting site, habitat, host, substrate, and fruiting body was recorded for each specimen. Samples were placed in specially prepared paper boxes and brought to the laboratory for identification. After being dried in air-vented ovens at 55℃ for 3~4 days, the specimens were deposited in the Seoul National University Fungus Collection (SFC).
The specimens were initially identified on the basis of their macro- and microscopic features according to published descriptions [24, 25, 26, 27, 28, 29, 30]. Taxonomic classification of species was as per the Fungal Tree of Life (http://aftol.org), and the nomenclature was based on Index Fungorum (http://www.indexfungorum.org/Names/Names.asp).
For the new records in Korea, microscopic structures of the specimens suspended in 3% (w/v) KOH, 1% (w/v) phloxine, and Melzer's reagent (IKI) [31] and mounted on slides were measured using a Nikon SMZ1500 dissecting microscope and a Nikon Eclipse 80i optical microscope (Nikon, Tokyo, Japan). Quotient (Q) was the ratio of the difference in the mean basidiospore length to the mean spore width of the specimens studied. The 'Methuen Handbook of Colour' [32] was used as the color standard.
DNA sequence-based identification
Genomic DNA was extracted using a modified CTAB extraction protocol [33]. The ITS region was amplified using the primer set ITS1F/ITS4B [34]. PCR was performed in a C1000 thermal cycler (Bio-Rad, Hercules, CA, USA) using the AccuPower PCR PreMix (Bioneer, Daejeon, Korea) in a final volume of 20 µL containing 10 pmol of each primer and 1 µL of DNA (10 ng/µL) by the method of Park et al. [15]. The PCR products were electrophoresed on 1% agarose gel with LoadingSTAR (Dyne Bio, Seoul, Korea) and purified using the Expin PCR SV purification kit (GeneAll Biotechnology, Seoul, Korea), according to the manufacturer's instructions. Sequencing was performed in the reverse direction using the ITS4B primer. DNA sequencing was performed at the DNA Synthesis and Sequencing Facility, Macrogen (Seoul, Korea), by using an ABI3700 automated DNA sequencer. All the sequences have been deposited in GenBank (accession Nos. KJ609155~KJ609174).
For molecular identification, each sequence was compared with reference sequences in GenBank by using BLAST [35]. Sequences were edited and aligned using MEGA5 [36]. A neighbor-joining phylogenetic analysis was also performed in MEGA5 using the Kimura 2-parameter model of evolution for tree inference and 500 bootstrap replicates.
RESULTS AND DISCUSSION
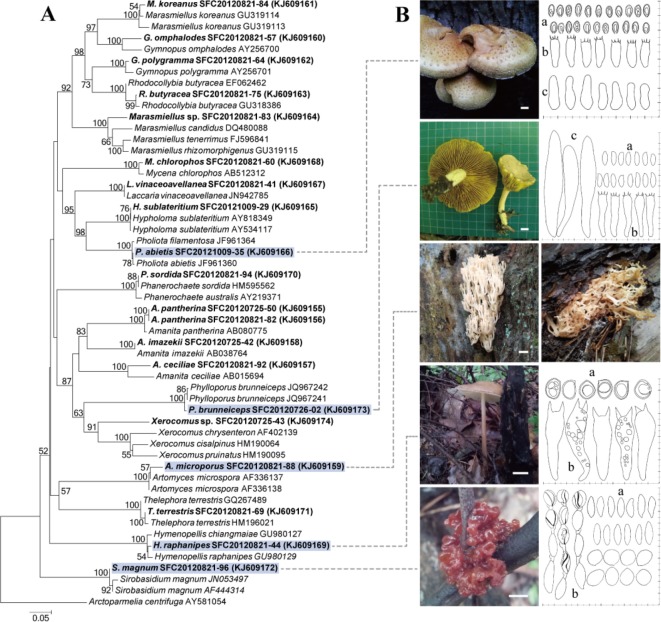
A total of 116 specimens of basidiomycetous macrofungi were collected during the surveys. Based on the macromorphological features alone, we were able to confidently identify 60 species. For the remaining specimens, DNA sequence analysis was performed because the morphological identification was inconclusive. For these unidentified specimens, a preliminary identification was performed using the ITS sequence analysis, and the identification was verified by comparing the observed morphological details with the published data. An additional 20 species were identified using the molecular data (Fig. 2). The morphological features and ITS sequences from 2 specimens did not match with the taxonomic information or sequences available in GenBank, and therefore, identification was limited to the genus level: Xerocomus sp. (SFC20120725-43) and Marasmiellus sp. (SFC20120821-83). Additionally, 5 species previously not recorded in Korea were identified from Mt. Oseo: Artomyces microsporus, Hymenopellis raphanipes, Pholiota abietis, Phylloporus brunneiceps, and Sirobasidium magnum (Fig. 2). Microscopic features of A. microsporus could not be observed because the specimen was immature.
Fig. 2.
Phylogenetic tree and morphologies of 5 new records in Korea. A, Phylogenetic tree inferred from internal transcribed spacer sequences. The topological structure is from neighbor-joining analysis. Bootstrap values exceeding 50% for nodes are indicated. Samples from our study are in bold font and the 5 new records in Korea are highlighted; B, Photographs of basidiocarps, and drawings of microscopic features (a, spores; b, basidia; c, cystidia) for the 5 new records in Korea. The scale bar is 1 cm in the basidiocarp images and 10 µm in the microscopic images.
In total, 80 species belonging to 57 genera and 25 families were identified from Mt. Oseo (Table 1). The most common genera were Russula (9 species), Amanita (5), and Lactarius (5). The families that contained the most number of species were Russulaceae (14 species), Coriolaceae (9), Tricholomataceae (8), Polyporaceae (6), and Amanitaceae (5), which together accounted for about 52% of the total fungal diversity in the collected specimens. The distribution of species by trophic group indicated a dominance of the saprotrophic species (68%), while parasitic species and symbionts were found in lower numbers (32%).
Table 1.
Checklist of basidiomycetous macrofungi from Mt. Oseo
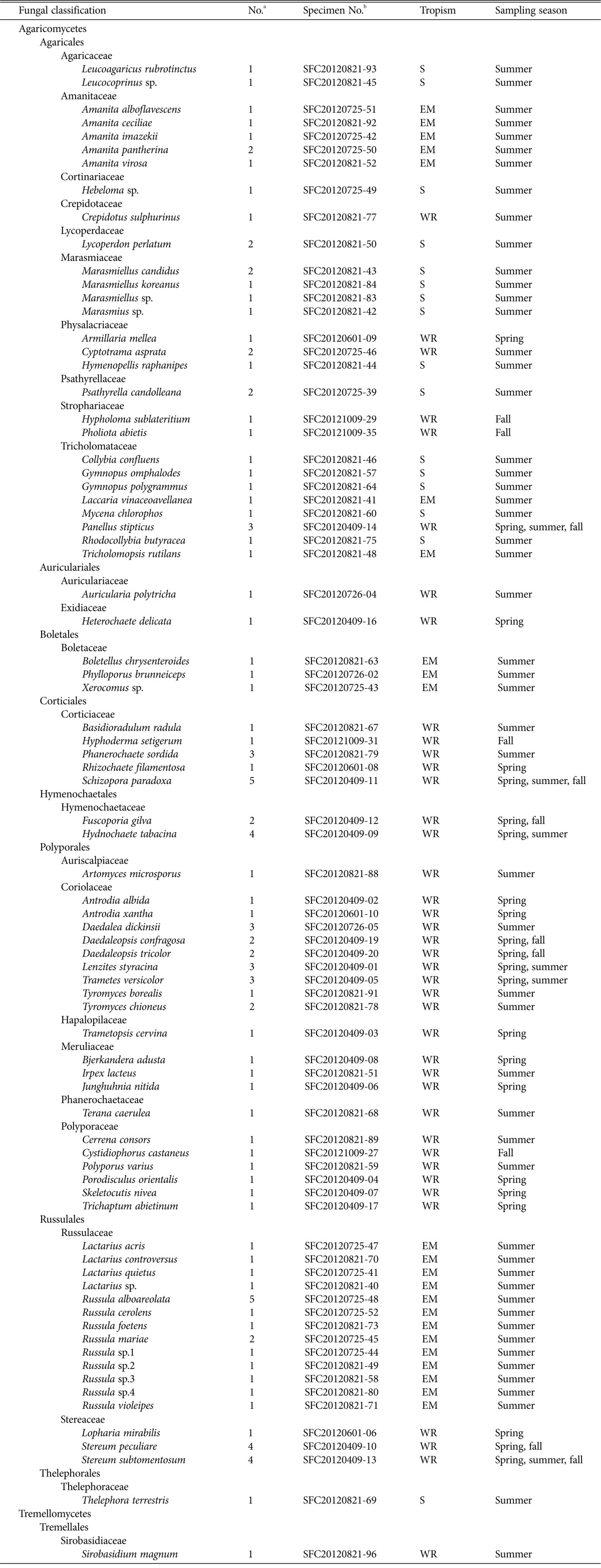
S, saprophyte; EM, ectomycorrhiza; WR, wood rotter.
aNo. of specimens collected.
bHerbarium number for representative specimen.
Species diversity and the type of tropism varied with the changing seasons. During the spring season, mostly wood-rotting fungi were observed. The distribution of the species in the genera was as follows: Antrodia (2 species), Daedaleopsis (2), and Stereum (2). During summer, we identified 59 species belonging to 40 genera and 21 families, which were mostly gilled fungi. The distribution of the species in the genera was as follows: Russula (9 species), Amanita (5), Lactarius (4), and Marasmiellus (3). Specimens collected during summer accounted for 75% of the total diversity and 66% of the total number of specimens. During fall, 11 species belonging to 9 genera and 7 families were identified, most of which were wood-rotting fungi. The most common genera were Daedaleopsis (2 species) and Stereum (2).
In a fungal survey of Mt. Oseo in 2010, 98 species belonging to 62 genera and 32 families were reported [22]. Although the 2010 study was limited to the summer season, it focused on different regions of Mt. Oseo than we did in our study. Comparing the results of our study with the results of the 2010 survey, we found that the majority of fungal diversity was unique to each survey, and only 8 species were determined in both the surveys. This shows that even with fungi that can be seen with the naked eye, new and not recorded macrofungal species may be waiting for discovery at Mt. Oseo. Precipitation patterns in 2012 were unique: spring and summer were drier and fall was wetter than usual. Rainfall during late summer and early fall was relatively high owing to 3 typhoons (Sanba, Bolaven, and Tembin). The lower summertime fungal diversity observed in 2012 compared with that in 2010 may be a result of these abnormal precipitation patterns.
Herein, we provide a checklist of the basidiomycetous macrofungi found in surveys of Mt. Oseo in 2012 as well as detailed descriptions of 5 new species recorded in Korea. In addition to exemplifying the fungal diversity of Mt. Oseo, our checklist provides baseline data for future comparisons [37]. Regular surveys of the fungal diversity in the area can provide valuable information on the effects of increased human activity and may be an adequate indicator of regional climate change, including global warming [38, 39]. These fungal species play essential roles in maintaining forest ecosystems and biodiversity [40, 41], and understanding these changes will aid in developing better strategies for management and conservation of these ecosystems [41, 42].
Artomyces microsporus (Qiu X. Wu & R. H. Petersen) Lickey, in Lickey, Hughes & Petersen, Sydowia 55: 227 (2003) [43]
Basidiocarp coral-like on crack in pine bark, beige to light ochraceous buff when fresh, 90mm in length, repeatedly branched, 2~4 branches per node, branches with crown-like shape and 2 mm diameter. Specimen examined, SFC20120821-88. Habitat on the bark of Pinus densiflora.
Hymenopellis raphanipes (Berk.) R. H. Petersen, in Petersen & Hughes, Beih. Nova Hedwigia 137: 213 (2010) [44]
Cap convex or plane, 50-mm wide, surface viscid, light brown. Gills adnexed, distant, white to cream when fresh and brown when dry. Stipe slightly cylindrical with swollen base, 65 × 5 mm, white to cream. Basidia clavate, 60.7~71.8 × 14.6~17.3 µm, with 2 sterigmata. Basidiospore subglobose to ellipsoid, 15.4~17.7 × 11.4~13.7 µm, Q = 1.2~1.4. Specimen examined, SFC20120821-44.
Pholiota abietis A. H. Sm. & Hesler, The North American species of Pholiota: 176 (1968) [45]
Cap broad, convex or plane, warts on surface, 40~98mm in diameter, brown in center and yellow around the edge, margin inrolled. Gills close and brown, connected to stipe. Stipe more densely scaled at base, 50 mm long, 20 mm thick, white to brown. Basidia clavate, 18.8~21.7 × 6.1~6.8 µm. Basidiospore 7.4~9.1 × 4.0~4.8 µm, Q = 1.6~1.9. Cheilocystidia capitulate or obclavate, 17~23.2 × 4.5~7.3 µm. Specimen examined, SFC20121009-35. Habitat on oak tree.
Phylloporus brunneiceps N. K. Zeng, Zhu L. Yang & L. P. Tang, Fungal Divers. 58: 82 (2013) [46]
Cap convex, slightly depressed, 50~90 mm in diameter, 30~40 mm thick, olive yellow, margin slightly curved inward. Gills yellow, attached to stem. Stipe 50~70 mm long, 7~10mm thick, surface yellow to brown. Basidia clavate, 34.7~41.5 × 7.8~9.1 µm. Basidiospore cylindrical, 11.4~13.1 × 3.8~4.5 µm, Q = 2.3~2.9. Pleurocystidia abundant, fusiform, 78.5~97.6 × 10.4~16.7 µm. Specimen examined, SFC20120726-02.
Sirobasidium magnum Boedijn, Bull. Jard. Bot. Buitenzorg, 3 Sér. 13: 266 (1934) [47]
Basidiocarps foliose, gelatinous when fresh, 1~4 cm in diameter, reddish brown. Basidia subglobose to oval, 2~6 basidia in chains, 11.4~16.5 × 10.8~11.8 µm, Q = 1.1~1.6, very short sterigmata. Basidiospore fusiform, 15.9~19.4 × 5.1~5.7 µm, Q = 2.5~3.4. Specimen examined, SFC20120821-96.
ACKNOWLEDGEMENTS
This work was supported by the Korea National Arboretum (Project No. KNA 1-1-10, 12-3).
References
- 1.Kirk PM, Cannon PF, Minter DW, Stalpers JA. Ainsworth and Bisby's dictionary of the fungi. Wallingford: CABI; 2008. [Google Scholar]
- 2.Thormann MN. Diversity and function of fungi in peatlands: a carbon cycling perspective. Can J Soil Sci. 2006;86:281–293. [Google Scholar]
- 3.Read DJ, Perez-Moreno J. Mycorrhizas and nutrient cycling in ecosystems: a journey towards relevance? New Phytol. 2003;157:475–492. doi: 10.1046/j.1469-8137.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang ST. The world mushroom industry: trends and technological development. Int J Med Mushrooms. 2006;8:297–314. [Google Scholar]
- 5.Chang ST, Miles PG. Recent trends in world production of cultivated edible mushrooms. Mushroom J. 1991;504:15–18. [Google Scholar]
- 6.Ooi VE, Liu F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr Med Chem. 2000;7:715–729. doi: 10.2174/0929867003374705. [DOI] [PubMed] [Google Scholar]
- 7.Daba A, Ezeronye OU. Anti-cancer effect of polysaccharides isolated from higher basidiomycetes mushrooms. Afr J Biotechnol. 2003;2:672–678. [Google Scholar]
- 8.Sullivan R, Smith JE, Rowan NJ. Medicinal mushrooms and cancer therapy: translating a traditional practice into Western medicine. Perspect Biol Med. 2006;49:159–170. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- 9.Lindequist U, Niedermeyer TH, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang ST, Buswell JA. Mushroom nutriceuticals. World J Microbiol Biotechnol. 1996;12:473–476. doi: 10.1007/BF00419460. [DOI] [PubMed] [Google Scholar]
- 11.Hebert PD, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci U S A. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CS, Jo JW, Kwag YN, Oh J, Shrestha B, Sung GH, Han SK. Four newly recorded Amanita species in Korea: Amanita sect. Amanita and sect. Vaginatae. Mycobiology. 2013;41:131–138. doi: 10.5941/MYCO.2013.41.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CS, Jo JW, Kwag YN, Kim JH, Shrestha B, Sung GH, Han SK. Taxonomic study of Amanita subgenus Lepidella and three unrecorded Amanita species in Korea. Mycobiology. 2013;41:183–190. doi: 10.5941/MYCO.2013.41.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park MS, Fong JJ, Lee H, Oh SY, Jung PE, Min YJ, Seok SJ, Lim YW. Delimitation of Russula subgenus Amoenula in Korea using three molecular markers. Mycobiology. 2013;41:191–201. doi: 10.5941/MYCO.2013.41.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang CG, Moon AR, Lee WB. Flora of Mt. Oseo. Korean J Nat Conserv. 2011;9:1–16. [Google Scholar]
- 17.Kim BW, Oh YJ, Kim CB. The vegetational structure of Mt. Oseo recreational forest in Chungcheongnam-do. Korean J Nat Conserv. 2011;9:99–109. [Google Scholar]
- 18.Kim H, Ko S, Shin H, Park H, Gang H, Han S, Yoon C. Analysis on the vegetation structure of Geumjabong to Byeongpug ridge in Mt. Oseosan. Proc Joint For Sci. 2012:669–672. [Google Scholar]
- 19.Oh HK, Kim DP, Oh KK, Kang KR, Bae JN. Management methods and vascular plants of the Ohseosan and the Bongsusan, Chungnam. J Korean Soc Environ Restor Technol. 2013;16:63–81. [Google Scholar]
- 20.Hong EJ, Jeon YL, Yoon JC, Kim JY, Lee MH, Kim JW, Park SJ, Kim KG, Kim JH, Kim BJ. Insect diversity of Mt. Oseosan. J Korean Nat. 2012;5:251–266. [Google Scholar]
- 21.Kim JI, Kim AY. A faunistic study on the insects from Mt. Oseo, Korea. Korean J Nat Conserv. 2011;9:19–26. [Google Scholar]
- 22.Cho DH, Bang KS. Genetic resources of fungi in Mt. Oseo areas. Korean J Nat Conserv. 2011;9:77–97. [Google Scholar]
- 23.Kim JH. Geomorphological features of Mt. Oseo, and its geology. Korean J Nat Conserv. 2011;9:67–75. [Google Scholar]
- 24.Lim YW, Lee JS, Jung HS. Fungal flora of Korea. Vol. 1. No. 1. Wood rotting fungi. Incheon: National Institute of Biological Resources; 2010. [Google Scholar]
- 25.Gilbertson RL, Ryvarden L. North American polypores. Vol. 1. Abortiporus-Lindtneria. North American Polypores. Oslo: Fungiflora A/S; 1986. [Google Scholar]
- 26.Park WH, Lee JH. New wild fungi of Korea. Seoul: Kyo-Hak Publishing Co.; 2011. [Google Scholar]
- 27.Hongo T, Izawa M. Yama-Kei field book. No. 10. Fungi. Tokyo: Yama-Kei Publishers; 1994. [Google Scholar]
- 28.Imazeki R, Otani Y, Hongo T, Izawa M, Mizuno N. Fungi of Japan. Yama-Kei Publishers: 1988. [Google Scholar]
- 29.Breitenbach J, Kränzlin F. The fungi of Switzerland. Vols. 1-5. Lucerne: Verlag Mykologia; 1984-2000. [Google Scholar]
- 30.Kränzlin F. Fungi of Switzerland. Vol. 6. Russulaceae: Lactarius, Russula. Lucerne: Verlag Mykologia; 2005. [Google Scholar]
- 31.Largent DL, Thiers HD. How to identify mushrooms to genus II: field identification of genera. Eureka: Mad River Press; 1977. [Google Scholar]
- 32.Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. London: Eyre Methuen; 1978. [Google Scholar]
- 33.Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoort RA, editors. Plant molecular biology manual D1. Boston: Kluwer Academic; 1994. pp. 1–8. [Google Scholar]
- 34.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 35.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raxworthy CJ, Pearson RG, Rabibisoa N, Rakotondrazafy AM, Ramanamanjato JB, Raselimanana AP, Wu S, Nussbaum RA, Stone DA. Extinction vulnerability of tropical montane endemism from warming and upslope displacement: a preliminary appraisal for the highest massif in Madagascar. Glob Chang Biol. 2008;14:1703–1720. [Google Scholar]
- 38.Vitousek PM. Beyond global warming: ecology and global change. Ecology. 1994;75:1861–1876. [Google Scholar]
- 39.Frankland JC, Magan N, Gadd GM. Fungi and environmental change; Symposium of the British Mycological Society; 1994 Mar; Cranfield University, Cambridge. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- 40.Hawksworth DL. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res. 1991;95:641–655. [Google Scholar]
- 41.Molina R, Pilz D, Smith J, Dunham S, Dreisbach T, O'Dell T, Castellano M. Conservation and management of forest fungi in the Pacic Northwestern United States: an integrated ecosystem approach. In: Moore D, Nauta NN, Evans SE, Rotheroe M, editors. Fungal conservation: issues and solutions. Cambridge: Cambridge University; 2008. pp. 19–63. [Google Scholar]
- 42.Richard F, Moreau PA, Selosse MA, Gardes M. Diversity and fruiting patterns of ectomycorrhizal and saprobic fungi in an old-growth Mediterranean forest dominated by Quercus ilex L. Can J Bot. 2004;82:1711–1729. [Google Scholar]
- 43.Lickey EB, Hughes KW, Petersen RH. Phylogenetic and taxonomic studies in Artomyces and Clavicorona (Homobasidiomycetes: Auriscalpiaceae) Sydowia. 2003;55:181–254. [Google Scholar]
- 44.Petersen RH, Hughes KW. The Xerula/Oudemansiella complex (Agaricales) Beih Nova Hedwigia. 2010;137:1–625. [Google Scholar]
- 45.Smith AH, Hesler LR. The North American species of Pholiota. New York: Hafner Publishing Co.; 1968. [Google Scholar]
- 46.Zeng NK, Tang LP, Li YC, Tolgor B, Zhu XT, Zhao Q, Yang ZL. The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Divers. 2013;58:73–101. [Google Scholar]
- 47.Boedijn KB. The genus Podostroma in the Netherlands indies. Bull Jard Bot Buitenzorg. 1934;13:269–275. [Google Scholar]