Abstract
During a search for neuraminidase inhibitors derived from medicinal fungi, we found that the fermentation broth of Phellinus linteus exhibited potent neuraminidase inhibitory activity. Through bioassay-guided fractionation, two active compounds were purified from the ethyl acetate-soluble portion of the fermentation broth of P. linteus. These structures were identified as inotilone (1) and 4-(3,4-dihydroxyphenyl)-3-buten-2-one (2) by spectroscopic methods. Compounds 1 and 2 inhibited H1N1 neuraminidase activity with IC50 values of 29.1 and 125.6 µM, respectively, in a dose-dependent manner. They also exhibited an antiviral effect in a viral cytopathic effect reduction assay using MDCK cells. These results suggest that compounds 1 and 2 from the culture broth of P. linteus would be good candidates for the prevention and therapeutic strategies towards viral infections.
Keywords: 4-(3,4-Dihydroxyphenyl)-3-buten-2-one; Anti-influenza agent; Inotilone; Neuraminidase inhibitor; Phellinus linteus
Influenza viruses are enveloped RNA viruses that belong to the family Orthomyxoviridae, and cause significant morbidity and mortality in humans through epidemics or pandemics [1]. Influenza viruses are classified into various serotypes on the basis of two surface glycoproteins: hemagglutinin and neuraminidase. Neuraminidase (EC 3.2.1.18) plays an important role in viral proliferation and is therefore a drug target for prevention of the spread of influenza [2]. Currently, the preferred treatment for influenza virus infection is the use of neuraminidase inhibitors such as oseltamivir (Tamiflu) and zanamivir (Relenza) [3]. However, toxicity due to long-term exposure to these drugs and the appearance of viral strains that are resistant to these antiviral drugs highlight the urgent need for next-generation neuraminidase inhibitors [4].
Phellinus linteus is a species of mushroom belonging to the Hymenochaetaceae family, which is indigenous mainly to tropical regions of America, Africa and East Asia [5]. It is one of many medicinal mushrooms that have been widely used in East Asia, especially in Korea, China, and Japan, as health booster and ancient herbal medicine [6]. P. linteus is known as Sangwhang in Korea [7] and produces abundant bioactive compounds such as protocatechuic acid, caffeic acid, hispidin, davallialactone, hypholomine B, interfungins A, and inoscavin A [8, 9, 10, 11]. The extract and compounds of P. linteus exhibit various biological activities including anti-cancer, anti-oxidative, anti-angiogenic, anti-inflammatory and anti-viral effects [6, 12, 13, 14, 15, 16, 17]. During the search for neuraminidase inhibitors from medicinal fungi, two neuraminidase inhibitors were isolated from the fermentation broth of P. linteus (Fig. 1). This paper describes the isolation, structure determination, and neuraminidase inhibitory activity of these compounds.
Fig. 1.
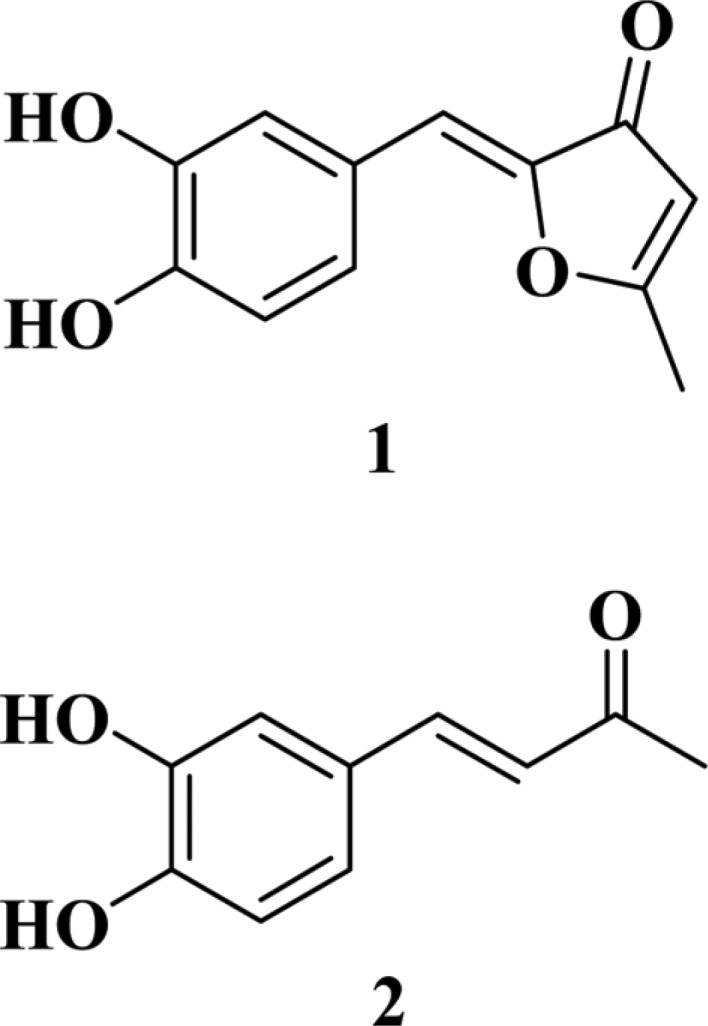
Structures of compounds 1 (inotilone) and 2 (4-(3,4-dihydroxyphenyl)-3-buten-2-one).
P. linteus was obtained from the Korea National College of Agriculture and Fisheries, Korea. The strain was fermented on potato dextrose broth (26 L) at 27℃ for 30 days. The fermentation broth was partitioned with ethyl acetate by vigorous shaking, and the ethyl acetate-soluble portion exhibited potent neuraminidase inhibitory activity at the concentration of 50 µg/mL. Following the concentration of the ethyl acetate-soluble portion under reduced pressure, the concentrate was subjected to a Sephadex LH-20 (Pharmacia, Uppsala, Sweden) column and eluted with methanol resulting in two active fractions. A Sephadex LH-20 column with 70% aqueous methanol was used for chromatography of one fraction, followed by purification with preparative reversed-phase high-performance liquid chromatography (HPLC) with 60% aqueous methanol/0.04% trifluoroacetic acid, which resulted in compound 1 (6.8 mg). The other fraction was purified by Sephadex LH-20 column chromatography eluted with 70% aqueous methanol, followed by preparative reversed-phase HPLC using the same solvent used for compound 1, to afford compound 2 (6.3 mg).
The structure of compound 1 was determined by the mass as well as the 1H and 13C nuclear magnetic resonance (NMR) measurements. The molecular weight of compound 1 was established by the electrospray ionization (ESI)-mass measurement, which provided a quasi-molecular ion peak at m/z 219.0 [M + H]+, suggesting a molecular weight of 218. The 1H NMR spectrum of compound 1 in CD3OD exhibited signals due to δ 7.34 (1H, d, J = 2.0 Hz, ArH), 7.16 (1H, dd, J = 8.4, 2.0 Hz, ArH), 6.80 (1H, d, J = 8.4Hz, ArH), 6.49 (1H, s, CH), 5.80 (1H, s, CH), and 2.55 (3H, s, CH3). In the 13C NMR spectrum, twelve carbons were evident including a carbonyl carbon at δ 187.0, four oxygenated sp2 carbons at δ 180.9, 148.4, 145.7, and 144.6, five sp2 methine carbons at δ 123.1, 118.2, 116.2, 112.3, 105.7, one sp2 quaternary carbon at δ 125.0, and one methyl carbon at δ 15.9. Consequently, compound 1 was identified as inotilone by comparing measured 1H and 13C NMR spectra with those reported in the literature [18].
The structure of compound 2 was determined by mass and 1H NMR measurements. The molecular weight of compound 2 was established by the ESI-mass, which provided a quasi-molecular ion peak at m/z 177.0 [M-H]-, suggesting a molecular weight of 178. The 1H NMR spectrum of compound 2 in CD3OD exhibited signals due to δ 7.51 (1H, d, J = 16.4 Hz), 7.07 (1H, d, J = 2.4 Hz), 6.98 (1H, dd, J = 2.4, 8.4 Hz), 6.78 (1H, d, J = 8.4 Hz), 6.54 (1H, d, J = 16.4 Hz), and 2.32 (3H, s, CH3). These spectroscopic data were well matched with those of 4-(3,4-dihydroxyphenyl)-3-buten-2-one.
We then investigated the inhibitory effects of compounds 1 and 2 against neuraminidase from recombinant influenza A virus H1N1 (rvH1N1). A previously reported method was used for the neuraminidase inhibition assay, with minor modifications [19]. In brief, 2-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid sodium salt (MUNANA, Cat. No M8639; Sigma, St. Louis, MO, USA), at the final concentration of 0.2 mM, was mixed with 90 µL of 50 mM Tris buffer (pH 7.5) at room temperature. Ten microliters of sample solution and 50 µL of rvH1N1 (50 ng/mL) were added to a well in a plate. The mixture was recorded at excitation and emission wavelengths of 365 nm and 445 nm, respectively, with a POLAR OPTIMA (BMG LABTECH, Ortenberg, Germany). Zanamivir (Relenza) and quercetin, which were used as positive controls, inhibited neuraminidase with IC50 values of 0.0015 and 37.2 µM, respectively, in this assay system. As a result, compounds 1 and 2 exhibited neuraminidase inhibitory activity with IC50 values of 29.1 and 125.6 µM, respectively, in a concentration-dependent manner (Table 1).
Table 1.
H1N1 neuraminidase inhibitory activity of compounds 1 and 2
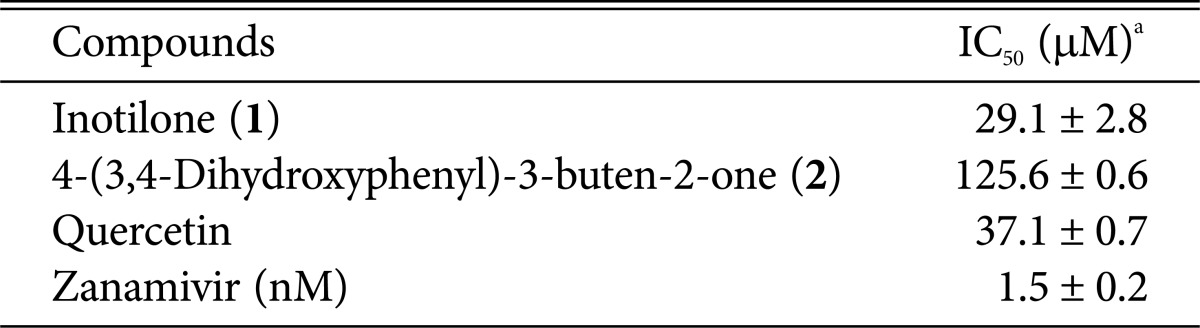
aResults are presented as mean IC50 values obtained from three independent experiments carried out in triplicate ± SD.
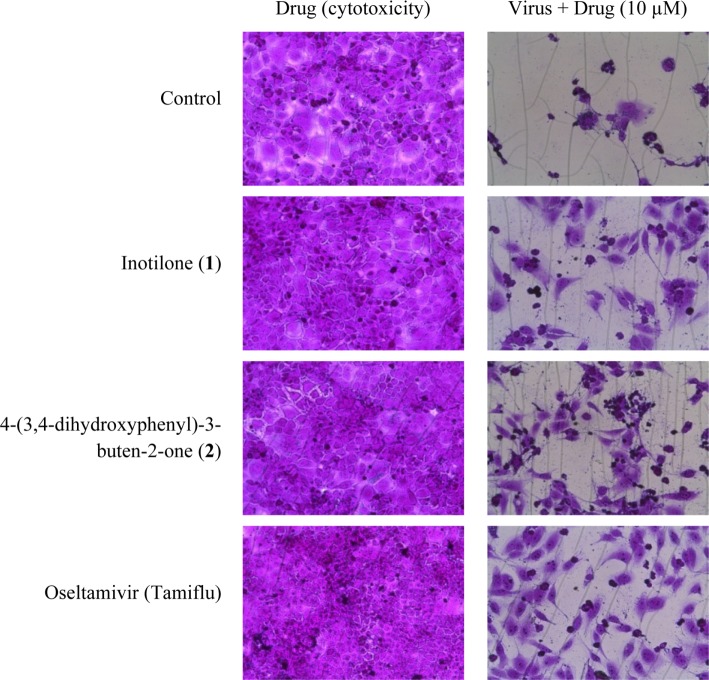
Antiviral effect and cytotoxicity were evaluated by the SRB method using the cytopathic effect (CPE) reduction method [20]. In brief, MDCK cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells/well. Then, 0.09 mL of diluted virus suspension and 0.01 mL of medium supplemented with trypsin-EDTA and containing 10 µg/mL of compounds 1 and 2 was added to each well. After incubation at 37℃ in 5% CO2 for 2 days, the morphology of cells was observed under a microscope at a magnification of 32 × 10 (AXIOVERT10; Zeiss, Jena, Germany), and images were recorded. After MDCK cells had undergone 2-day infection with the influenza A/WS/33 virus, mock cells or cells treated with compounds 1, 2 or oseltamivir showed typical spread-out shapes and normal morphology. At this concentration, no signs of cytotoxicity were observed. Infection with influenza A/WS/33 virus in the absence of compounds resulted in a severe CPE (Fig. 2). Addition of compounds 1 and 2 to influenza A/WS/33 virus-infected MDCK cells inhibited the formation of a visible CPE with IC50 values of 61.5 and 52.3 µM, respectively, while oseltamivir prevented CPE formation with an IC50 value of 64.7 µM. These results revealed that compounds 1 and 2 were more effective than the positive control oseltamivir against influenza virus H1N1 (Table 2).
Fig. 2.
Effects of compounds 1 and 2 on influenza A/WS/33 virus-induced cytopathic effect.
Table 2.
Antiviral activity of compounds 1 and 2 against influenza A virus in MDCK cellsa
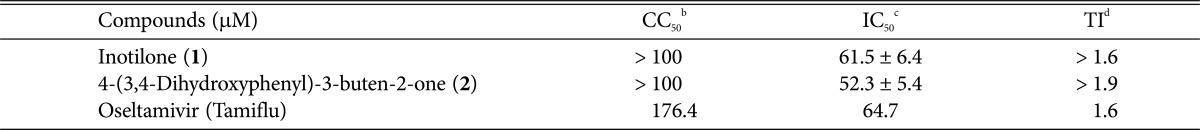
aResults are presented as mean IC50 values obtained from three independent experiments carried out in triplicate ± SD.
bConcentration required to reduce cell growth by 50%.
cConcentration required to inhibit virus-induced cytopathic effect by 50%.
dTherapeutic index = CC50/IC50.
In conclusion, inotilone and 4-(3,4-dihydroxyphenyl)-3-buten-2-one isolated from the fermentation broth of P. linetus were shown to be effective against H1N1 neuraminidase and the influenza A/WS/33 virus. Therefore, the potential of these compounds for use in the treatment of viral influenza infections merits additional attention.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Technology Development Program for Bio-industry, Ministry for Food, Agriculture, Forestry and Fisheries as well as support from the Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ009796012014), Rural Development Administration, Republic of Korea.
References
- 1.Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1647–1689. [Google Scholar]
- 2.Zhang J, Yu K, Zhu W, Jiang H. Neuraminidase pharmacophore model derived from diverse classes of inhibitors. Bioorg Med Chem Lett. 2006;16:3009–3014. doi: 10.1016/j.bmcl.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 3.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng K, Zhang Y, Xie L, Peng W, Gan B, Ren Z. Simultaneous determination of five fatty acids in Phellinus sp. by high-performance liquid chromatography with photodiode-array detection. J Med Plant Res. 2011;5:2816–2821. [Google Scholar]
- 6.Zhu T, Kim SH, Chen CY. A medicinal mushroom: Phellinus linteus. Curr Med Chem. 2008;15:1330–1335. doi: 10.2174/092986708784534929. [DOI] [PubMed] [Google Scholar]
- 7.Yeo WH, Hwang EI, So SH, Lee SM. Phellinone, a new furanone derivative from the Phellinus linteus KT&G PL-2. Arch Pharm Res. 2007;30:924–926. doi: 10.1007/BF02993957. [DOI] [PubMed] [Google Scholar]
- 8.Zheng YB, Lu CH, Shen YM. New abscisic acid-related metabolites from Phellinus vaninii. J Asian Nat Prod Res. 2012;14:613–617. doi: 10.1080/10286020.2012.681379. [DOI] [PubMed] [Google Scholar]
- 9.Yeom JH, Lee IK, Ki DW, Lee MS, Seok SJ, Yun BS. Neuraminidase inhibitors from the culture broth of Phellinus linteus. Mycobiology. 2012;40:142–144. doi: 10.5941/MYCO.2012.40.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IK, Yun BS. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J Antibiot (Tokyo) 2011;64:349–359. doi: 10.1038/ja.2011.2. [DOI] [PubMed] [Google Scholar]
- 11.Lee IK, Jung JY, Kim YH, Yun BS. Phellinins B and C, new styrylpyrones from the culture broth of Phellinus sp. J Antibiot (Tokyo) 2010;63:263–266. doi: 10.1038/ja.2010.25. [DOI] [PubMed] [Google Scholar]
- 12.Cho JY, Kwon YJ, Sohn MJ, Seok SJ, Kim WG. Phellinstatin, a new inhibitor of enoyl-ACP reductase produced by the medicinal fungus Phellinus linteus. Bioorg Med Chem Lett. 2011;21:1716–1718. doi: 10.1016/j.bmcl.2011.01.080. [DOI] [PubMed] [Google Scholar]
- 13.Jung JY, Lee IK, Seok SJ, Lee HJ, Kim YH, Yun BS. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J Appl Microbiol. 2008;104:1824–1832. doi: 10.1111/j.1365-2672.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 14.Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chem. 2003;82:593–597. [Google Scholar]
- 15.Lee YS, Kim YH, Shin EK, Kim DH, Lim SS, Lee JY, Kim JK. Anti-angiogenic activity of methanol extract of Phellinus linteus and its fractions. J Ethnopharmacol. 2010;131:56–62. doi: 10.1016/j.jep.2010.05.064. [DOI] [PubMed] [Google Scholar]
- 16.Kim HG, Yoon DH, Lee WH, Han SK, Shrestha B, Kim CH, Lim MH, Chang W, Lim S, Choi S, et al. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J Ethnopharmacol. 2007;114:307–315. doi: 10.1016/j.jep.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Ichinohe T, Ainai A, Nakamura T, Akiyama Y, Maeyama J, Odagiri T, Tashiro M, Takahashi H, Sawa H, Tamura S, et al. Induction of cross-protective immunity against influenza A virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia. J Med Virol. 2010;82:128–137. doi: 10.1002/jmv.21670. [DOI] [PubMed] [Google Scholar]
- 18.Huang GJ, Huang SS, Deng JS. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS One. 2012;7:e35922. doi: 10.1371/journal.pone.0035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Jeong HJ, Park JY, Kim YM, Park SJ, Cho JK, Park KH, Ryu YB, Lee WS. Selective and slow-binding inhibition of shikonin derivatives isolated from Lithospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorg Med Chem. 2012;20:1740–1748. doi: 10.1016/j.bmc.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Choi HJ, Song JH, Park KS, Kwon DH. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]