Abstract
A model synthetic peptide substrate of the cyclic AMP-dependent protein kinase (ATP:protein phosphotransferase; EC 2.7.1.37), Leu-Arg-Arg-Ala-Ser-Leu-Gly, closely resembling the local phosphorylation site sequence in porcine hepatic pyruvate kinase, was shown to be phosphorylated in vivo after microinjection into Xenopus oocytes. This result demonstrates that the microinjection technique, utilizing a synthetic peptide substrate, or possibly a synthetic substrate analog inhibitor [Kemp, B. E., Benjamini, E. & Krebs, E. G. (1976) Proc. Natl. Acad. Sci. USA 73, 1038--1042], can be used to study protein phosphorylation-dephosphorylation reactions in living oocytes. This follows, since it is clear that the injected peptide was accessible to the cellular compartment containing the protein kinase.
Full text
PDF
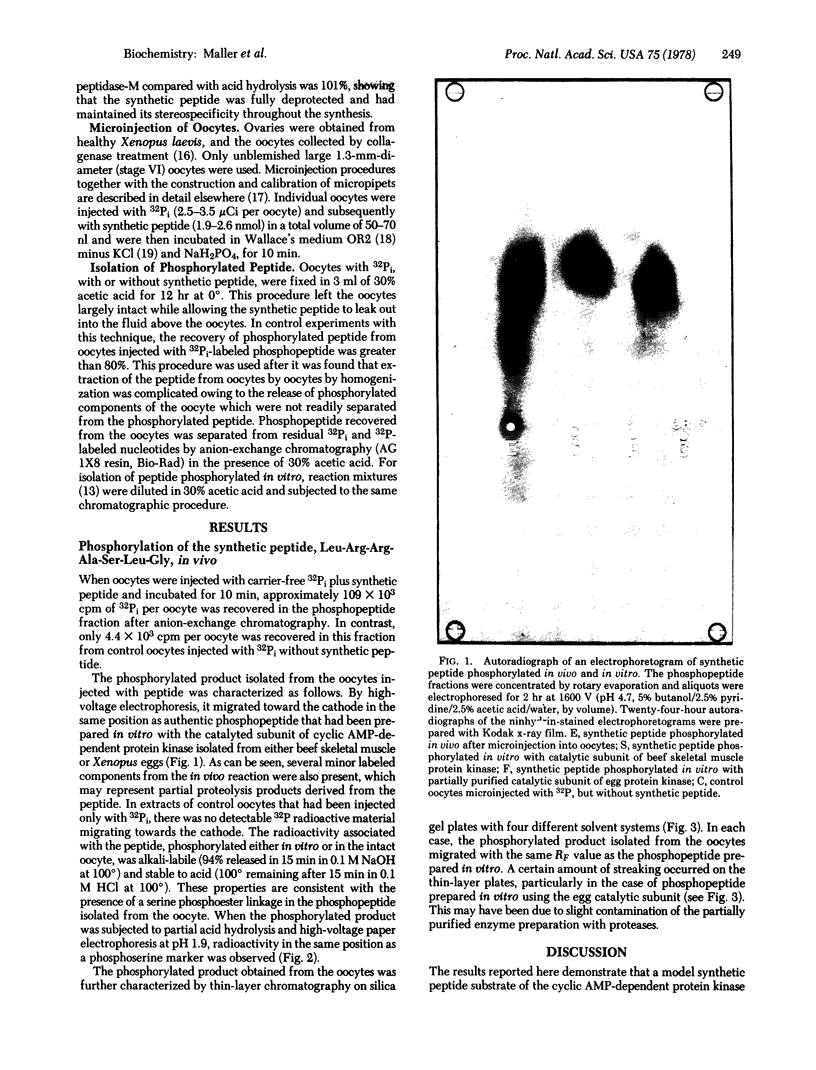
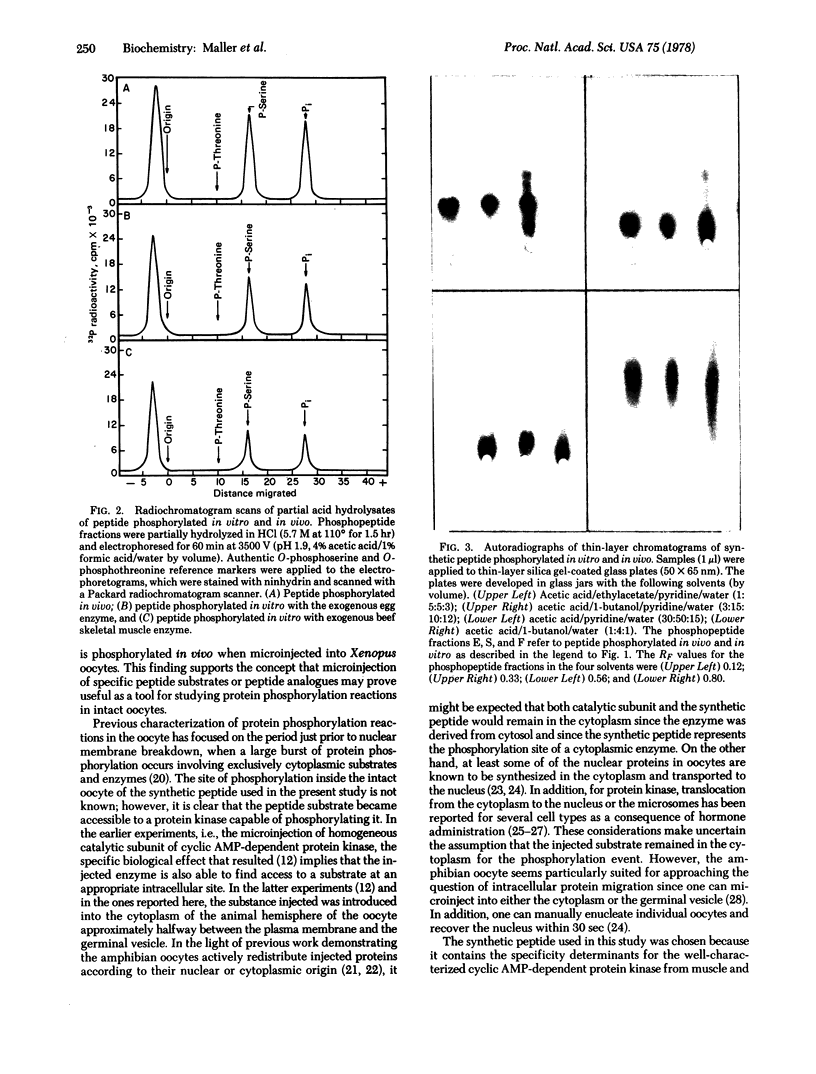

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Palmer W. K., Walsh D. A. Nuclear protein-kinase activity in perfused rat liver stimulated with dibutyryl-adenosine cyclic 3':5'-monophosphate. Eur J Biochem. 1975 Jun 16;55(1):193–199. doi: 10.1111/j.1432-1033.1975.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R. Peptides from myelin basic protein as substrates for adenosine 3', 5'-cyclic monophosphate-dependent protein kinases. Biochem Biophys Res Commun. 1974 Dec 11;61(3):852–858. doi: 10.1016/0006-291x(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M. The uptake of endogenous proteins by oocyte nuclei. Exp Cell Res. 1975 Jul;93(2):411–419. doi: 10.1016/0014-4827(75)90467-x. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier P., Moreau M., Dorée M. Inhibition de la réinitiation de la méiose des ovocytes de Xenopus laevis par trois antiprotéases naturelles, l'antipïne, la chymostatine et la leupeptine. C R Acad Sci Hebd Seances Acad Sci D. 1977 Jan 24;284(4):317–319. [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Humble E., Berglund L., Titanji V., Ljungström O., Edlund B., Zetterqvist O., Engström L. Non-dependence on native structure of pig liver pyruvate kinase when used as a substrate for cyclic 3',5'-AMP-stimulated protein kinase. Biochem Biophys Res Commun. 1975 Sep 16;66(2):614–621. doi: 10.1016/0006-291x(75)90554-9. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Hiestand P. C., Schweppe J. S. Mechanism of action of gonadotropin. IV. Cyclic adenosine monophosphate-dependent translocation of ovarian cytoplasmic cyclic adenosine monophosphate-binding protein and protein kinase to nuclear acceptor sites. Endocrinology. 1974 Jan;94(1):168–183. doi: 10.1210/endo-94-1-168. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenman S. G., Bhalla R. C., Sanborn B. M., Stevens R. H. Protein kinase translocation as an early event in the hormonal control of uterine contraction. Science. 1974 Feb 1;183(4123):430–432. doi: 10.1126/science.183.4123.430. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. An adenosine 3',5'-monophosphate-dependent protein kinase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3417–3419. [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. Adenosine 3':5'-cyclic monophosphate- and guanosine 3':5'-cyclic monophosphate-dependent protein kinases: possible homologous proteins. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3239–3243. doi: 10.1073/pnas.74.8.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Maller J., Wu M., Gerhart J. C. Changes in protein phosphorylation accompanying maturation of Xenopus laevis oocytes. Dev Biol. 1977 Jul 15;58(2):295–312. doi: 10.1016/0012-1606(77)90093-8. [DOI] [PubMed] [Google Scholar]
- Masui Y. Relative roles of the pituitary, follicle cells, and progesterone in the induction of oocyte maturation in Rana pipiens. J Exp Zool. 1967 Dec;166(3):365–375. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- Merriam R. W. Movement of cytoplasmic proteins into nuclei induced to enlarge and initiate DNA or RNA synthesis. J Cell Sci. 1969 Sep;5(2):333–349. doi: 10.1242/jcs.5.2.333. [DOI] [PubMed] [Google Scholar]
- POSNER J. B., STERN R., KREBS E. G. EFFECTS OF ELECTRICAL STIMULATION AND EPINEPHRINE ON MUSCLE PHOSPHORYLASE, PHOSPHORYLASE B KINASE, AND ADENOSINE 3',5'-PHOSPHATE. J Biol Chem. 1965 Mar;240:982–985. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Vitto A., Jr, wallace R. A. Maturation of Xenopus oocytes. I. Facilitation by ouabain. Exp Cell Res. 1976 Jan;97:56–62. doi: 10.1016/0014-4827(76)90654-6. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]