Abstract
Two new yeast records, Cryptococcus adeliensis YJ19-2 and Cryptococcus uzbekistanensis YJ10-4 were screened from 60 yeasts strains that were isolated and identified from wild flowers in Yokjido, Gyeongsangnam-do, Korea. The morphological and cultural characteristics of the newly recorded yeasts and the physiological functionalities of the supernatants and cell-free extracts obtained from their cultures were investigated. The two newly recorded yeasts did not form ascospores and pseudomycelia. They also grew well in yeast extract-peptone-dextrose broth. C. uzbekistanensis YJ10-4 grew in a vitamin-free medium and was also tolerant to sugar and salt. Antihypertensive angiotensin I-converting enzyme inhibitory activity of the supernatant from C. adeliensis YJ19-2 was high (71.8%) and its cell-free extract also showed very high (81.2%) antidiabetic á-glucosidase inhibitory activity.
Keywords: Morphological characteristics, New yeast records, Wild flowers, Yokjido
The GRAS strains of yeasts are traditionally used in the preparation of various Korean fermented foods including traditional rice wines and soy sauces [1, 2, 3]. Recently, some bioactive agents, such as antihypertensive angiotensin I-converting enzyme (ACE) inhibitors [4], ribonucleotides [5, 6] anti-angiogenic compounds [7], and antidementia β-secretase inhibitors [8], have been produced using Saccharomyces cerevisiae. However, almost all of the fungal strains were isolated only from soy sauce, traditional rice wine and their by-productts (meju or nuruk). Only a few researchers have isolated useful yeast strains from natural sources such as wild flowers, fruits, or cereals. It is necessary to isolate and characterize new yeast strains from natural sources and to screen them for their potential industrial use, as well as to further establish a yeast mycoflora map. We have previously isolated various new yeast strains from wild flowers in Daejeon city, Gejoksan [9, 10], Oseosan, Baekamsan [11, 12], coastal and inland areas [13], Gyeonggi-do and the Jeju island [14] in Korea. Furthermore, we have reported the production of the antigout xanthine oxidase inhibitor from one of these strains [14].
We have also isolated several yeast strains from wild flowers in Ulleungdo and Yokjido, Korea [15]. In this study, we describe screening of new records of yeasts from Yokjido, Gyeongsangnam-do, Korea, and evaluated their morphological characteristics and the physiological functions of the supernatants and cell-free extracts obtained from their cultures for the production of bioactive agents.
The morphological and cultural characteristics of the new records of yeasts were investigated according to theprotocols described in a previous paper [16]. Ascospore and pseudomycelium formation test were performed as follows: the new yeast strains were cultured in yeast extract-peptone-dextrose (YPD) medium at 30℃ for 24 hr and, then cultured for 5 days in an ascospore medium containing potassium acetate 1%, yeast extract 0.1% and dextrose 0.05%. The ascospores were then observed by microscopy. Furthermore, the new yeast strains were cultured at 30℃ for 7 days in YPD medium, yeast extract-malt extract medium, potato dextrose medium, and glucose-peptone-yeast extract agar containing dextrose 4%, peptone 0.5% and yeast extract 0.5%. Pseudomycelium formation was determined by observation of the shape of each cell in these cultures. The physiological functionality of supernatants and cell-free extracts from the two new yeast strains were investigated as follows: the newly recorded yeasts were cultured in the YPD medium at 30℃ for 2 days. After centrifugation at 10,000 ×g for 15 min, the supernatants and cells were obtained. The cells were disrupted by vortexing with sonication, centrifuged at 12,000 ×g for 20 min. The mixture was filtered to obtain the cell-free extract and supernatants. The physiological functionalities of these cell-free extracts and supernatants were determined as described in previous papers [8, 17, 18, 19].
Screening for unrecorded yeasts
New records of yeasts from Yokjido, Korea were screened from 60 yeast strains from Yokjido by searching KERIS, PubMed, and other fungal taxonomy databases [9, 11, 20]. Cryptococcus uzbekistanensis YJ10-4 and Cryptococcus adeliensis YJ19-2, which were isolated from Chrysanthemum coronarium in Yokjido, Korea, were finally screened as two new records of yeasts.
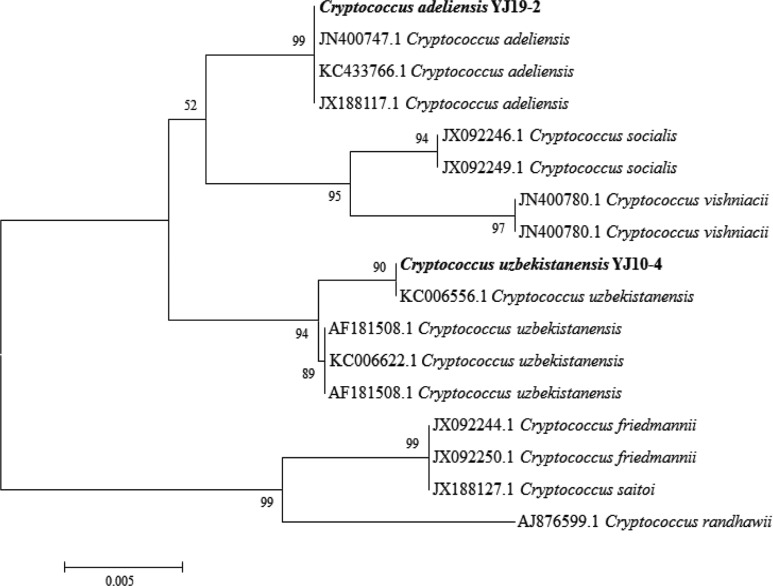
Phylogenetic analyses on these two newly recorded yeasts were performed using MEGA 5.1. The phylogenetic tree was constructed based on the large-subunit rDNA D1/D2 domain sequence. C. adeliensis YJ19-2 was closely grouped to C. adeliensis JN400747.1, KC433766.1 and JX188117.1 (Fig. 1). C. uzbekistanensis YJ10-4 was closely grouped to C. uzbekistanensis KC006556.1 (Fig. 1). Finally, we reconfirmed the two newly recorded yeasts as C. adeliensis YJ19-2 and C. uzbekistanensis YJ10-4 and submitted their sequences to the GenBank database with accession nos. KJ410348 (C. adeliensis YJ19-2) and KJ410347 (C. uzbekistanensis YJ10-4).
Fig. 1.
Phylogenetic tree of Cryptococcus adeliensis YJ19-2 and Cryptococcus uzbekistanensis YJ10-4 based on nucleotide sequences of large subunit rDNA. The tree was generated by the neighbor-joining method using MEGA 5.1.
C. uzbekistanensis was first isolated by Powel et al. [21] from an immunocompromised patient with lymphoma, and Yalcyn et al. [22] reported the molecular characterization and lipase profiling of C. uzbekistanensis isolated from environments contaminated with petroleum. Sipiczki [23] condueted a molecular taxonomic analysis of C. adeliensis isolated from Verbascum flowers, and Velázquez et al. [24] and Scorzetti et al. [25] reported xylanase production from C. adeliensis.
Characteristics of the newly recorded yeasts
The morphological and cultural characteristics of the two newly recorded yeasts are summarized in Table 1 and Fig. 2. C. uzbekistanensis YJ10-4 was round in shape, while C. adeliensis YJ19-2 was oval-shaped. Neither of the strains, formed ascospores or pseudomycelia.
Table 1.
Morphological and cultural characteristics of the newly reported yeast strains isolated from wild flowers in Yokjido, Gyeongsangnam-do, Korea
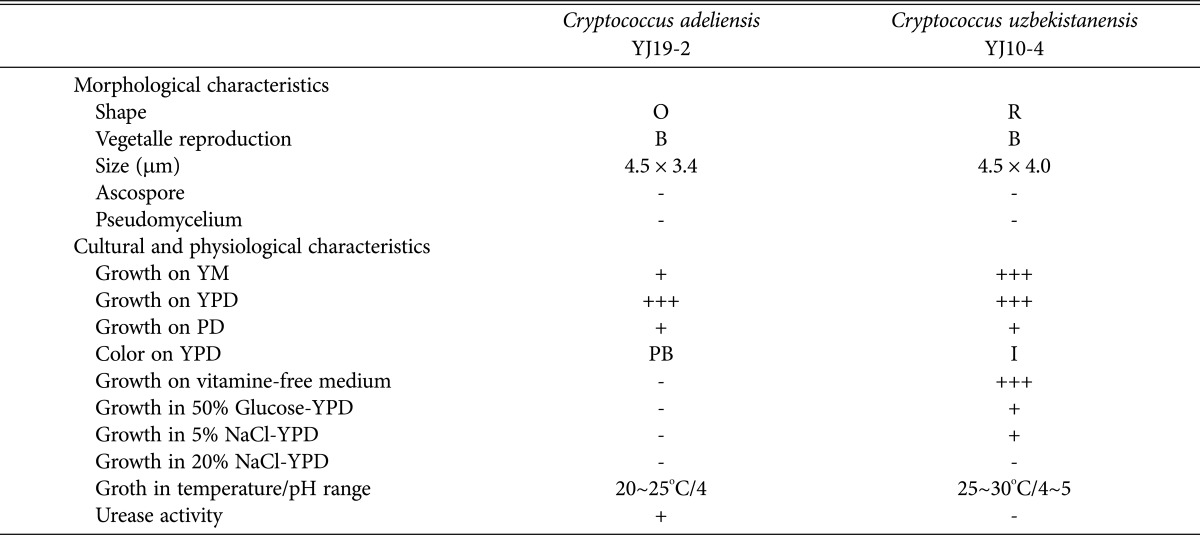
O, oval; R, round; B, budding; YM, yeast malt; YPD, yeast extract-peptone-dextrose; PD, peptone-dextrose; PB, pink beige; I, ivory.
Fig. 2.
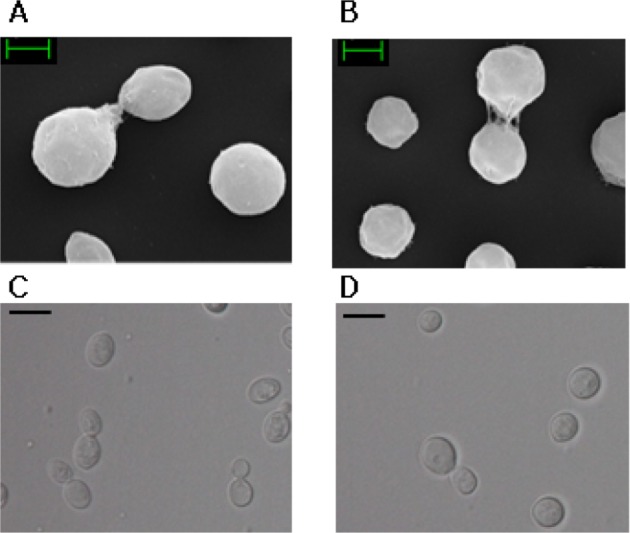
Morphological characterization of Cryptococcus uzbekistanensis YJ10-4 (A, C) and Cryptococcus adeliensis YJ19-2 (B, D). A, B, Scanning electron microscopy; C, D, Optical microscopy (scale bars: A, B = 5 µm, C, D = 2 µm).
The two newly recorded yeasts grew well in YPD medium, yeast extract-malt extract medium, and potato-dextrose broth. C. uzbekistanensis YJ10-4 grew in vitamin-free medium, was tolerant to sugar and salt and also grew in 50% glucose and 5% NaCl-YPD broth. The cell extracts dbtained from C. adeliensis YJ19-2 also showed urease activity.
We investigated the assimilation of various carbon sources by the two newly recorded yeasts using the API 20C AUX yeast identification kit (BioMériux, Marcy-I'Etoile, France) (Table 2). Both the yeasts utilized D-glucose, 2-keto-D-gluconate, L-arabinose, D-cellobiose, D-maltose, D-saccharose and D-raffinose. However, D-xylose, D-sorbitol, methyl-α-D-glucopyranoside and D-melezitose were only utilized by C. uzbekistanensis YJ10-4. In particular, C. adeliensis YJ19-2 utilized only seven kinds of carbon sources that were utilized by C. uzbekistanensis YJ10-4. Furthermore, the two newly recorded yeasts did not ferment all of the carbon sources tested, such as D-glucose, D-galactose, D-fructose, sucrose, maltose, lactose, mannose, raffinose, starch, cellobiose, and sorbitol (data not shown).
Table 2.
Carbon source assimilation by Cryptococcus uzbekistanensis YJ10-4 and Cryptococcus adeliensis YJ19-2
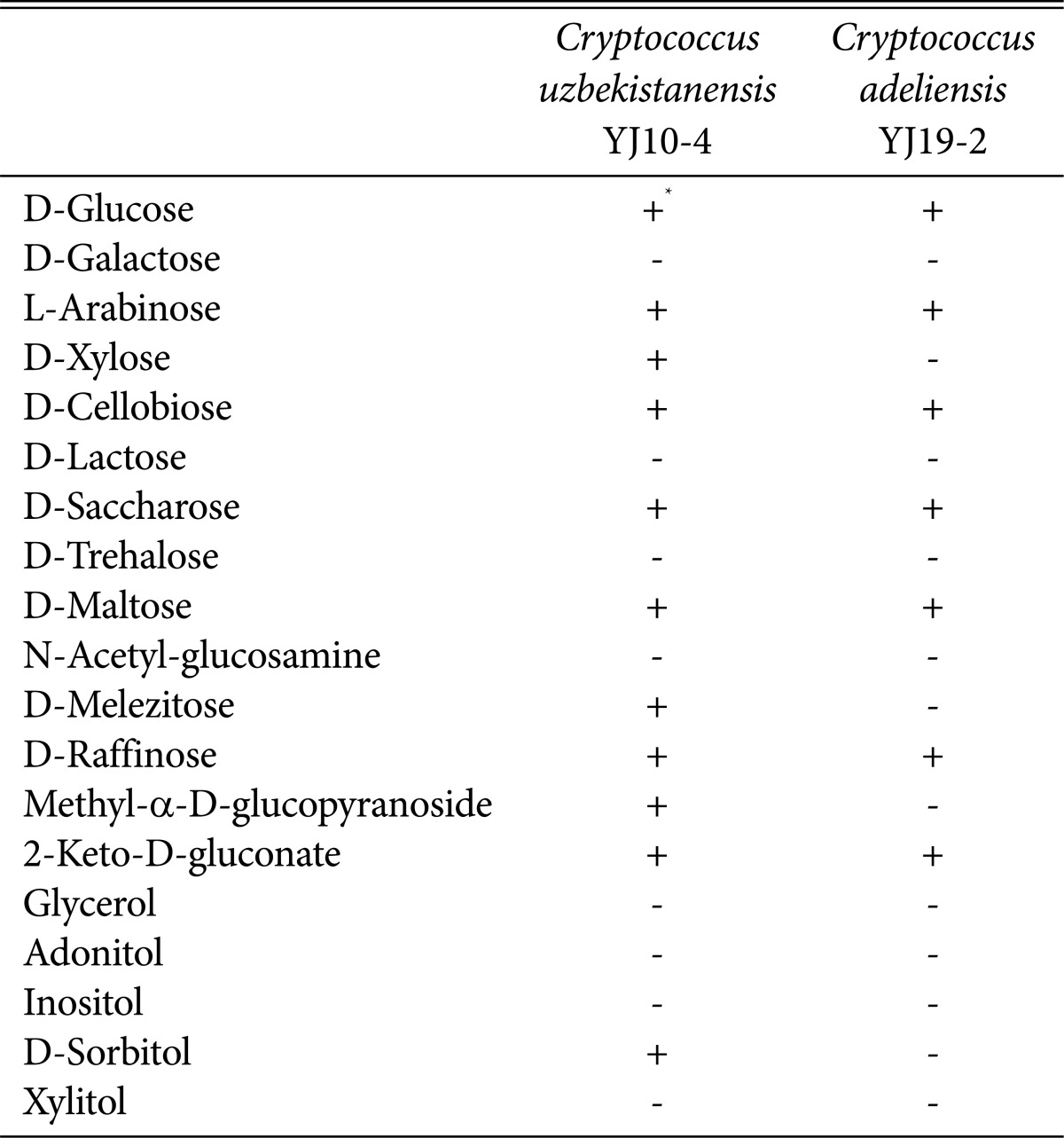
+, growth (assimilation); -, no growth (no assimilation).
We compared the morphological and cultural characteristics of these new yeast strains with those of the known strains of the respective species. C. adeliensis from Antarctica [25], which was obtained from the CBS-KNAW Fungal Biodiversity Center was very similar to C. adeliensis YJ19-2 in that both the strains appeared globose to subglobose, measured (3~9) × (2~7) µm in 5% malt extract medium, and looked cream colored after being cultured for 30 days. C. adeliensis also did not form ascospores and pseudomycelium or true mycelium. Its optimal growth temperature ranged from 25~30℃ and it did not grow in 50% glucose-YPD and 20% NaCl-YPD medium.
The first isolated C. uzbekistanensis strain was from s patient with lymphoma [21]; it was obtained from the CBS-KNAW Fungal Biodiversity Center, and the yeast extrac was very similar to the extract of the new yeast strain (C. uzbekistanensis YJ10-4) in terms of urease activity and growth of the yeast in 50% glucose-YPD. C. uzbekistanensis was round, approximately 5 µm in diameter, and asexually reproduced by budding. It did not form ascospores and pseudomycelia.
Physiological functionalities of the newly recorded yeasts
The physiological functionalities of the supernatants and cell-free extracts obtained from the newly recorded yeasts were investigated (Table 3). The antihypertensive ACE-inhibitory activities of supernatants from C. adeliensis YJ19-2 and C. uzbekistanensis YJ10-4 were 71.8% and 61.4%, respectively, and the inhibitory activities of the supernatants were also higher than those of the cell-free extracts (37.2% and 29.5%, respectively). These results were higher than those of the biomass from S. cerevisiae KCTC 7904 (42.1%), Pichia anomala (31.0%) [17], and its mutant (16.0%) [6]. Moreover, these results were similar to those for Pichia anomala KCCM 11473 (72.0%) and Kluyveromyces fragilis KCTC 7260 (68.9%) but lower than that for edible mushrooms, Pleurotus cornucopiae (78.0%) [16].
Table 3.
Physiological functionalities of the supernatants and cell-free extracts of the cultures of the newly reported yeast strains obtained from Yokjido, Korea
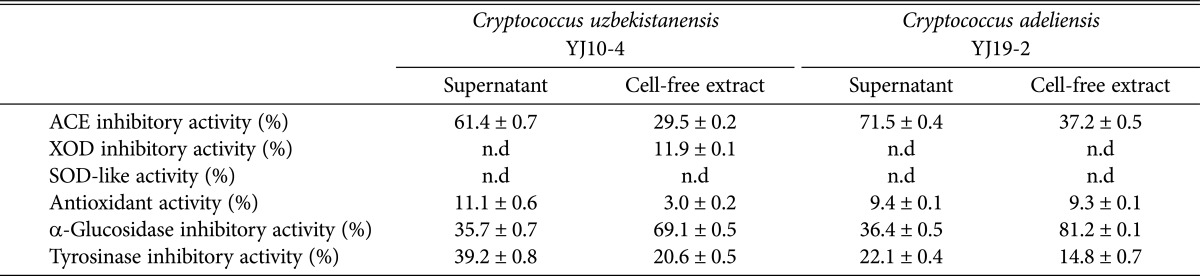
ACE, angiotensin I-converting enzyme; n.d, not detected; XOD, xanthine oxidase; SOD, superoxide dismutase.
The antidiabetic α-glucosidase-inhibitory activity of the cell-free extract from C. adeliensis YJ19-2 was very high (81.2%). This inhibitory activity was higher than that of Aspergillus oryzae N157-1 (48.3%) [26], but was lower than that of Pichia burtonii Y257-7 (90.9%) isolated from traditional Korean fermented foods [18]. Tyrosinase-inhibitory activity in the C. uzbekistanensis YJ10-4 supernatant was 39.2%, and the other physiological functionalities were either not detected or weak (15µ).
From the results described above, we conclude that antihypertensive ACE-inhibitory activity (71.8%) and antidiabetic α-glucosidase-inhibitory activity (81.2%) of a new yeast strain, C. adeliensis YJ19-2, were higher than those of other known yeasts and the other new yeast strain, C. uzbekistanensis YJ10-4. Therefore, C. adeliensis YJ19-2 would be very useful for food or medicinal applications as a potent bioactive compound-producing yeast.
ACKNOWLEDGEMENTS
This study was funded by the project on survey and excavation of Korean indigenous species of NIBR under the Ministry of Environment, Republic of Korea.
References
- 1.Jang IT, Kim YH, Yi SH, Lim SI, Lee JS. Screening of a new fibrinolytic substances-producing yeast. Korean J Mycol. 2011;39:227–228. [Google Scholar]
- 2.Kim JH, Kim NM, Lee JS. Physiological characteristics and ethanol fermentation of thermotolerant yeast Saccharomyces cerevisiae OE-16 from traditional meju. Korean J Food Nutr. 1999;12:490–495. [Google Scholar]
- 3.Lee JS, Choi YJ, Kwon SJ, Yoo JY, Chung DH. Screening and characterization of osmotolerant and gas-producing yeasts from traditional Doenjang and Kochujang. Food Biotechnol. 1996;5:54–58. [Google Scholar]
- 4.Kim JH, Lee DH, Jeong SC, Chung KS, Lee JS. Characterization of antihypertensive angiotensin 1-converting enzyme inhibitor from Saccharomyces cerevisiae. J Microbiol Biotechnol. 2004;14:1318–1323. [Google Scholar]
- 5.Kim JH, Lee BH, Lee JS. Production of ribonucleotides by autolysis of Hansenula anomala grown on Korean ginseng steaming effluent. J Biosci Bioeng. 2002;93:318–321. doi: 10.1016/s1389-1723(02)80035-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Hyun KW, Jeong SC, Kim JH, Choi YJ, Miguez CB. Production of ribonucleotides by autolysis of Pichia anomala mutant and some physiological activities. Can J Microbiol. 2004;50:489–492. doi: 10.1139/w04-032. [DOI] [PubMed] [Google Scholar]
- 7.Jeong SC, Lee DH, Lee JS. Production and characterization of an anti-angiogenic agent from Saccharomyces cerevisiae K-7. J Microbiol Biotechnol. 2000;16:1904–1911. [Google Scholar]
- 8.Lee DH, Lee DH, Lee JS. Characterization of a new antidementia β-secretase inhibitory peptide from Saccharomyces cerevisiae. Enzyme Microb Technol. 2007;42:83–88. [Google Scholar]
- 9.Kang MG, Hyun SH, Ryu JJ, Min JH, Kim HK, Lee JS. Note on newly isolated yeasts from wild flowers in Daejeon city, Korea. Korean J Mycol. 2012;40:174–176. [Google Scholar]
- 10.Min JH, Hyun SH, Kang MG, Lee HB, Kim CM, Kim HK, Lee JS. Isolation and identification of yeasts from wild flowers of Daejeon city and Chungcheongnam-do in Korea. Korean J Mycol. 2012;40:141–144. [Google Scholar]
- 11.Hyun SH, Lee HB, Kim CM, Lee JS. New records of yeasts from wild flowers in coast near areas and inland areas, Korea. Korean J Mycol. 2013;41:74–80. [Google Scholar]
- 12.Min JH, Ryu JJ, Kim HK, Lee JS. Isolataion and identification of yeasts from wild flowers in Gyejoksan, Oseosan and Beakamsan of Korea. Korean J Mycol. 2013;41:47–51. [Google Scholar]
- 12.Kim JH, Lee BH, Lee JS. Production of ribonucleotides by autolysis of Hansenula anomala grown on Korean ginseng steaming effluent. J Biosci Bioeng. 2002;93:318–321. doi: 10.1016/s1389-1723(02)80035-4. [DOI] [PubMed] [Google Scholar]
- 13.Min JH, Lee HB, Lee JS, Kim HK. Identification of yeasts isolated from wild flowers collected in coast areas of Korea based on the 26S rDNA sequences. Korean J Mycol. 2013;41:185–191. [Google Scholar]
- 14.Hyun SH, Mun HY, Lee HB, Kim HK, Lee JS. Isolation of yeasts from wild flowers in Gyonggi-do Province and Jeju Island in Korea and production of anti-gout xanthine oxidase inhibitor. Korean J Microbiol Biotechnol. 2013;41:383–390. [Google Scholar]
- 15.Hyun SH, Min JH, Lee HB, Kim HK, Lee JS. Isolation and diversity of yeasts from wild flowers in Ulleungdo and Yokjido, Korea. Korean J Mycol. 2014;42:28–33. [Google Scholar]
- 16.Jang JH, Jeong SC, Kim JH, Lee YH, Ju YC, Lee JS. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011;127:412–418. doi: 10.1016/j.foodchem.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim NM, So SH, Lee SG, Song JE, Seo DS, Lee JS. Physiological functionality and enzyme activity of biomass from Pichia anomala grown on ginseng-steaming effluent. Mycobiology. 2008;36:148–151. doi: 10.4489/MYCO.2008.36.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YH. Production and in-vivo anti-diabetic activity of α-glucosidase inhibitor from Pichia burtonii Y257-7 [dissertation] Daejeon: University of Paichai; 2014. [Google Scholar]
- 19.Noro T, Oda Y, Miyase T, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem Pharm Bull (Tokyo) 1983;31:3984–3987. doi: 10.1248/cpb.31.3984. [DOI] [PubMed] [Google Scholar]
- 20.Hyun SH, Lee HB, Lee JS. Characteristics of unrecorded yeasts, Rhodosporidium fluviale, Rhodosporidium paludigenum, Candida sp. 80-J-3 and Kluyveromyces thermotolerans isolated from wild flowers in Korea. Korean J Mycol. 2013;41:181–184. [Google Scholar]
- 21.Powel MS, Alizadeh AA, Budvytiene I, Schaenman JM, Banaei N. First isolation of Cryptococcus uzbekistanensis from an immunocompromised patient with lymphoma. J Clin Microbiol. 2012;50:1125–1127. doi: 10.1128/JCM.05678-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yalçın HT, Corbacý C, Uçar FB. Molecular characterization and lipase profiling of the yeasts isolated from environments contaminated with petroleum. J Basic Microbiol. 2013 May 26; doi: 10.1002/jobm.201300029. [Epub]. http://dx.doi.org/10.1002/jobm.201300029. [DOI] [PubMed] [Google Scholar]
- 23.Sipiczki M. Detection of yeast species also occurring in substrates associated with animals and identification of a novel dimorphic species in Verbascum flowers from Georgia. Antonie Van Leeuwenhoek. 2013;103:567–576. doi: 10.1007/s10482-012-9841-9. [DOI] [PubMed] [Google Scholar]
- 24.Velázquez E, del Villar M, Grondona I, Monte E, González-Villa T. Ultrastructural and chemotaxonomic analysis of a xylanolytic strain of Cryptococcus adeliensis isolated from sheep droppings in Spain. Arch Microbiol. 2006;186:195–202. doi: 10.1007/s00203-006-0134-4. [DOI] [PubMed] [Google Scholar]
- 25.Scorzetti G, Petrescu I, Yarrow D, Fell JW. Cryptococcus adeliensis sp. nov., a xylanase producing basidiomycetous yeast from Antarctica. Antonie Van Leeuwenhoek. 2000;77:153–157. doi: 10.1023/a:1002124504936. [DOI] [PubMed] [Google Scholar]
- 26.Kang MG, Yi SH, Lee JS. Production and characterization of a new α-glucosidase inhibitory peptide from Aspergillus oryzae N159-1. Mycobiology. 2013;41:149–154. doi: 10.5941/MYCO.2013.41.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]